Abstract
Articular cartilage lesions of the distal femur and patella are common. In order to provide an accurate diagnosis of a clinically symptomatic cartilage lesion and subsequent appropriate planning for potential treatment options a proper staging is required. This includes clinical exam, radiographic imaging, and arthroscopy. Once the staging is completed other co-morbidities may need to be addressed that may require additional surgical procedures. These can either be planned as staged procedures of concomitantly with a cartilage repair procedure. This article will discuss this staging and evaluation process in depth in order to serve as a guideline to the orthopaedic surgeon engaged in the treatment of cartilage defects in patients with early posttraumatic osteoarthritis.
Keywords: Cartilage, staging, treatment, diagnosis
Introduction
Articular cartilage injuries are common.1-3 The spectrum of these injuries ranges from small, superficial defects (focal chondral defects) to complete degenerative delamination of entire condyles with or without involvement of the subchondral bone and adjacent structures (Osteoarthritis). In an ideal world, focal chondral defects exist in isolation, have clearly defined borders, are solitary defects and are located in ideally accessible anatomic locations in young patients that are physically active. These types of lesions are the standard that is currently being used to enroll patients into randomized clinical trials investigating the efficiency of articular cartilage procedures. While these studies are important and necessary in order to compare different techniques, the reality is that most patients (95%) that are presenting with clinically symptomatic cartilage lesions do not fit these clear cut criteria.4 This presents a dilemma to the surgeon as the cartilage lesions most commonly treated are usually less clear cut and often involve “best clinical judgment” in order to perform an adequate assessment. This assessment process, or “staging”, is necessary in order to guide both, patient and physician, towards a clinically feasible and satisfying solution for the knee cartilage injury patient. The staging process requires knowledge about frequency and prevalence of cartilage defects, their clinical symptoms, arthroscopic grading and sizing as well as assessment of the joint environment. Furthermore specific co-morbidities have to be taken into account prior to performing cartilage repair procedures, as many of them require additional staged or concomitant surgical procedures. In this article we will sequentially discuss the most pertinent factors that influence the decision making process in patients with symptomatic cartilage lesions of the knee.
Frequency and prevalence of cartilage injuries
Damage to articular cartilage is common and can result from acute traumatic injuries, early posttraumatic degenerative changes, developmental factors affecting the subchondral bone such as Osteochondritis Dissecans (OCD) lesions or acquired metabolic factors such as avascular necrosis (AVN).1-3
Articular cartilage lesions are frequently encountered in routine knee arthroscopies. Curl et al. reported articular cartilage lesions in as many as 63% of over 35,000 knee arthroscopies in the US.3 This high incidence was corroborated by Hjelle et al. in Norway and Widuchowski et al. in Poland who reported and incidence of 61% and 60% respectively1, 2. The average age of patients reported in these studies is high and thus the percentage of treatable lesions in younger patients is likely much lower. In fact, upon further sub-analysis of Curl’s data, 60% of the reported lesions were grade III lesions and thus are potentially treatable lesions. Only 1750 patients out of 31,516 were under the age of 40 and had Outerbridge grade III lesions. Based upon this study one can estimate that approximately 5% of all patients under 40 undergoing arthroscopies may present with a chondral lesion that can technically be treated. These studies, however, document the prevalence of these lesions; no information is available how many of those lesions may be clinically symptomatic. Interesting is that the mere presence of a lesion does not seem to lead to an increase in OA rate over time in large cross-sectional studies, as the long-term natural history study conducted by Widuchowski in 2010 suggests.5 However, Shelbourne et al. found that 123 out of 2700 patients with ACL injuries and cartilage lesions at the time of surgery showed lower subjective Noyes’ scores 8 years after ACL reconstruction compared to patients who did not have cartilage lesions at the time of surgery.6 The presence of cartilage lesions does lead to rapid progression of radiographic OA as documented by Messner and Maletius.7 These findings underline the importance of identifying the patient who has a clinically symptomatic cartilage lesions that may benefit from early treatment.
