Abstract
The presence of signet-ring cells in an endometrial adenocarcinoma is extremely uncommon and it is always necessary to rule out a metastatic neoplasm. We report a FIGO grade 2 endometrial carcinoma with a signet-ring cell component found in the curettage performed to a 53-year-old woman. The neoplastic proliferation was also found in the endometrium of the radical hysterectomy with bilateral salpingo-oophorectomy and pelvic and para-aortic lymphadenectomy. The uterine neoplasm invaded less than one-half of the myometrium (FIGO stage I B). Alcian blue showed the presence of mucin in the signet-ring cells. The patient was alive and without evidence of recurrence 14 months after surgery. Polymerase chain reaction method from paraffin-embedded tissue revealed the presence of human papilloma virus type 11. We have discussed the differential diagnosis of this kind of neoplasm and we have reviewed the literature on signet-ring cell carcinoma of the endometrium.
1. Introduction
Signet-ring cell (SRC) carcinoma is defined as a tumor composed predominantly or exclusively of SRCs, characterized by a central, optically clear, globoid droplet of cytoplasmic mucin with an eccentrically placed nucleus. SRCs are generally rare in primary adenocarcinomas of the female genital tract.
To the best of our knowledge primary carcinoma of the endometrium with SRCs has only been observed in four previous cases [1–3] (Table 1). In fact primary pure endometrial SRCs adenocarcinomas of the genital tract are extremely rare, while more often they may be seen admixed with other more conventional types. In this paper we report a new case of endometrial adenocarcinoma (EA) with SRCs component. We have reviewed the literature in order to emphasize the histological criteria in the diagnosis of this very unusual malignancy.
Table 1.
Endometrial adenocarcinoma with signet-ring cells: review of the literature.
| Case | Authors | Age/years | Diagnosis | Treatment | Stage | Followup |
|---|---|---|---|---|---|---|
| 1 | Mooney et al., 1997 [1] | 65 | SRC | Hysterectomy, B.S., pelvic and para-aortic L. partial O. abdominal, pelvic washing | NS | Free of disease 6 months after surgery |
|
| ||||||
| 2 | Chebib et al., 2010 [2] | 51 | Primary SRC | Hysterectomy, B.S., L. abdominal, pelvic washing | FIGO IVB | Death of metastatic disease 6 months after surgery |
|
| ||||||
| 3 | Boyd et al., 2010 [3] | 46 | Primary mucinous adenocarcinoma of the endometrium with signet-ring cells arising in adenomyosis | Subtotal hysterectomy | NS | NS |
|
| ||||||
| 4 | Boyd et al., 2010 [3] | 59 | Primary endometrioid adenocarcinoma of the endometrium with signet-ring cells | Hysterectomy | NS | NS |
|
| ||||||
| Our case | 53 | Primary endometrioid adenocarcinoma of the endometrium with signet-ring cells | Radical hysterectomy with B S L | FIGO stage IB | Free of disease 14 months after surgery | |
LLegend
SRCE: signet-ring cell carcinoma of the endometrium.
B.: bilateral.
S.: salpingo-oophorectomy.
L.: lymphadenectomy.
O.: omentectomy.
NS: not specified.
2. Materials and Methods
A 53-year-old multiparous (gravida 4, para 4) woman was referred to the Department of Obstetrics and Gynecology for persistent abnormal vaginal bleeding of three-month duration. An endometrial curettage was performed. An extensive search for an extrapelvic primary cancer was undertaken, but abdominopelvic computed tomography (CT), mammography, cystoscopy, esophagogastroduodenoscopy, and colonoscopy revealed no evidence of malignancy. The patient underwent a radical hysterectomy with bilateral salpingo-oophorectomy and pelvic and para-aortic lymph node dissection.
