Summary
Peptide neuromodulators are released from a unique organelle: the dense-core vesicle. Dense-core vesicles are generated at the trans-Golgi, and then sort cargo during maturation before being secreted. To identify proteins that act in this pathway, we performed a genetic screen in Caenorhabditis elegans for mutants defective in dense-core vesicle function. We identified two conserved Rab2-binding proteins: RUND-1, a RUN domain protein, and CCCP-1, a coiled-coil protein. RUND-1 and CCCP-1 colocalize with RAB-2 at the Golgi, and rab-2, rund-1 and cccp-1 mutants have similar defects in sorting soluble and transmembrane dense-core vesicle cargos. RUND-1 also interacts with the Rab2 GAP protein TBC-8 and the BAR domain protein RIC-19, a RAB-2 effector. In summary, a new pathway of conserved proteins controls the maturation of dense-core vesicles at the trans-Golgi network.
Introduction
The nervous system has two modes of chemical signaling at synapses. Fast signaling occurs by the release of small molecule neurotransmitters that activate ligand-gated ion channels. Slow signaling occurs by the release of neuromodulators, such as neuropeptides or monoamines, that activate G-protein-coupled receptors. Fast neurotransmitters are released from synaptic vesicles, whereas many neuromodulators are released from dense-core vesicles (DCVs). A wide variety of biological processes are regulated by DCV secretion, including cell survival, neuronal development, synaptic plasticity, and excitability.
The pathway for the generation of DCVs in neurons is likely to be related to the generation of secretory vesicles in neuroendocrine cells such as chromaffin cells or the insulin-secreting cells of the pancreas (Borgonovo et al., 2006; Kim et al., 2006; Park and Loh, 2008; Tooze et al., 2001). The secretory granules are generated at the trans-Golgi and undergo several maturation steps, including the homotypic fusion of immature secretory granules, and removal of some soluble and transmembrane cargo (Ahras et al., 2006; Dittie et al., 1996; Kakhlon et al., 2006; Klumperman et al., 1998; Tooze et al., 1991; Urbé et al., 1998; Wendler et al., 2001). Cargo sorting relies on poorly defined motifs that probably interact with multiple sorting receptors (Dikeakos and Reudelhuber, 2007; Dikeakos et al., 2009). It is likely that DCVs in neurons undergo similar maturation processes at the Golgi, but some mechanisms may differ since these cells have a very different architecture.
The rich variety of small Rab GTPase proteins provide vesicles in all trafficking pathways with identity. Rabs orchestrate numerous aspects of vesicle trafficking including vesicle budding, transport, tethering, uncoating and fusion (Stenmark, 2009; Zerial and McBride, 2001). There are about 60 different Rabs in humans and 26 in C. elegans (Lundquist, 2006; Pereira-Leal and Seabra, 2001). Rabs are localized to different intracellular compartments and help provide specificity to vesicular trafficking (Chavrier et al., 1990). In C. elegans, Rab2 (RAB-2) localizes to the Golgi (Sumakovic et al., 2009) and rab-2 mutants (also known as unc-108) have defects in sorting soluble and transmembrane DCV cargos (Edwards et al., 2009; Sumakovic et al., 2009). Thus, there are likely to be Rab2 effectors involved in DCV cargo sorting.
Here we use a genetic screen to identify proteins involved in DCV function. We identify two proteins that are Rab2 interactors: RUND-1 and CCCP-1. RUND-1 and CCCP-1 are conserved proteins that colocalize with RAB-2 at the trans-Golgi. RUND-1 and CCCP-1 bind to activated RAB-2, and like rab-2 mutants, rund-1 and cccp-1 mutants exhibit defects in sorting soluble and transmembrane DCV cargo. We conclude that RUND-1 and CCCP-1 may function as RAB-2 effectors in DCV maturation at the trans-Golgi.
Results
A Genetic Screen for Regulators of Dense-core Vesicle Function
In C. elegans, loss-of-function mutants in the trimeric G-protein Gαq (encoded by egl-30) have a straight posture and are almost immobile on food (Brundage et al., 1996). Activated Gαq mutants exhibit the opposite phenotype; these mutants are hyperactive and exhibit tightly coiled body bends (Bastiani et al., 2003). Mutants in the DCV secretion protein CAPS (encoded by the unc-31 gene) have a straight posture and are almost immobile on food (Avery et al., 1993), similar to egl-30(lf) mutants. Mutations in CAPS suppress activated alleles of Gαq (Charlie et al., 2006), suggesting that these proteins act in a pathway. Thus, mutations in other genes involved in DCV biogenesis and secretion should also suppress activated alleles of egl-30. We screened for genetic suppressors of the activating Gq mutation egl-30(tg26) (Doi and Iwasaki, 2002). egl-30(tg26) mutants have hyperactive, loopy locomotion and are smaller and slower-growing than the wild type (Movies S1 and S2). We searched for less hyperactive animals among the F2 progeny of ENU-mutagenized egl-30(tg26). We isolated six independent alleles of unc-31, validating the strategy of the screen. In addition, we isolated recessive mutations in six complementation groups with locomotion phenotypes reminiscent of unc-31, though somewhat weaker. These mutants exhibit a novel locomotion phenotype, which we call “unmotivated.” When separated from the activated Gq mutation, the unmotivated mutants have a fairly normal posture but exhibit little spontaneous movement on food (Movie S3). However, they are capable of coordinated locomotion when stimulated. Conditions that stimulate movement include starvation, harsh touch and UV light (Movies S3 and S4). Thus, these genes appear to function in the regulation of movement rather than the execution of coordinated movements.