Lesion location and size
The location of cartilage lesions is spread between the three compartments of the knee. Lesions are most commonly found in the weight-bearing femoral condyle (43-58%). Patella lesions are frequently encountered and account for 11-36% of all lesions. Trochlea lesions overall are less frequent (6-16%).1-3
When analyzed for lesion size Hjelle et al. were able to show that the majority of lesions (88%) were below 4 cm.1,2
Widuchowski et al. found that 60% of knees (average 39 years old) contained chondral/osteochondral lesions, 68% of which were focal chondral lesions, 3% being OCD lesions and 29% being osteoarthritic lesions.8
History and Physical examination
The clinical evaluation of patients with symptomatic cartilage lesions in the knee is difficult and follows the recommendations of a thorough history and physical exam of the knee joint. No true evidence based approach is available to guide the clinician but several factors that may be important should be pointed out.
Upon initial evaluation it is important to discover the history of symptoms that may be related to a cartilage lesion. Length of symptoms has been associated with clinical outcome in patients undergoing microfracture. Mithoefer et al. could show that patients with symptoms longer than 1 year had lower overall subjective outcome results than patients with more acute cartilage injuries.9 There is a correlation of worse overall clinical outcomes after cartilage procedures in patients who receive workmen’s compensation.10,11 History of smoking and family history of OA are often considered negative predictive factors for cartilage repair procedures, however, no clear evidence exists to actually link those two isolated factors to clinical outcomes.
History should include the documentation of the body mass index (BMI). While a BMI up to 35 does not seem to affect overall outcomes in patients undergoing cell based cartilage procedures12,13 a higher BMI clearly affects the results of patients undergoing microfracture treatment.14 Similar consideration needs to be given to the age factor. Several studies have shown that higher age influences clinical outcome negatively in patients undergoing microfracture procedures.14,15 The data for cell based procedures is somewhat conflicting. A clear correlation between age and clinical outcome has not been shown. Basic science studies, however, suggest that chondrocytes from older donors (>40 years of age) have a lower proteoglycan and collagen production and thus may respond slower and less vigorous to the challenging intra-articular environment after implantation.16 A little researched topic that is of importance is the willingness and compliance with post-operative treatment protocols and rehabilitation procedures. Current protocols are not based upon evidence but rather on anecdotal experience or small case series by individual surgeons and rehabilitation specialists.17-19 Nevertheless it is felt that adherence to these basic protocols is important. A history of non-compliance may therefore be a warning sign to the cartilage surgeon potentially indicating the patients’ lack of understanding or a significant difference in the goals that the treatment is aiming to achieve.
Pain
Pain assessment is an important part of the pre-operative exam. Localized pain may be able to pinpoint a specific area of articular cartilage damage or it may indicate injury to associated structures such as the meniscus. The shorter the history of pain is the more reliably it can be considered to indicate the affected area. A clinical sign that utilizes this concept is the “Wilson” sign. This test is performed originally to diagnose OCD lesions in the medial femoral condyle. The knee is flexed to 90°. The tibia is forced into internal rotation. Under gradual extension and external rotation of the tibia the patient may report pain when the lesion rotates into the area of the soft spot of the medial femoral condyle.20 This test can be modified by pushing the thumb slightly into the soft spot. Another helpful test is the direct palpation of the medial and lateral patella facette. If palpation is reproducing the patients’ pain this can be a sign for a clinically symptomatic lesion in this area and will need to be correlated with imaging results. Cartilage lesions do not typically hurt directly at the joint line. Direct palpation at the joint line is more likely associated with meniscal pathology.
No reliable data exists about the correlation of pain with a symptomatic cartilage lesion. However, the more chronic in nature the pain is, the less likely it is that a cartilage procedure alone is going to address the problem. In the orthopaedic setting it is difficult to fully assess pain. Most commonly utilized are visual analogue scales (VAS) or a “Likert” scale for pain.
In absence of any clear evidence based guidelines regarding pain there are some pearls of wisdom that may help the less experienced cartilage surgeon. The ideal patient should not report maximal pain other than perhaps with heavy exertion. Likewise, patients with minimal or no pain are less likely to benefit from cartilage surgery. Typically, the patient reporting pain in the midrange is considered an acceptable patient for treatment. It is also important to assess pain with and without medication (particularly narcotic pain medication) in this context.
Physical examination
The physical exam should evaluate the overall dynamic and static alignment, antalgic gait, range of motion, and muscle envelope as well as ligamentous stability of the tibio-femoral and patellofemoral joint.