The patient provided written informed consent to perform the study. Tissue specimens were fixed in 10% neutral-buffered formalin and were paraffin-embedded according to standard procedures. Three-micrometer sections of representative blocks were deparaffinized in xylene, rehydrated, and treated with 3% H2O2 in TBS for 5 minutes to block endogenous peroxidase activity. Antigen retrieval procedure was performed by microwave oven heating in citrate buffer (pH 6) for each antibody. Cells expressing estrogen receptor (ER) (clone 6F11; Novocastra, Leica Byosystems Newcastle Ltd., UK), progesterone receptor (PR) (clone PGR-312; Novocastra, Leica Byosystems Newcastle Ltd., UK), and Ki-67 (clone MM1; Novocastra, Leica Byosystems Newcastle Ltd., UK) were identified after overnight incubation at 4°C. Sections were incubated with a secondary poly-HRP anti-mouse/rabbit IgG reagent (Bond Polimer Refine Detection; Leica Byosystems Newcastle Ltd., UK) against ER, PR, and Ki-67. The slides were developed with diaminobenzidine (DAB), counterstained with Mayer hematoxylin, dehydrated in ethanol and xylene, and finally mounted. Immunohistochemical staining was performed using the avidin-biotin complex method with antibodies direct against the following antigens: synaptophysin (1 : 100, Dako, Glostrup, Denmark), chromogranin A (1 : 700, Dako), neuron-specific enolase (1 : 200, Dako), carcinoembryonic antigens (CEA) (1 : 100, Dako), vimentin (1 : 700, Dako), and E-cadherin (1 : 60, Dako). P16 immunostain was performed using a monoclonal antibody to the p16INK4A antigen (E6H4, 1 : 100 dilution; CINtec Histology, Heidelberg, Germany). All immunostains were performed using a Ventana Benchmark LT and XT automated immunostainers (Ventana Medical Systems, Tucson, AZ). Polymerase chain reaction (PCR) method was made to send the presence of HPV. DNA was extracted from the neoplastic tissue included in paraffin blocks. DNA amplification was made using a “Rotor-Gene Q” (Qiagen, Germany). DNA extraction from formalin-fixed and paraffin-embedded samples was performed using a Qiagen kit. Molecular analysis was performed by nested polymerase chain reaction (PCR) method using primer outer MY09/11 and primers inner GP5+/6+ for amplification L1 region using AB-Analitica Kit. Mucicarmine, PAS, and alcian blue were used to highlight the mucin content in the tumor signet-ring cells.
3. Results
FIGO grade 2 endometrioid adenocarcinoma (EA) of usual type with SRCs component was diagnosed in endometrial curettage (Figures 1 and 2). Foci of atypical endometrial hyperplasia were found. In the hysterectomy specimen, the moderately differentiated G2 endometrioid adenocarcinoma comprised 70% of the tumour, while 30% was represented by a SRCs component. The uterine neoplasm invaded less than one-half of the myometrium (FIGO stage I B). Isthmus and uterine cervix were free of disease. Special stains showed the presence of mucin in the SRCs component (Figure 3). ER and PR immunostains were negative in the SRCs tumoral component while they showed weak positivity in the endometrioid adenocarcinoma. The results of the additional immunostains were similar in the two components (Figure 4) (Table 2). Polymerase chain reaction method from paraffin-embedded tissue revealed the presence of human papilloma virus type 11 (Figures 5 and 6). The patient was alive and without evidence of recurrence 22 months after surgery.
Figure 1.
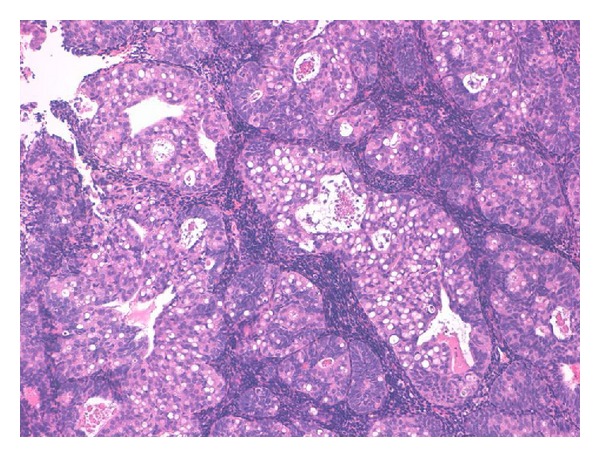
Endometrial curettage showed a neoplasm composed of glandular elements and solid areas with signet-ring cells (10x, hematoxylin and eosin).