We mapped and characterized in detail two of the six strong unmotivated mutants, rund-1 and cccp-1. Two mutations (ox281 and ox328) define the new gene rund-1. Mutations at a second locus, named cccp-1 (conserved coiled-coil protein-1), caused a similar phenotype. The mutation cccp-1(ox334) was isolated in the screen, and we found that it failed to complement an existing locomotion mutant e1122 (previously known as unc-81).
rund-1 and cccp-1 Encode Novel Conserved Proteins
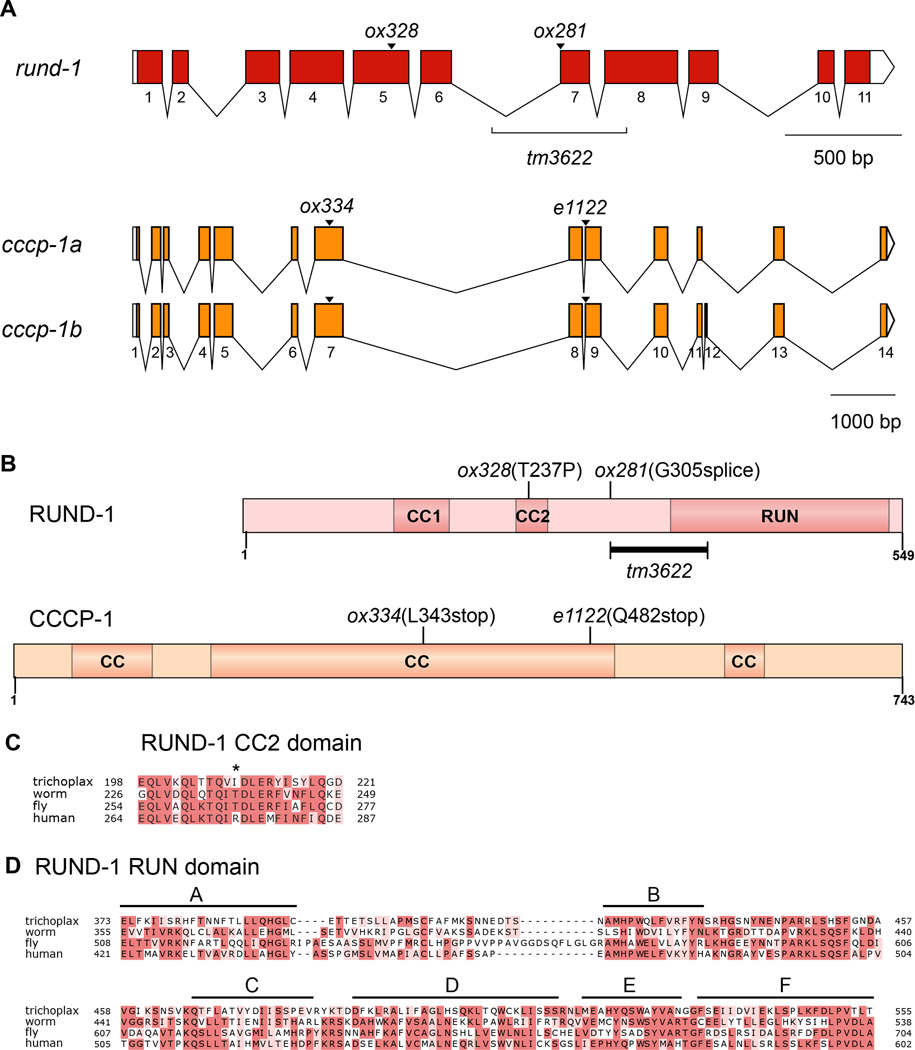
We cloned rund-1 and cccp-1 by standard methods (Experimental Procedures). The rund-1 phenotype is fully rescued by the single gene T19D7.4. This open reading frame encodes a novel protein of 549 amino acids that has two coiled-coil domains in its N-terminal half and a RUN domain (RPIP8, UNC-14, and NESCA) near the C-terminus (Figures 1A and 1B). RUN domains have been found in several proteins that bind to activated small GTPases of the Rab and Rap families and have been proposed to be effectors of these small G proteins (Callebaut et al., 2001). The mutation ox281 is a splice site mutation, ox328 introduces a proline into the second coiled-coil domain. We subsequently obtained a deletion allele tm3622 which deletes the entire A block of the RUN domain (Figures 1A–D). RUND-1 has a single conserved ortholog in other metazoans, including humans and Drosophila, and even the primitive metazoan Trichoplax adhaerens (Figure S1). RUND-1 shows conservation throughout the length of the protein; the second coiled-coil domain and the RUN domain are especially well-conserved (Figures 1C and 1D)..
Figure 1. rund-1 and cccp-1 encode conserved proteins.
(A) Gene structures of rund-1 and cccp-1. Colored boxes show coding segments. White boxes show untranslated regions. cccp-1 has two transcripts.
(B) Domain structures of RUND-1 and CCCP-1. RUND-1 is a 549 amino acid protein with two coiled-coil (CC) domains and a RUN domain. ox328 is a missense mutation in the second coiled-coil domain. ox281 is a splice site mutation. The tm3622 deletion is marked, but because this deletion starts in an intron (Figure 1A), its effect on the protein is unknown. CCCP-1b is a 743 amino acid protein with multiple coiled-coil (CC) domains. The ox334 and e1122 mutations lead to premature stop codons.
(C) Alignment of the second coiled-coil domain of RUND-1 and its orthologs from Trichoplax adhaerens (Trichoplax), C. elegans (worm), Drosophila melanogaster (fly) and Homo sapiens (human). The position of the ox328 T237P mutation is shown by an asterisk.
(D) Alignment of the RUN domain of RUND-1. The six conserved blocks A-F of the RUN domain (Callebaut et al., 2001) are marked with black bars. The tm3622 deletion removes the A block, but ends before the beginning of the B block. See also Figures S1 and S2.