A crude visual gait analysis in the office usually allows for detection of an antalgic gait, quadriceps avoidance gait or a dynamic varus thrust. Any of these findings, if present, can point the examiner towards further underlying pathologies that may have a significant impact on the chosen treatment options. A varus thrust for example may point out an insufficiency of the lateral ligamentous structures (Posterolateral corner, LCL) and a triple varus. A quadriceps avoidance gait may indicate chronic anterior instability.
Knee joint effusions are generally felt to be a significant sign for symptomatic cartilage injuries. It is important to understand, however, that intra-articular effusions can exist without pain and therefore can be present longer than the actual onset of pain.
Range of motion assessment should be a routine part of the physical examination and has to be assessed in comparison to the uninjured side. While small deficits in knee flexion can be observed with knee joint effusions, they are not normal in patients who have no effusion. An extension deficit is an important finding as these are very difficult to correct and may indicate progression to osteoarthritis already beyond the scope of cartilage repair. Significant loss of motion is considered a relative contraindication for cartilage repair procedures.
Mechanical symptoms, locking during the range of motion exam or acute inability to flex or extend the knee joint may indicate an unstable meniscus or articular cartilage fragment or a loose body. Ligamentous stability is a prerequisite for cartilage procedures. It is therefore necessary to perform a full ligament examination of the knee joint. This usually includes varus and valgus stress at 0 and 30 to test the collateral ligaments, the Lachman-test and pivot shift exam evaluates ACL competency, the posterior drawer test at 90 of knee flexion and the posterior sag sign evaluates PCL sufficiency. In case of a potential posterolateral corner injury the dial up test and the flexion rotation drawer can be performed. Often forgotten is the stability exam of the patella. The medial and lateral patella glide and tilt as well as the competency of the Medial PatelloFemoral Ligament (MPFL) should be assessed. The patella apprehension test is helpful to rule out previous patella sub/dislocation. Q-angle and patella stability throughout flexion should be carefully evaluated.
The “character” of the lesion
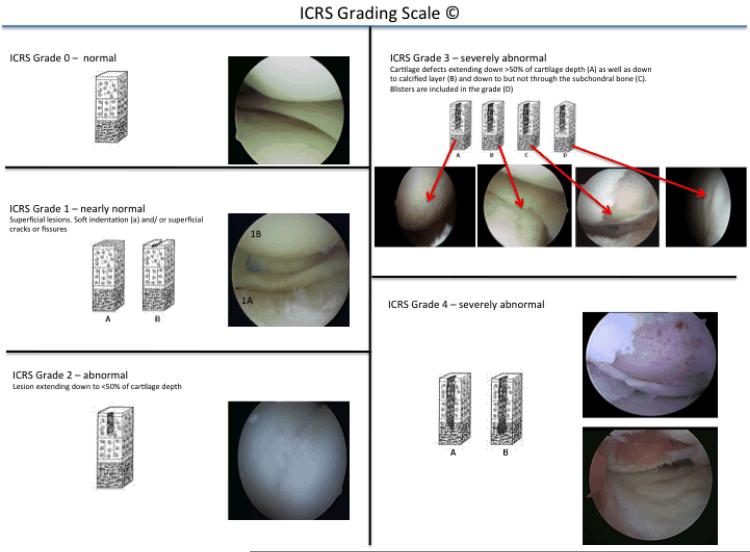
In order to assess the actual severity of a lesion arthroscopic evaluation is imperative. The grading of the severity can be done using several different classification systems. The International Cartilage Research Society (ICRS) has developed a universally accepted and comprehensive grading system that should be utilized in order to allow for generalisation of arthroscopic findings (Figure 1: ICRS grading).
Figure 1.
ICRS grading scheme for cartilage defects.