Figure 2.
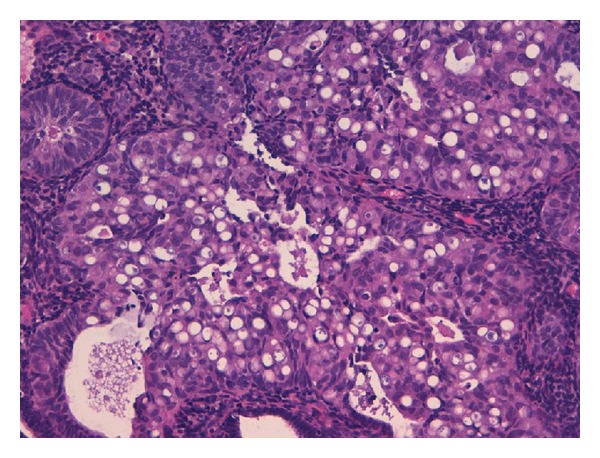
Signet-ring cells with vacuolated cytoplasm (20x, hematoxylin and eosin).
Figure 3.
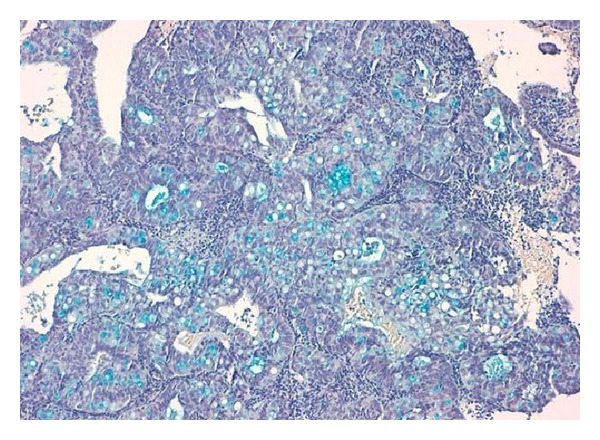
Alcian blue shows the presence of mucin in the signet-ring cells (10x alcian blue).
Figure 4.
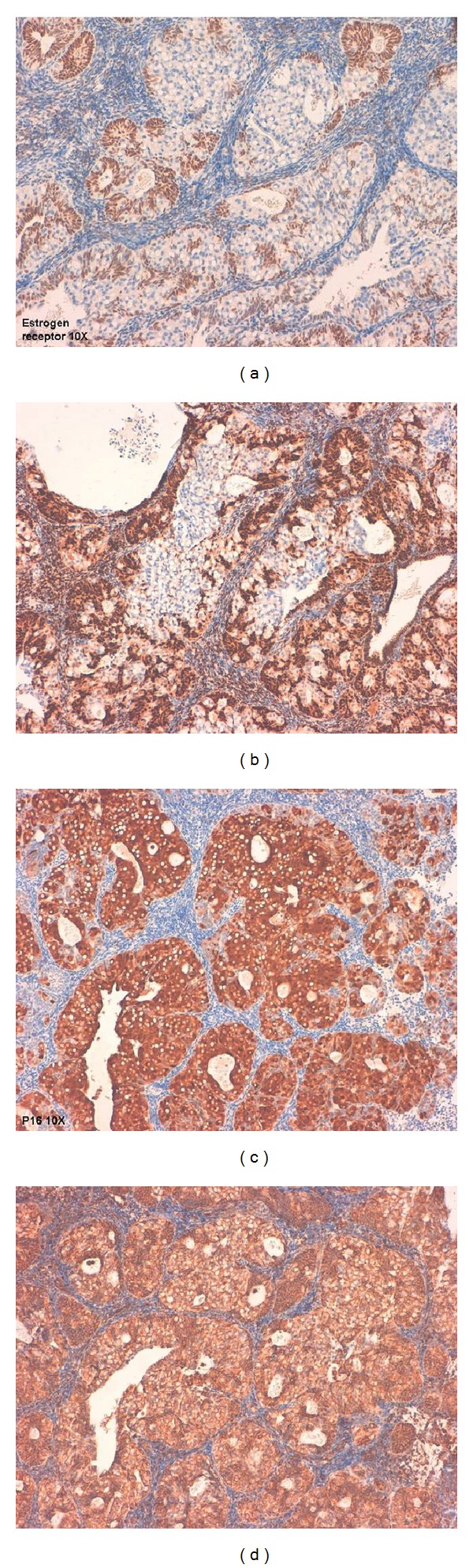
Estrogen Receptor (a) and Progesteron Receptor (b) immunostains are negative in the signet-ring cells component. P16 (c) and vimentin (d) immunostains are strongly positive in the signet-ring cell component (10x).
Table 2.
Endometrioid carcinoma of the endometrium with signet-ring cells: immunohistochemical results in two tumoral components of our case.