RUND-1 and its human ortholog RUNDC1 are about 32% identical (Figure S1). We found that human RUNDC1 could rescue the worm rund-1 mutants (Table S1; two-tailed P<0.0001 for both strains, Fisher’s exact test). RUNDC1 was tagged with tagRFP and exhibited a cellular localization pattern similar to the native RUND-1 (data not shown). Thus, the function and localization of RUND-1 are conserved in the human ortholog RUNDC1.
cccp-1 corresponds to the open reading frame Y49E10.23. cccp-1 has two alternatively-spliced transcripts, cccp-1a and cccp-1b, which encode novel proteins of 734 or 743 amino acids with multiple coiled-coil domains (Figures 1A and 1B). Like RUND-1, CCCP-1 has a single conserved ortholog in other metazoans including flies and humans, and is not found outside metazoans (Figure S2). cccp-1(ox334) and cccp-1(e1122) both carry mutations leading to early stop codons.
rund-1 Mutants Exhibit Unmotivated Locomotion
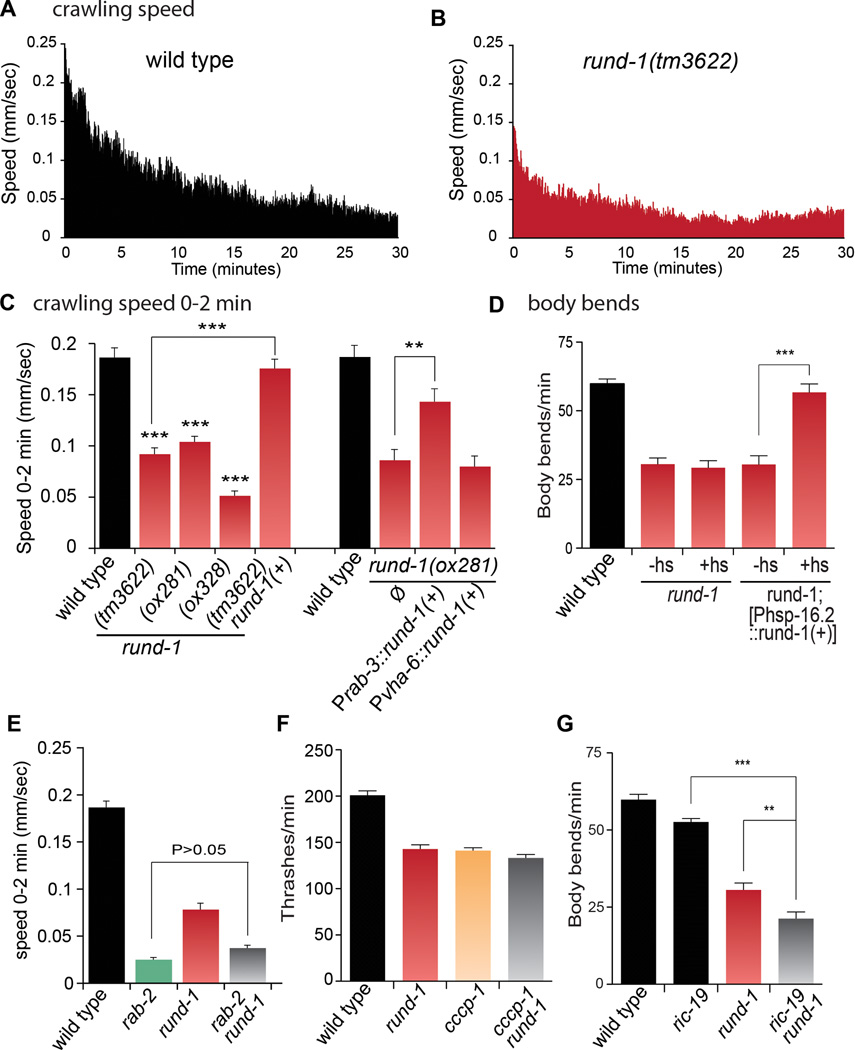
The unmotivated phenotype is typified by inactivity on food (Movie S3). We used a computer-tracking system to characterize the movement of animals over a 30-minute period. The speed of wild-type animals is highest immediately after a harsh mechanical stimulation and then decays over approximately the next 20 to 30 minutes to a stable baseline rate (Figure 2A). For rund-1 mutants, the speed after stimulation is about half that of the wild type (Figures 2B and 2C and Figure S3B–D) and the animals become mostly inactive after 15 minutes; the phenotype is fully rescued by a single-copy insertion of the wild-type rund-1(+) gene (Figure 2C and Figure S3E).
Figure 2. rund-1 mutants exhibit unmotivated locomotion, but respond to stimulation.
(A and B) Locomotion of wild-type and rund-1 mutants. The graphs plot mean speed during the 30 minutes after transfer to a new plate. Wild-type locomotion is stimulated by transfer and decays to baseline in twenty minutes. rund-1 mutants are stimulated by transfer, but less than wild type, and have a reduced baseline locomotion rate. n=21–24 animals.
(C) Left, mean crawling speed during the first two minutes after transfer to a new plate. All three rund-1 mutants exhibit reduced speed (*** P<0.001 vs wild type) and the rund-1(tm3622) mutant is fully rescued by a rund-1(+) single-copy transgene. Right, expression of rund-1(+) in the nervous system rescues the rund-1 mutant. The rund-1 cDNA was expressed in either the nervous system (Prab-3) or the intestine (Pvha-6). Expression in the nervous system rescued (** P<0.01), but expression in the intestine did not (P>0.05). Error bars=SEM; n=21–27 (left) and n=9 (right).
(D) The rund-1 locomotion defect is rescued by expression of a rund-1(+) transgene in mature animals. The frequency of body bends was measured during the first two minutes after transfer to a new plate. Heatshock-induced expression of rund-1(+) fully rescued the rund-1 mutant defect (*** P<0.001 vs no heatshock). Heatshock did not affect rund-1 (P>0.05). +hs: heatshock, -hs: no heatshock. Error bars=SEM; n=10.