In order to restore articular cartilage it is important to understand the reason for the initial failure of the cartilage surface to maintain its integrity. In few cases this can be associated with an acute injury (Figure 2: lesion femoral condyle after direct penetrating trauma). In many cases, however, the underlying reason is more subtle. Even more importantly it is imperative to assess the true extent of the chondral lesion. Radiographic imaging has made incredible advances over the last decade and is invaluable to characterize the lesion and its surrounding better. While it is not the focus of this article it needs to be understood that imaging provides information about the articular cartilage as well as the subchondral bone, the synovial envelope and ligamentous structures of the knee joint. All of those need to be assessed in order to create an overall picture or “character” of a knee joint. An invaluable tool to help synthesize all of the above mentioned aspects of information about the patients’ knee is the arthroscopic evaluation of the knee. For some procedures that allow for immediate point-of-care intervention such as the microfracture or the cartilage autologous implant system (CAIS) this evaluation will be followed by an immediate final treatment approach. In other cases it will be necessary to either perform a biopsy with or without minor procedure such as a chondroplasty, a partial meniscectomy or a removal of a loose body. The arthroscopy offers the unique opportunity to assess and verify the location grading and actual size of the lesion. Additionally it allows for assessment of the entirety of a compartment including the status of the articular cartilage surfaces of the tibial and femoral condyles surrounding a full thickness lesions as well as the status of the meniscus which often has been treated in a prior procedure (the majority of patients undergoing cartilage repair procedures have had more than one previous surgical procedure). Particularly globalized findings such as compartment wide grade one or two changes (ICRS) can elude radiographic assessment but may indicate a more generalized chondropenia in the affected compartment. Development of osteophytes along the medial or lateral condylar rim is another sign for more generalized changes in the knee that can easily be missed in x-ray and MRI examination but may be a factors to be taken into account for the assessment of the future success of a cartilage procedure. This arthroscopic evaluation may also help to advise the patient regarding return to higher level activities post-surgically. Figure 3 is an example of an isolated focal chondral defect in an otherwise pristine knee joint (Figure 3a). This is contrasted with an example of an isolated lesion in a knee joint displaying grade 2 changes throughout the entire compartment (3b) indicating beginning chondropenia.
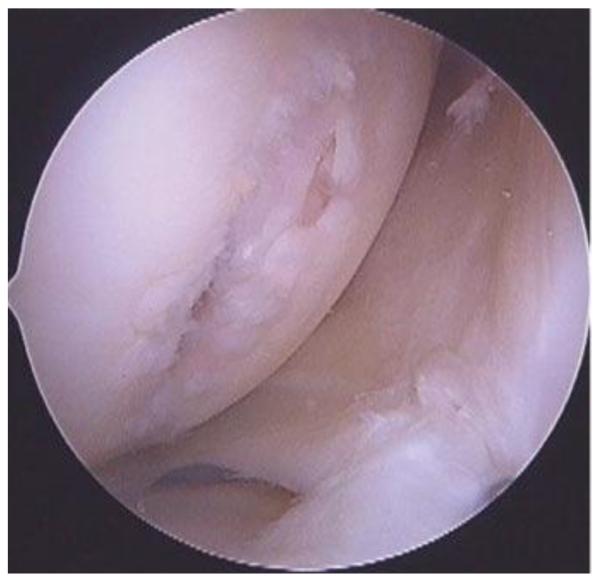
Figure 2.
This is a Grade 4b lesion in a medial femoral condyle after direct trauma. This patient was involved in an MVA 3 months prior to this image and had a penetrating trauma to the knee.
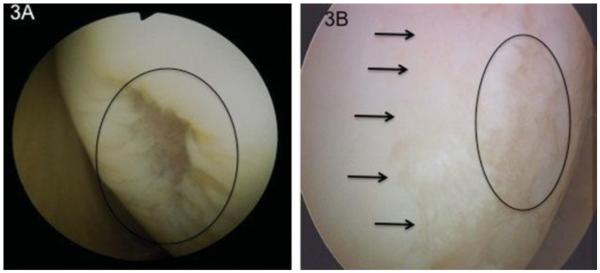
Figure 3.
Lesion 3A is an isolated Grade 3b defect in an otherwise pristine appearing knee joint. This patient went on to receive a microfracture and did well. Figure 3B is a similar size Grade 3b lesion (indicated with the circle) surrounded by areas of Grade 2 lesions. This patient failed an initial microfracture and went on to receive an autologous chondrocytes implantation involving the majority of her condyle (2.2×4.8cm). Even though this is obvious on the video of this lesion it is difficult to document this significant difference in character of this lesion in pictures.
As a final pearl regarding the arthroscopic examination it should be noted that a “video documentation of the lesion and the involved compartment says more than a thousand pictures”. In today’s world video documentation should be the standard as it facilitates communication with colleagues and greatly improves the surgeons’ recall of the character of a lesion in case of a likely time delay between the initial arthroscopy and the final restorative procedure.
Co-morbidities
Prior to considering a cartilage repair procedure it is essential to perform a thorough analysis of co-morbidities that potentially influence the success of the procedure or may even be contra-indications.