| Immunostaining | Endometrioid adenocarcinoma | Signet-ring cell carcinoma |
|---|---|---|
| CD56 | Negative | Negative |
| Synaptophysin | Negative | Negative |
| CEA | Negative | Negative |
| Ki-67 | 5% | <2% |
| Chromogranin A | Negative | Negative |
| E-cadherin | Positive | Positive |
| HER-2 | Negative | Negative |
| Estrogen receptor | Focally positive | Negative |
| Progesterone receptor | Weak positivity | Negative |
| Vimentin | Positive | Positive |
| p16 | Positive | Positive |
Figure 5.
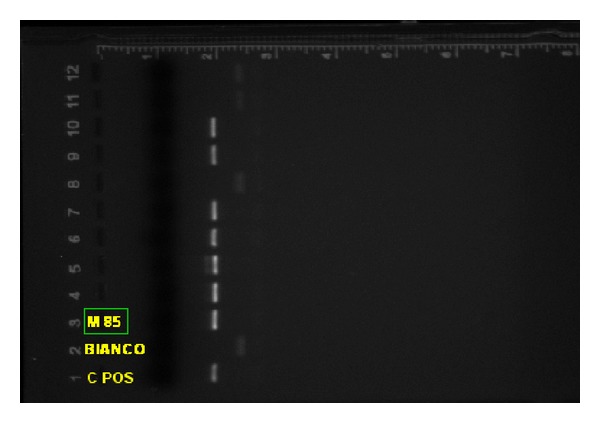
HPV positive view on agarose gel 2% (case identified as M-85).
Figure 6.
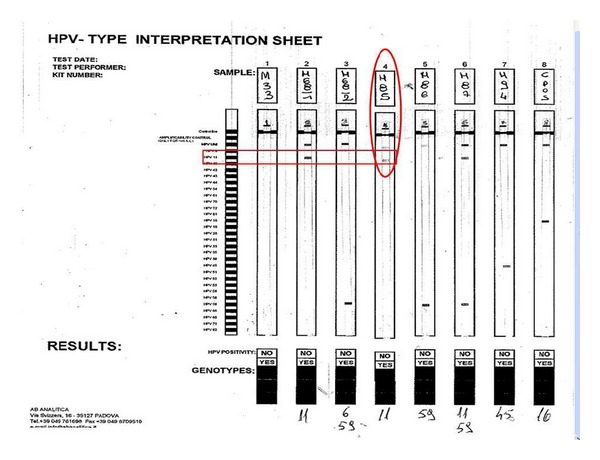
Genotyping case M-85 with HPV 11 positive.