(E) rund-1 and rab-2 act in the same genetic pathway to regulate locomotion. Mean speed was measured for two minutes after transfer to a new plate. The rab-2 rund-1 double mutant is similar to the rab-2 single mutant. Error bars=SEM; n=24–31.
(F) rund-1 and cccp-1 act in the same genetic pathway to regulate locomotion. Frequency of body bends (“thrashes”) in liquid was reduced for rund-1(ox281), cccp-1(ox334) and cccp-1(ox334); rund-1(ox281) mutants compared to wild type (P<0.001). The cccp-1 rund-1 double mutant is similar to the single mutants (P>0.05). Error bars=SEM; n=8–9.
(G) rund-1 and ric-19 act in parallel to regulate locomotion. Frequency of body bends was measured for two minutes after transfer to a new plate. ric-19(pk690) rund-1(tm3622) animals show a more severe defect than either single mutant (*** P<0.001; ** P<0.01). ric-19 is not significantly different from wild type (P>0.05). The wild type and rund-1 data are identical to Figure 2D; these experiments were performed together. Error bars=SEM; n =10.
See also Figures S3 and S7, and Table S2.
The unmotivated phenotype is reminiscent of the locomotion of unc-31/CAPS mutants. CAPS is required for DCV fusion (Ann et al., 1997; Gracheva et al., 2007; Speese et al., 2007; Walent et al., 1992; Zhou et al., 2007), and may also regulate synaptic vesicle exocytosis (Gracheva et al., 2007; Jockusch et al., 2007). Mutants lacking CAPS/UNC-31 are paralyzed on food but not off food (Avery et al., 1993; Speese et al., 2007); rund-1 mutants also exhibit increased speed off food. rund-1 and unc-31 mutants also have defects in egg-laying and defecation, and enter the dauer diapause stage at high temperature (Ailion et al., 1999; Avery et al., 1993; Speese et al., 2007; data not shown). Also, both unc-31 and rund-1 mutants can be suppressed by an activating mutation in the Gαs ortholog gsa-1 (Charlie et al., 2006; data not shown). Thus, rund-1 likely regulates a DCV pathway shared by CAPS.
Although the unmotivated phenotype resembles a worm behavior called ‘quiescence’, the resemblance is superficial (Van Buskirk and Sternberg, 2007; Gaglia and Kenyon, 2009; Singh et al., 2011; You et al., 2008). First, unlike rund-1 mutants, quiescent animals do not forage for food or eat. Second, quiescence can be suppressed by mutations in the cyclic GMP-dependent protein kinase egl-4, but rund-1 mutants are not suppressed by egl-4 (data not shown). Third, quiescence depends on function of the ALA neuron and is suppressed by mutations that affect ALA development such as ceh-17. rund-1 mutants, however, are not suppressed by ceh-17 (data not shown). We conclude that unmotivated locomotion is a novel worm phenotype.
rund-1 Acts in Mature Neurons to Regulate Locomotion
We determined the cellular expression patterns of the rund-1 and cccp-1 genes by fusing their promoters to GFP. Prund-1::GFP was expressed in most or all neurons, the pharynx, the intestine, the spermatheca, and the uterus (Figures S4A and S4B) but was not seen in skin or muscle cells. Pcccp-1::GFP was also expressed in most or all neurons, the intestine, and the spermatheca, but was not observed in the pharynx, skin or muscle (Figure S4C).
To determine the cellular focus of the locomotory phenotype, we expressed rund-1 and cccp-1 under the control of tissue-specific promoters. Expression of rund-1(+) under the neuronal specific rab-3 promoter rescued rund-1 mutant locomotion, but expression of rund-1 in the intestine using the vha-6 promoter did not rescue (Figure 2C and Figures S3G–I). Under its own promoter, the tagged cccp-1 cDNA rescued the cccp-1 mutant locomotion defect (data not shown). This transgene was primarily expressed in the nervous system but at low levels. Interestingly, the cccp-1 gene caused defects in locomotion in wild-type worms when expressed under the more highly expressed rab-3 promoter (data not shown). Thus, both reduced expression and overexpression of cccp-1 in neurons cause defects in locomotion.
To determine whether rund-1 affects the development of neurons, we first examined neuronal architecture using the GABA neuron marker Punc-47::GFP (McIntire et al., 1997). No defects were seen in the rund-1 mutant, indicating that rund-1 is not generally required for axon guidance or maintenance (data not shown). Second, we examined the distribution of synaptic varicosities by imaging fluorescently tagged synaptobrevin, a synaptic vesicle protein (Punc-129::mCherry::SNB-1). No defects were observed in rund-1 mutants, indicating that rund-1 is not required for synapse development (Figures S5A and S5B).
The lack of a role in development was confirmed by rescuing the phenotype post-developmentally. We expressed the rund-1 cDNA fused to tagRFP under a heat-shock promoter. rund-1 mutant animals carrying this construct were heat-shocked at various stages of larval development. Twenty-four hours after heat-shock, adults were fully rescued for locomotion on food or in liquid (Figure 2D and data not shown). However, five hours after heat-shock, animals exhibited no discernible rescue, even though RFP expression was detected in neurons. Thus, rund-1 acts in mature neurons, but in a rather slow process.
RUND-1 and CCCP-1 Act in the Same Genetic Pathway as RAB-2
The presence of a RUN domain suggested that RUND-1 may interact with a small GTPase in either the Rab or Rap family. RAB-2 is a Golgi Rab that functions in DCV maturation in C. elegans (Edwards et al., 2009; Sumakovic et al., 2009). Mutants lacking the rab-2 gene (also called unc-108) exhibit similar but somewhat stronger locomotion defects than rund-1 (Chun et al., 2008; Edwards et al., 2009; Sumakovic et al., 2009). Like rund-1, rab-2 mutants exhibit little spontaneous movement on food, but move normally when stimulated by UV light (Edwards et al., 2009). Unlike rund-1, rab-2 mutants cannot be stimulated by a harsh mechanical stimulus (Figure S7A).