Absolute contraindications for cartilage repair procedures are the documented presence of inflammatory arthritis (i.e. psoriatic, gouty, rheumatoid) or established compartmental OA with radiographic changes indicating joint space collapse (Kellgren Lawrence III+IV) or malignancy in the involved limb. Uncorrected axial malalignment is an absolute contra-indication for tibiofemoral cartilage repair procedures as is chronic uncorrected ligamentous instability. Malalignment or instability in the patellofemoral is not considered a contraindication, however, most cartilage surgeons will address obvious patella malalignment and instability in face of a cartilage repair in the patellofemoral joint21. Significant loss of range of motion or arthrofibrosis is also considered to be an absolute contraindication.
Higher Age is a relative contraindication. Consensus exists that in the younger patient the potential for a successful outcome is higher. For this reason most surgeons will consider the age of 50 a cut-off point for cell based procedures or allografts, however, some autologous procedures may be performed in patients up to the age of 60.22-24 It needs to be understood that the biological age of the patient plays a larger role than the chronologic age. This may account for the relatively soft recommendation of the age cut off for these procedures.25-27 Malalignment, meniscus deficiency or ligamentous instability, even though they represent contra-indications to a cartilage repair procedure they can be overcome by either a staged or simultaneous operation to correct the condition.
Axial Malalignment
Varus or valgus malalignment of the knee is the major contributing factor to compartment overload and thus has to be addressed when a cartilage repair procedure is considered to address a cartilage defect in the overloaded compartment.25-27 When addressing a cartilage defect surgically the goal is to restore the normal load distribution that allow the repair cartilage to adjust to physiologic rather than non-physiologic loads. The goal for axial alignment correction in cartilage repair procedures is therefore not an overcorrection as popularized by Coventry and others28 but rather to correct back to neutral alignment. The weight bearing axis should pass through the center of the knee joint allowing for a mechanical axis of 1.2° degrees of varus.29 It is imperative that the origin of the malalignment is identified. Most commonly varus alignment originates in the proximal tibia and valgus alignment in the distal femur. However, in some cases this may be different. It is therefore prudent to do a full axial alignment measurement of tibia and femur rather than just the overall mechanical axis evaluation on the long leg alignment full cassette x-ray. With today’s hardware options low profile plates can be utilized to perform well controlled open wedge high tibial or distal femoral osteotomies to address varus or valgus alignment up to 10°. Malalignment correction above 10° may require additional bone grafting or alternate techniques.
Patellofemoral malalignment
Cartilage injuries in the patellofemoral joint are amongst the most difficult to treat. Technically these lesions are easily accessible but the analysis of concomitant pathologies is difficult. This fact explains the initially disappointing results that Brittberg et al. reported. He saw 5 out of 7 patients undergoing ACI of the patellofemoral joint fail.30 The authors recognized the importance of PF alignment and tracking at a later time point and advocated the combination of the ACI procedure with concomitant, or staged, unloading and normalization of the patella tracking in the PF joint. In 2011, cell based cartilage procedures in patients with PF malalignment are routinely combined with an anteromedialization of the tibial tubercle (AMZ).31
Since, the clinical experience has been promising. Brittberg et al. reported 11 of 17 patients with good and excellent results at 2 years and slightly better results (13/19) at 9 years, indicating a long initial postoperative recovery time with improvement over one year postoperatively.27 In Minas’ study of 45 patients he performed an AMZ in over 60% and reported 71% good and excellent results.32 Henderson and Lavigne reported their results in a group of patients that was divided into ACI (patients with normal PF alignment) and ACI with AMZ (patients with clinically present PF malalignment).33 Interestingly, the group that did not receive the AMZ because they did not have patellofemoral malalignment did worse than the group with patellofemoral alignment requiring an AMZ. This study suggested that there is either an additional effect of the anterior unloading of the patellofemoral joint or perhaps some subtle patellofemoral malalignment that was not detected as this study was published prior to the establishment of the Tibial tubercletrochlear groove (TT-TG) measurements that are used today to determine patellofemoral alignment.34 The potential to unload the patellofemoral joint by doing an anteriorization of the tibial tubercle by less than 1cm has been shown by Rue et al., who concluded that the patellofemoral contact pressures measured by Tekscan can be reduced by 20%.35 Overall, cartilage procedures in the patellofemoral joint can be considered a valuable treatment option as long as an adequate evaluation and concomitant treatment of an underlying PF malalignment is performed.