4. Discussion
Among primary adenocarcinomas of the genital tract, SRCs tumours are generally rare. The World Health Organization (WHO) Classification [4] of the uterine cervix tumours describes SRC carcinoma as a rare variant in “pure form.” SRCs more commonly occur as a focal finding in poorly differentiated mucinous adenocarcinomas and adenosquamous carcinomas. WHO Classification [5] and the main gynaecologic pathology textbooks [6] do not report SRC carcinoma of the uterine corpus. To date four cases of endometrial adenocarcinoma with SRCs have been described in the literature. The tumour reported by Chebib et al. [2] was formed by two components and the percentage of each component was not specified. We think that the title of the paper is not accurate because the neoplasm is a mixed tumour composed of SRC carcinoma and endometrioid adenocarcinoma. Mooney et al. [1] report foci of atypical endometrial hyperplasia and FIGO grade I endometrioid carcinoma of the usual type in the endometrial curettage of a 51-year-old postmenopausal woman. Occasionally, vacuolated cytoplasm and SRCs were found. Mooney et al. [1] did not specify the percentage of the SRC component. We believe that the title of the paper is not accurate, so that the tumour would be defined as “endometrioid adenocarcinoma of the uterine corpus with SRCs component.” The title of the case described by Boyd et al. [3] is correct. The authors described a grade I EA in an endometrial polyp, with presence of small foci of solid tumour composed of SRCs. The subsequent hysterectomy did not reveal residual EA. It is evident that the histotype of the tumour is “endometrioid adenocarcinoma with SRCs.” The authors avoided the title “primary SRCs adenocarcinoma of the endometrium.” We believe that uterine neoplasm should be termed as “SRC adenocarcinoma of the endometrium” when the lesion is exclusively composed of SRCs. To date no case of true “primary SRC carcinoma of the endometrium” has been reported. The presence of SRCs cells in a carcinoma of the uterine corpus strongly raises the possibility of a metastasis. The most common extrauterine carcinomas that metastasize to or extend into the endometrium arise in the ovary, breast, or gastrointestinal tract, especially the colon. Therefore, abnormal uterine bleeding can be the first clinically apparent manifestation of disseminated disease [7, 8]. The myometrium is invaded by metastases more often than the endometrium. The extragenital malignancy that most frequently metastasizes to uterus is breast carcinoma; it is followed by primary gastrointestinal carcinomas, especially from stomach and colon. Metastatic breast carcinoma in the uterus is frequently characterized by polygonal cells or SRCs, often in a linear or single-file arrangement. An immunostain for gross cystic disease fluid protein-15 would help to identify the primary site of a breast metastasis. Metastatic gastrointestinal carcinomas are also characterized by SRCs or well-formed glandular structures. Immunohistochemical stains for CEA may be helpful in establishing whether the tumour is metastatic from the gastrointestinal tract. Generally, colonic primary tumours are diffusely positive for CEA while EAs are not. Also, endometrial carcinomas usually are positive for cytokeratin 7 and negative for cytokeratin 20, while metastatic colonic adenocarcinoma is usually strongly positive for cytokeratin 20 and negative for cytokeratin 7. HPV has emerged as one of the most important risk factors for human cancer and is recognized as an etiologic agent in virtually all cases of cervical cancers. Furthermore, HPV is also linked to other anogenital cancers as well as to a subset of head and neck cancers [9]. However, the relationship between HPV and other malignancies including upper genital tract, respiratory tract, digestive tract, and breast carcinomas is not clear [10, 11]. The role of HPV in endometrial carcinomas has been investigated giving contradictory results. The studies showed that the