The phenotypic similarity between rund-1, cccp-1 and rab-2 mutants suggests that they may act in the same pathway, and analysis of double mutants supports this conclusion. First, rab-2, rund-1 and cccp-1 mutations all suppress the hyperactive locomotion of the activated Gq mutant egl-30(tg26) (data not shown). Second, double mutants among rab-2, rund-1, and cccp-1 do not show enhanced locomotion defects (Figure 2E,F; Figure S7A, and data not shown). These data suggest that RAB-2, RUND-1 and CCCP-1 function together in DCV maturation.
DCV maturation involves the sorting of neuropeptide precursors into vesicles; the vesicles are then acidified, which activates the endopeptidases that cleave the precursors into mature neuropeptides. In C. elegans, there are four proprotein convertases that process neuropeptides. Most peptides in neurons are processed by the convertase EGL-3 (Husson et al., 2006; Kass et al., 2001), though other convertases can contribute to the processing of peptides and some peptides are not processed at all (Leinwand and Chalasani, 2013; Pierce et al., 2001). Interestingly, egl-3 mutants have a weaker locomotion defect than the unmotivated mutants rab-2, rund-1, and cccp-1. However, when egl-3 is combined with rab-2, rund-1, or cccp-1, the double mutants are essentially paralyzed (Figures S7B and S7C and data not shown), indicating that egl-3 acts in parallel to these genes. The double mutants are both qualitatively and quantitatively similar to unc-31/CAPS mutants (Edwards et al., 2009; Sumakovic et al., 2009; Figures S7B–D and data not shown). These data support a model in which the unmotivated class of proteins, RAB-2, RUND-1 and CCCP-1 act in parallel to EGL-3 to regulate locomotion, perhaps via different DCV cargos; both pathways may require UNC-31/CAPS for secretion.
RUND-1 and CCCP-1 are Required to Sort Dense-Core Vesicle Cargo
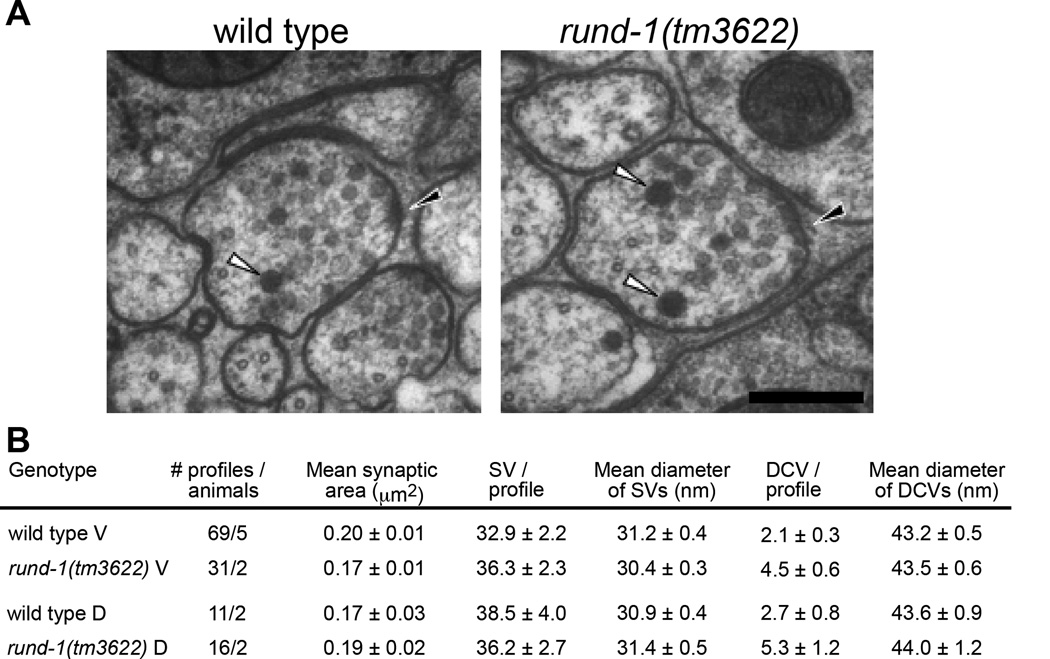
To test whether rund-1 affects synaptic or dense-core vesicle transport, we examined the ultrastructure of rund-1(tm3622) using electron microscopy. rund-1 does not exhibit defects in synaptic vesicle number (Figure 3) or localization of synaptic vesicle cargo (Figures S5A and S5B). Nor is synaptic function defective in rund-1 and cccp-1 mutants as determined by electrophysiological recordings (Figures S5C–G). Also, DCVs of a normal size and appearance are present at synapses in both the ventral and dorsal nerve cords of the rund-1(tm3622) mutant (Figure 3). In fact, there is actually a small increase in the number of DCVs. Thus, RUND-1 is not required for synaptic vesicle or DCV biogenesis or transport to synapses. These data support the idea that RUND-1 and CCCP-1 are required for sorting of DCV cargos.
Figure 3. RUND-1 is not required to make DCVs.
(A) Electron microscopy of synapses in the ventral nerve cord of wild type and rund-1(tm3622). White arrowheads point to DCVs. Black arrowheads point to the presynaptic density. Scale bar: 200 nm.
(B) Quantification of synaptic vesicles (SVs) and dense-core vesicles (DCVs) at synapses in the ventral cord (V) or dorsal cord (D).