Meniscal deficiency
The menisci are critical for load sharing and shock absorption. The act as a transmission within the knee linking the femoral condyle with the tibia. They also contribute to joint lubrication and knee stability. Particularly the medial meniscus has been shown to be the most important secondary stabilizer against anterior translation of the knee36. This critical role is commonly impaired as meniscus injuries are the most common knee injury requiring arthroscopic surgery in the US. Biochemical, biomechanical as well as clinical, radiographic and patient related outcomes data has clearly established a direct relation of loss of meniscus tissue to impairment of all these parameters.37 Impressive data has been published by Baratz et al. who showed an increase in contact pressures of 75% and an overall increase of 235% in peak-contact pressures after subtotal meniscectomy.38,39 Lee et al. showed that the periphery of the meniscus is more important for the overall pressure distribution in the compartment than the central portion.40 This data is encouraging and may indicate that patients after partial meniscectomy still have a nearly normal pressure distribution in the joint. An isolated partial meniscectomy therefore may not pose a significant short term risk for a cartilage repair procedure. However, long-term data exists linking partial meniscectomies to the development of OA over a 15 year time span. This data is even more compelling in conjunction with a ligamentous instability.40
Patients who have undergone a subtotal or complete meniscectomy or have suffered a nonrepairable radial tear have pathologic pressure distribution that is detrimental to the weight bearing articular cartilage and any repair tissue. In these cases a meniscus transplant may need to be considered. While the indications for meniscal transplant are still evolving, they are generally considered in patients who are young, have unicompartmental pain, a history of previous meniscectomy, normal ligamentous stability and normal or correctable alignment. Gomoll et al. have published their series of 7 patients undergoing cartilage restoration, high tibial osteotomy and meniscus transplantation. They reported encouraging results in this small series with significant improvement of the IKDC subjective score, KOOS and Lysholm score after 24 months (average) follow up.42 While these patients are a very challenging group they can achieve significant improvement if all three major factors (axial alignment, focal chondral defect and meniscal deficiency) are addressed adequately.
Ligamentous instability
A knee ligament insufficiency such as an ACL insufficiency has been clearly linked to an increased risk of OA over time.41 Articular cartilage lesions in ACL injured patients are not uncommon. Not all of these lesions are acute and clinically symptomatic,43 however, ACL instability will over time contribute to a significant increase in the size of the cartilage lesion as Murrell could show. He evaluated patients 2 months and 2 years after ACL tear prior to stabilisation and found a six times larger loss of cartilage in patients with longer standing ACL insufficiency (Murrell et al.). In patients who had a combination of ACL injury and meniscal tear this rate increased to 18 times after 2 years. It has been shown that knee ligament stability is important to preserve meniscal integrity. Particularly the interaction of the medial meniscus and the ACL is important as the lack of the medial meniscus may lead to early failure of the ACL graft due to the meniscus’ function as a secondary restraint to anterior tibial translation.36 In patients with chronic ACL instability and pain it is important to evaluate the primary factor, pain or instability. Patients who only have instability related pain episodes might be served well with a correction of the instability alone. Patients who have pain only may benefit from an osteotomy. Lattermann et al. showed in a retrospective study that ACL insufficient patients with varus alignment who have predominantly pain but no instability may significantly improve after high tibial osteotomy and may not require any other procedure. In these cases a staged approach may be beneficial.44
Conclusions
The careful evaluation of patients undergoing cartilage repair procedures is of foremost importance since these patients generally require very individualized care. Thorough examination and judgment of co-morbidities and their impact on the cartilage procedure is imperative. Unfortunately there are no evidence based guidelines or clear cut recommendations for the majority of patients that are encountered in the practice setting.4 However, with careful clinical decision making, evaluation of malalignment, and other co morbidities and careful staging of the lesion during arthroscopy the cartilage surgeon can make good choices that will lead to good clinical outcomes as reported in the literature. It is important to communicate the complexity of the decision making process to the patient and make the patient aware that the proposed treatment is not a “routine” straight forward standardized procedure.
Abbreviations
- OA
Osteoarthritis
- AMZ
anteromedialisation of the tibial tubercle
- MPFL
Medial Patellofemoral Ligament
- ICRS
International Cartilage Repair Society
Contributor Information
Christian Lattermann, Director: Center for Cartilage Repair and Reconstruction University of Kentucky 740 South Limestone Kentucky Clinic K401 Lexington KY 40536.