presence of HPV DNA in endometrial cancers differed in a range from 9% to 24%. In most cases HPVs were defined as high-risk type. HPV DNA, mostly 16 and 18 subtypes, was more intensively present in areas of squamous differentiation [12–16]. Karadayi et al. [17] believe that HPV does not play any role in the pathogenesis of endometrial carcinoma, since endometrium may not be a suitable host for HPV replication [17]. In our case the polymerase chain reaction (PCR) method revealed the presence of type 11 HPV. This finding is very surprising because the demonstration of the presence of HPV subtypes is usually used to support the cervical primitive origin of the adenocarcinoma. In our case the uterine cervix was free of tumour in the hysterectomy specimen. Immunohistochemical and molecular studies can help to distinguish endometrial from endocervical primary tumours. ER and PR immunostains were positive in EA, while they are negative in cervical adenocarcinomas. The combination of hormone receptors and HPV molecular detection appears to be very useful in this differential diagnosis. In our case ER and PR were negative in the SRC neoplastic component and weakly positive in the endometrioid adenocarcinoma. Preliminary data suggest that strong diffuse expression of p16ink4, which occurs in close to 100% of cervical squamous carcinomas and adenocarcinomas, is either absent or only patchy in endometrioid carcinomas [18]. p16ink4 is expressed strongly in lesions associated with intermediate- and high-risk HPV types, in contrast to low-risk HPV infection [19]. In selected head and neck squamous cell carcinomas, mainly from the oropharynx and sinonasal cavity, p16ink4 positivity correlates well with high-risk HPV infection. P16ink4 is not a reliable indicator of high-risk HPV infection in squamous cell carcinomas of the lung, Skin urinary bladder, and esophagus [20, 21].
5. Conclusion
In conclusion the expression of immunohistochemical markers was similar in the two components of the malignancy examined in the present study. The endometrial origin is documented by the histopathological examination of the hysterectomy specimen because uterine cervix and isthmus were free of disease.
Conflict of Interests
There is no conflict of interests regarding the publication of this paper.
References
- 1.Mooney EE, Robboy SJ, Hammond CB, Berchuck A, Bentley RC. Signet-ring cell carcinoma of the endometrium: a primary tumor masquerading as a metastasis. International Journal of Gynecological Pathology. 1997;16(2):169–172. doi: 10.1097/00004347-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Chebib I, Chu P, Duggan MA, Difrancesco LM. Primary signet-ring cell adenocarcinoma of the endometrium: case report and review of the literature. International Journal of Gynecological Pathology. 2010;29(3):269–272. doi: 10.1097/PGP.0b013e3181c1cbf2. [DOI] [PubMed] [Google Scholar]
- 3.Boyd C, Cameron I, McCluggage WG. Endometrial adenocarcinoma with signet ring cells: report of two cases of an extremely rare phenomenon. International Journal of Gynecological Pathology. 2010;29(6):579–582. doi: 10.1097/PGP.0b013e3181e20c66. [DOI] [PubMed] [Google Scholar]
- 4.Wells M, Ostor AG, Crum CP, et al. Epithelial tumours. In: Fattaneh A, Tavassoli, Devilee P, editors. Pathology & Genetics of Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press; 2003. pp. 272–276. (World Health Organization Classification of Tumours). [Google Scholar]
- 5.Silverberg SG, Kurman RJ, Nogales F, Mutter GL, Kubik-Huch RA, Tavassoli FA. Epithelial tumours and related lesions. In: Fattaneh A, Tavassoli, Devilee P, editors. Pathology & Genetics of Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press; 2003. pp. 221–228. (World Health Organization Classification of Tumours). [Google Scholar]
- 6.Crum CP, Duska LR, Nucci MR. Adenocarcinoma, carcinosarcoma, and other epithelial tumors of the endometrium. In: Crum CP, Duska LR, Nucci MR, editors. Diagnostic Gynecologic and Obstetric Pathology. 2th edition. Philadelphia, Pa, USA: Elsevier Saunders; 2006. pp. 517–574. [Google Scholar]