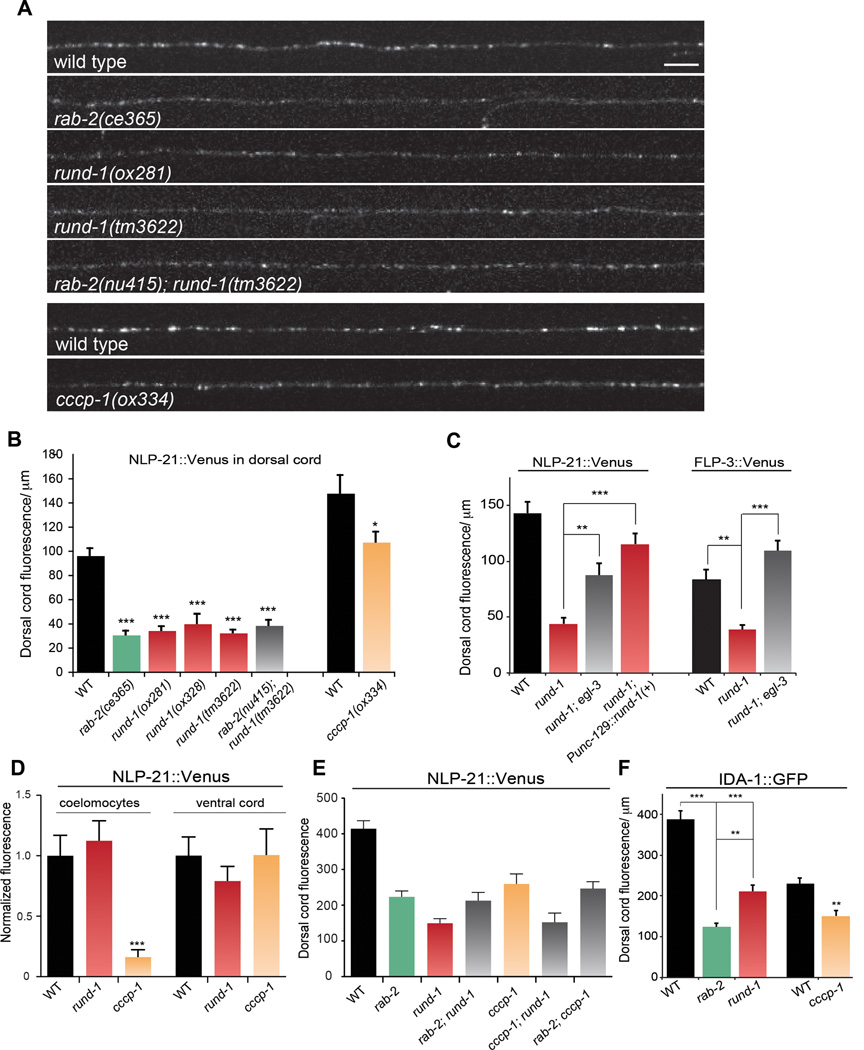
We tested whether rund-1 and cccp-1 play a role in DCV cargo trafficking. IDA-1 is the C. elegans ortholog of the DCV transmembrane protein phogrin (Cai et al., 2004). rund-1 and cccp-1 mutants exhibit a defect in axonal trafficking of IDA-1::GFP (Figure 4F), but it is weaker than that observed in rab-2 mutants (Edwards et al., 2009).
Figure 4. rund-1 and cccp-1 mutants have defects in trafficking DCV cargos.
(A) Images of NLP-21::Venus fluorescence in motor neuron axons of the dorsal nerve cord. rab-2, rund-1, and cccp-1 mutants have decreased levels of fluorescence in the dorsal cord, indicative of an NLP-21::Venus trafficking defect. The final two panels (WT and cccp-1) were from a separate experiment. Scale bar: 5 µm.
(B) NLP-21::Venus fluorescence levels are decreased in the dorsal cord in rab-2, rund-1, and cccp-1 mutants. The mean fluorescence intensity per µm is given in arbitrary units. All single mutants are defective in trafficking NLP-21::Venus to the dorsal cord (*** P<0.001 ; * P<0.05 vs wild type). The rab-2 rund-1 double mutant is not significantly different from the single mutants (P>0.05). Error bars=SEM; n=9–22.
(C) Trafficking of NLP-21::Venus and FLP-3::Venus is rescued by egl-3 mutants. The peptide processing mutant egl-3 suppresses NLP-21::Venus and FLP-3::Venus trafficking defects of the rund-1(tm3622) mutant (** P<0.01, *** P<0.001 respectively). The rund-1 NLP-21::Venus defect is rescued by expression of the wild-type rund-1 cDNA in the dorsal motor neurons (*** P<0.001). Error bars=SEM; n=6–11.
(D) Secreted NLP-21::Venus fluorescence levels in coelomocytes. cccp-1(ox334) has decreased coelomocyte fluorescence (*** P<0.001) but rund-1(tm3622) has no defect (P>0.05). Neither rund-1 nor cccp-1 has decreased fluorescence in the ventral cord (P>0.05). Error bars=SEM; n=13–21.
(E) rab-2, rund-1, and cccp-1 act in the same genetic pathway. Double mutants of rab-2(nu415), rund-1(tm3622) and cccp-1(ox334) are similar to single mutants in trafficking NLP-21::Venus to the dorsal cord (P>0.05 for all pairwise comparisons except cccp-1; rund-1 vs. cccp-1, P<0.05). All mutants exhibit significant defects compared to wild type (P<0.001). Error bars=SEM; n=10–13.
(F) IDA-1::GFP fluorescence levels in the dorsal cord. The rab-2(nu415), rund-1(tm3622), and cccp-1(ox334) mutants are defective in trafficking IDA-1::GFP (*** P<0.001 and ** P<0.01 vs WT). Error bars=SEM; n=9–13.