Matthew R Luckett, Resident Physician University of Kentucky 740 South Limestone Kentucky Clinic K401 Lexington KY 40536 Tel: 8592183065 Fax: 8593232412.
Literature
- 1.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002 Sep;18(7):730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 2.Widuchowski W, Lukasik P, Kwiatkowski G, et al. Isolated full thickness chondral injuries. Prevalance and outcome of treatment. A retrospective study of 5233 knee arthroscopies. Acta Chir Orthop Traumatol Cech. 2008 Oct;75(5):382–386. [PubMed] [Google Scholar]
- 3.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997 Aug;13(4):456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 4.Engen CNE L, Aroen A. Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopaedic practice. Cartilage. 2010;1(4):312–319. doi: 10.1177/1947603510373917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widuchowski W, Widuchowski J, Faltus R, et al. Long-term clinical and radiological assessment of untreated severe cartilage damage in the knee: a natural history study. Scand J Med Sci Sports. 2011 Feb;21(1):106–110. doi: 10.1111/j.1600-0838.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 6.Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85-A(Suppl 2):8–16. doi: 10.2106/00004623-200300002-00002. [DOI] [PubMed] [Google Scholar]
- 7.Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand. 1996 Apr;67(2):165–168. doi: 10.3109/17453679608994664. [DOI] [PubMed] [Google Scholar]
- 8.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007 Jun;14(3):177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Mithoefer K, Williams RJ, 3rd, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006 Sep;34(9):1413–1418. doi: 10.1177/0363546506288240. [DOI] [PubMed] [Google Scholar]
- 10.McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008 Dec;16(4):196–201. doi: 10.1097/JSA.0b013e31818cdb82. [DOI] [PubMed] [Google Scholar]
- 11.Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 2010 Jan;468(1):147–157. doi: 10.1007/s11999-009-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaslav K, Cole B, Brewster R, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009 Jan;37(1):42–55. doi: 10.1177/0363546508322897. [DOI] [PubMed] [Google Scholar]
- 13.Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008 Sep;36(9):1770–1778. doi: 10.1177/0363546508317122. [DOI] [PubMed] [Google Scholar]
- 14.Mithoefer K, Williams RJ, 3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005 Sep;87(9):1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 15.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidencebased systematic analysis. Am J Sports Med. 2009 Oct;37(10):2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 16.Adkisson HDt, Martin JA, Amendola RL, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010 Jul;38(7):1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hambly K, Bobic V, Wondrasch B, Van Assche D, Marlovits S. Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. Am J Sports Med. 2006 Jun;34(6):1020–1038. doi: 10.1177/0363546505281918. [DOI] [PubMed] [Google Scholar]
- 18.Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T. Traditional vs accelerated approaches to post-operative rehabilitation following matrix-induced autologous chondrocyte implantation (MACI): comparison of clinical, biomechanical and radiographic outcomes. Osteoarthritis Cartilage. 2008 Oct;16(10):1131–1140. doi: 10.1016/j.joca.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Hirschmuller A, Baur H, Braun S, Kreuz PC, Sudkamp NP, Niemeyer P. Rehabilitation After Autologous Chondrocyte Implantation for Isolated Cartilage Defects of the Knee. Am J Sports Med. 2011 May 21; doi: 10.1177/0363546511404204. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JN. A diagnostic sign in osteochondritis DISSECANS OF THE KNEE. J Bone Joint Surg Am. 1967 Apr;49(3):477–480. [PubMed] [Google Scholar]
- 21.Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop Relat Res. 2007 Oct;463:187–194. [PubMed] [Google Scholar]
- 22.Hangody L, Kish G, Karpati Z, Udvarhelyi I, Szigeti I, Bely M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics. 1998 Jul;21(7):751–756. doi: 10.3928/0147-7447-19980701-04. [DOI] [PubMed] [Google Scholar]