- 7.Kumar A, Schneider V. Metastases to the uterus from extrapelvic primary tumors. International Journal of Gynecological Pathology. 1983;2(2):134–140. doi: 10.1097/00004347-198302000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kumar NB, Hart WR. Metastases to the uterine corpus from extragenital cancers. A clinicopathologic study of 63 cases. Cancer. 1982;50(10):2163–2169. doi: 10.1002/1097-0142(19821115)50:10<2163::aid-cncr2820501032>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Javier RT, Butel JS. The history of tumor virology. Cancer Research. 2008;68(19):7693–7706. doi: 10.1158/0008-5472.CAN-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yavuzer D, Karadayi N, Salepci T, Baloglu H, Dabak R, Bayramicli OU. Investigation of human papillomavirus DNA in colorectal carcinomas and adenomas. Medical Oncology. 2011;28(1):127–132. doi: 10.1007/s12032-010-9416-4. [DOI] [PubMed] [Google Scholar]
- 11.Hoory T, Monie A, Gravitt P, Wu T-C. Molecular epidemiology of human papillomavirus. Journal of the Formosan Medical Association. 2008;107(3):198–217. doi: 10.1016/S0929-6646(08)60138-2. [DOI] [PubMed] [Google Scholar]
- 12.Giatromanolaki A, Sivridis E, Papazoglou D, Koukourakis MI, Maltezos E. Human papillomavirus in endometrial adenocarcinomas: infectious agent or a mere “passenger”? Infectious Diseases in Obstetrics and Gynecology. 2007;2007:4 pages. doi: 10.1155/2007/60549.60549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang L, Malpica A, Deavers MT, et al. Endometrial endometrioid adenocarcinoma of the uterine corpus involving the cervix: some cases probably represent independent primaries. International Journal of Gynecological Pathology. 2010;29(2):146–156. doi: 10.1097/PGP.0b013e3181b8e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ansari-Lari MA, Staebler A, Zaino RJ, Shah KV, Ronnett BM. Distinction of endocervical and endometrial adenocarcinomas: immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. American Journal of Surgical Pathology. 2004;28(2):160–167. doi: 10.1097/00000478-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Chinen K, Kamiyama K, Kinjo T, et al. Morules in endometrial carcinoma and benign andometrial lesions differ from squamous differentiation tissue and are not infected with human papillomavirus DNA in malignant lesions from Chinese women with carcinomas of the upper genital tract. Gynecologic Oncology. 2008;87(1):104–111. [Google Scholar]
- 16.Jones MW, Onisko A, Dabbs DJ, Elishaev E, Chiosea S, Bhargava R. Immunohistochemistry and HPV in situ hybridization in pathologic distinction between andocervical and endometrial adenocarcinoma. A comparative tissue microarray study of 76 tumors. International Journal of Gynecological Cancer. 2013;23(2):380–384. doi: 10.1097/IGC.0b013e31825cc8ee. [DOI] [PubMed] [Google Scholar]
- 17.Karadayi N, Gecer M, Kayahan S, et al. Association between human papillomavirus and endometrial adenocarcinoma. Medical Oncology. 2013;30(3, article 597) doi: 10.1007/s12032-013-0597-5. [DOI] [PubMed] [Google Scholar]
- 18.McCluggage WG, Jenkins D. p16 immunoreactivity may assist in the distinction between endometrial and endocervical adenocarcinoma. International Journal of Gynecological Pathology. 2003;22(3):231–235. doi: 10.1097/01.PGP.0000055172.04957.2F. [DOI] [PubMed] [Google Scholar]
- 19.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. American Journal of Pathology. 1998;153(6):1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doxtader EE, Katzenstein A-LA. The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: a study of 137 cases. Human Pathology. 2012;43(3):327–332. doi: 10.1016/j.humpath.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Alexander RE, Hu Y, Kum JB, et al. p16 expression is not associated with human papillomavirus in urinary bladder squamous cell carcinoma. Modern Pathology. 2012;25(11):1526–1533. doi: 10.1038/modpathol.2012.103. [DOI] [PubMed] [Google Scholar]