We followed soluble components of DCVs by tagging the Venus fluorescent protein to the processed propeptides NLP-21 and FLP-3, and the nonprocessed peptide INS-22. During vesicle maturation, the processed propeptides are cleaved and the fluorescent protein is released within the vesicle. The mature vesicle is then transported into the axon. rund-1 and cccp-1 mutations reduced axonal fluorescence of Venus (Figures 4A–C, 4E and Figure S6A). The loss of fluorescence in the axons was not caused by a defect in polarity since there was no increase of fluorescence in the dendrites (Figure 4D). Expression of the rund-1 cDNA in the same cells fully rescued the rund-1 trafficking defect (Figure 4C), indicating that RUND-1 acts cell autonomously.
rab-2 mutants show soluble cargo trafficking defects similar to rund-1 and cccp-1 mutants (Edwards et al., 2009; Sumakovic et al., 2009). To determine if these genes act in the same pathway, we built double mutants and measured axonal fluorescence. In all cases, double mutants showed defects comparable to the single mutants (Figures 4A, 4B and 4E), providing additional evidence that rab-2, rund-1, and cccp-1 function in the same genetic pathway.
Despite the loss of soluble fluorescent proteins from vesicles in these mutants, neuropeptide trafficking into axons does not seem to be blocked. The Venus tag can be forced to remain with neuropeptides by mutation of the egl-3 proprotein convertase. In the absence of the convertase, Venus cannot be cleaved from the condensed peptides. In the rund-1 egl-3 double mutant, Venus-tagged NLP-21 and FLP-3 is observed in the axons (Figure 4C), as was previously observed in rab-2 egl-3 double mutants (Edwards et al., 2009; Sumakovic et al., 2009). However, in both rab-2 egl-3 and rund-1 egl-3 double mutants, there is still a reduction in axonal fluorescence compared to egl-3 single mutants (Edwards et al., 2009; Sumakovic et al., 2009; data not shown), suggesting that there is also reduced trafficking of unprocessed cargo in rab-2 and rund-1 mutants.
To assay the release of DCV cargo, we measured the accumulation of Venus fluorescence in coelomocytes, scavenger cells residing in the body cavity of the worm (Sieburth et al., 2007; Speese et al., 2007). cccp-1 mutants have reduced fluorescence in coelomocytes, suggesting that missorted cargo is not released in cccp-1 mutants. rund-1 mutants lack fluorescence in axons but accumulate fluorescence in the coelomocytes at wild-type levels (Figure 4D), suggesting that an alternative release pathway in the cell body is still intact in rund-1 mutants. This may be due to missorting of cargo to the constitutive secretory pathway.
RUND-1 and CCCP-1 are Localized to the Trans-Golgi in Neuronal Cell Bodies
The rund-1 gene was tagged at its C-terminus with GFP, tagRFP, or tdEos, and inserted as a single copy in the genome. These constructs fully rescued the rund-1 mutant locomotion defect, (Figure 2C and Figure S3E). RUND-1 localized mainly to two or three perinuclear puncta in neuronal cell bodies; only extremely faint fluorescence was observed in axons (Figure 5 and Figure S8A).
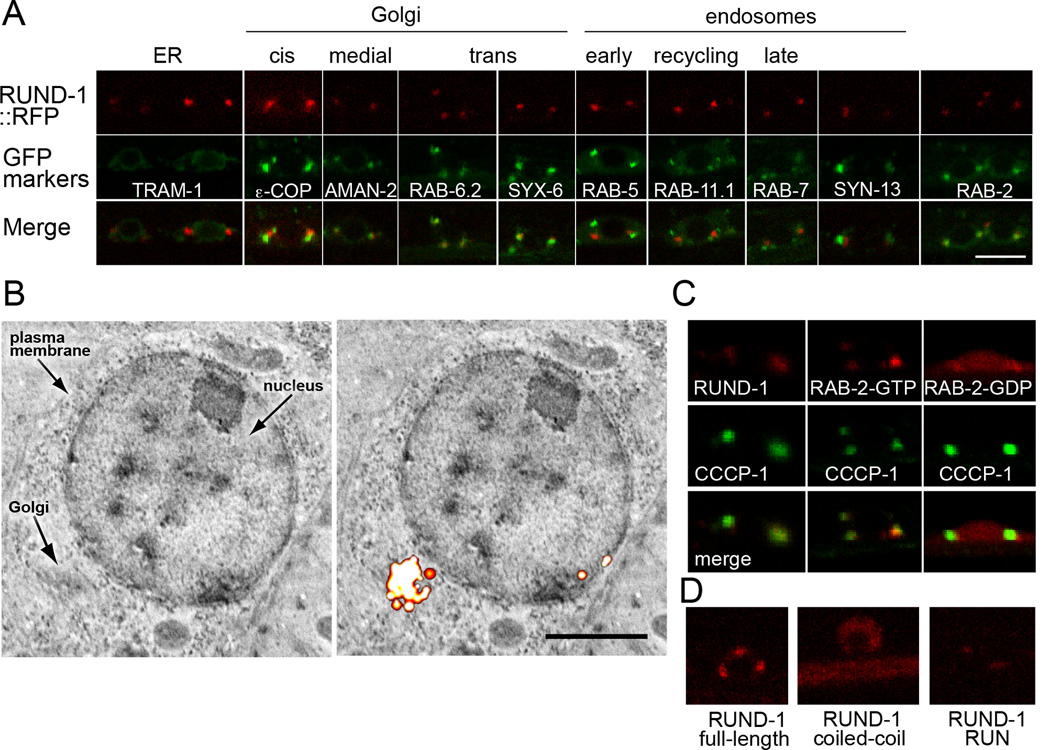
Figure 5. RUND-1 colocalizes with RAB-2 at the trans-Golgi network.
(A) RUND-1 colocalizes with RAB-2 and trans-Golgi markers. Each panel shows a single slice of a confocal image of motor neuron cell bodies in the ventral cord of young adult animals. The top boxes show the localization of a single-copy rescuing RUND-1::tagRFP-T fusion protein (oxIs590). RUND-1 localizes almost exclusively to two or three perinuclear puncta per cell. The middle boxes show the localization of single-copy GFP-tagged compartment markers. The bottom boxes show the merged images. Scale bar: 5 µm, applies to all panels. RUND-1 shows tightest colocalization with RAB-6.2, a trans-Golgi Rab protein, and with SYX-6, the ortholog of the trans-Golgi SNARE syntaxin 6. RUND-1 puncta also colocalize well with RAB-2 puncta. RUND-1 partially overlaps with the medial-Golgi marker mannosidase II (AMAN-2) and the cis-Golgi marker εCOP. RUND-1 is not colocalized with the rough ER marker TRAM-1 nor with the endosomal markers RAB-5, RAB-11.1, RAB-7 and SYN-13.