- 23.Steadman JR, Ramappa AJ, Maxwell RB, Briggs KK. An arthroscopic treatment regimen for osteoarthritis of the knee. Arthroscopy. 2007 Sep;23(9):948–955. doi: 10.1016/j.arthro.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 24.Braun S, Steadman JR, Rodkey WG, Briggs KK. [Microfracture and specific rehabilitation for treating osteoarthritis of the knee. Indications, surgical technique, and rehabilitation protocol] Z Rheumatol. 2009 Dec;68(10):811–818. doi: 10.1007/s00393-009-0551-2. [DOI] [PubMed] [Google Scholar]
- 25.Ghodadra NB, S, Verma NN. Cole BGA. 1 ed SLACK Incorporated; Thorofare NJ: 2009. Biologic Joint Reconstruction: Alternatives to Arthroplasty; pp. 13–22. [Google Scholar]
- 26.Lindahl A, Brittberg M, Peterson L. Cartilage repair with chondrocytes: clinical and cellular aspects. Novartis Found Symp. 2003;249:175–186. discussion 186-179, 234-178, 239-141. [PubMed] [Google Scholar]
- 27.Brittberg M, Tallheden T, Sjogren-Jansson B, Lindahl A, Peterson L. Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop Relat Res. 2001 Oct;(391 Suppl):S337–348. doi: 10.1097/00003086-200110001-00031. [DOI] [PubMed] [Google Scholar]
- 28.Coventry MB. Proximal tibial varus osteotomy for osteoarthritis of the lateral compartment of the knee. J Bone Joint Surg Am. 1987 Jan;69(1):32–38. [PubMed] [Google Scholar]
- 29.Hsu RW, Himeno S, Coventry MB, Chao EY. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res. 1990 Jun;(255):215–227. [PubMed] [Google Scholar]
- 30.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994 Oct 6;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 31.Gomoll AH, Minas T, Farr J, Cole BJ. Treatment of chondral defects in the patellofemoral joint. J Knee Surg. 2006 Oct;19(4):285–295. doi: 10.1055/s-0030-1248121. [DOI] [PubMed] [Google Scholar]
- 32.Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005 Jul;(436):30–39. doi: 10.1097/01.blo.0000171916.40245.5d. [DOI] [PubMed] [Google Scholar]
- 33.Henderson IJ, Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006 Aug;13(4):274–279. doi: 10.1016/j.knee.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Schöttle PB, Zanetti M, Seifert B, Pfirrmann CW, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006 Jan;13(1):26–31. doi: 10.1016/j.knee.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Rue JP, Colton A, Zare SM, et al. Trochlear contact pressures after straight anteriorization of the tibial tuberosity. Am J Sports Med. 2008 Oct;36(10):1953–1959. doi: 10.1177/0363546508317125. [DOI] [PubMed] [Google Scholar]
- 36.Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res. 2000 Jan;18(1):109–115. doi: 10.1002/jor.1100180116. [DOI] [PubMed] [Google Scholar]
- 37.Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009 Aug;35(3):579–590. doi: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Baratz ME, Rehak DC, Fu FH, Rudert MJ. Peripheral tears of the meniscus. The effect of open versus arthroscopic repair on intraarticular contact stresses in the human knee. Am J Sports Med. 1988 Jan-Feb;16(1):1–6. doi: 10.1177/036354658801600101. [DOI] [PubMed] [Google Scholar]
- 39.Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986 Jul-Aug;14(4):270–275. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Aadalen KJ, Malaviya P, et al. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med. 2006 Aug;34(8):1334–1344. doi: 10.1177/0363546506286786. [DOI] [PubMed] [Google Scholar]
- 41.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007 Oct;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 42.Gomoll AH, Kang RW, Chen AL, Cole BJ. Triad of cartilage restoration for unicompartmental arthritis treatment in young patients: meniscus allograft transplantation, cartilage repair and osteotomy. J Knee Surg. 2009 Apr;22(2):137–141. doi: 10.1055/s-0030-1247738. [DOI] [PubMed] [Google Scholar]
- 43.Rotterud JH, Sivertsen EA, Forssblad M, Engebretsen L, Aroen A. Effect of gender and sports on the risk of full-thickness articular cartilage lesions in anterior cruciate ligament-injured knees: a nationwide cohort study from sweden and norway of 15 783 patients. Am J Sports Med. 2011 Jul;39(7):1387–1394. doi: 10.1177/0363546510397813. [DOI] [PubMed] [Google Scholar]
- 44.Lattermann C, Jakob RP. High tibial osteotomy alone or combined with ligament reconstruction in anterior cruciate ligament-deficient knees. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):32–38. doi: 10.1007/BF01565995. [DOI] [PubMed] [Google Scholar]