(B) RUND-1 localizes to the Golgi. The left panel shows a backscatter scanning electron micrograph of the cell body of a neuron in the nerve ring. The right panel shows the same image overlaid with the corresponding fluorescence PALM image of RUND-1::tdEos. Scale bar: 1 µm.
(C) CCCP-1 colocalizes with RUND-1 and RAB-2. CCCP-1 colocalizes with RUND-1 and RAB-2(GTP). CCCP-1 is still punctate when coexpressed with RAB-2(GDP), which is diffusely expressed.
(D) The RUN domain of RUND-1 mediates its localization. Full length RUND-1, the coiled-coil domain, and the RUN domain were tagged at their C-termini with tagRFP-T and integrated in the genome. The truncated proteins were expressed at lower levels. See also Figure S8.
We examined colocalization of RUND-1::tagRFP with GFP-tagged markers for various compartments (Figure 5A). All markers were expressed as single-copy insertions since overexpression of some markers can change the size of the corresponding compartment (Bucci et al., 1992, 2000). RUND-1 did not localize to compartments that communicate with the Golgi, including the endoplasmic reticulum (TRAM-1), the early endosome (RAB-5), recycling endosome (RAB-11.1), late endosome (RAB-7), or with the general endosome marker syntaxin-13 (SYN-13). RUND-1 showed partial overlap with a cis-Golgi marker (the COP I vesicle marker ε-COP) and more overlap with a medial-Golgi marker (AMAN-2, mannosidase II). In neuronal cell bodies, RUND-1 showed almost perfect colocalization with the trans-Golgi markers RAB-6.2 and syntaxin 6 (SYX-6). RAB-6.2 is localized predominantly to the trans-Golgi and is also found on exocytotic vesicles leaving the Golgi (Goud et al., 1990; Grigoriev et al., 2007); syntaxin 6 is a trans-Golgi SNARE also found on immature secretory granules in neuroendocrine cells (Bock et al., 1997; Klumperman et al., 1998). We conclude that RUND-1 localizes to the trans-Golgi or to vesicles closely associated with the trans-Golgi. Localization of RAB-6.2 and syntaxin 6 were not altered in a rund-1 mutant background (data not shown), suggesting that rund-1 is not required for normal Golgi structure.
To confirm that RUND-1 localized to the Golgi, we performed correlative nanometer-resolution fluorescence electron microscopy (Watanabe et al., 2011). Fluorescence from the tdEos-tagged RUND-1 was localized using super-resolution PALM imaging and superimposed on scanning electron micrographs. RUND-1 was localized to the Golgi in neuronal cell bodies (Figure 5B) and in pharyngeal cells (data not shown).
To determine which part of RUND-1 mediates its localization, we split RUND-1 into two pieces and tagged each with tagRFP. The coiled-coil domain of RUND-1 was spread diffusely throughout the cell body and axons (Figure 5D). In contrast, the RUN domain of RUND-1 was sufficient for localization to perinuclear puncta (Figure 5D). Neither truncation rescued a rund-1 mutant, suggesting that both domains are required for normal function, but that the RUN domain is sufficient for localization.
We also tagged CCCP-1 with GFP and expressed it using the Prab-3 neuronal promoter. CCCP-1 is localized mainly to perinuclear puncta in neuronal cell bodies (Figure 5C), but unlike RUND-1 is also more diffusely spread throughout cell bodies and axons. This pattern resembles that of RAB-2 (Edwards et al., 2009; Sumakovic et al., 2009). To confirm the broader localization of RAB-2, we inserted a GFP::RAB-2 single-copy transgene in the genome (Figure S8C). Like CCCP-1, RAB-2 was found at perinuclear puncta and also diffusely in the cell body and axons. RUND-1 and RAB-2 colocalize at perinuclear puncta in neuronal cell bodies (Figure 5A).
The localization of RUND-1 and CCCP-1 to the Golgi is not disrupted in rab-2 mutants (Figures S8A, B, D, and E). Nor is CCCP-1 localization affected by GTP-bound or GDP-bound RAB-2 (Figure 5C). Conversely, RAB-2 is not mislocalized in rund-1 and cccp-1 mutants (Figure S8C). Finally, RUND-1 is localized normally in a cccp-1 mutant and CCCP-1 is localized normally in a rund-1 mutant (Figures S8A and S8B). Thus, RUND-1, CCCP-1 and RAB-2 are not required for each other’s localization.
RUND-1 and CCCP-1 Bind RAB-2 Specifically
Using yeast two-hybrid assays, we examined the interactions of full-length RUND-1 and CCCP-1 with three different forms of RAB-2: (1) the constitutively active GTP-bound form RAB-2(Q65L), (2) the constitutively inactive GDP-bound form RAB-2(S20N), and (3) the wild-type RAB-2. RUND-1 interacted with GTP-bound RAB-2, but not with the GDP-bound RAB-2 or the wild-type RAB-2 (Figure 6A). Thus, like other RUN-domain proteins, the interaction of RUND-1 with RAB-2 is specific to the activated GTP-bound form of the Rab. CCCP-1 interacted with both the GTP-bound and wild-type RAB-2, but not with the GDP-bound RAB-2 (Figure 6A). This suggests a stronger interaction of RAB-2 and CCCP-1 that is still GTP-dependent, given that some of the wild-type RAB-2 is expected to be GTP-bound. RUND-1 and CCCP-1 did not interact with each other in two-hybrid assays (data not shown).
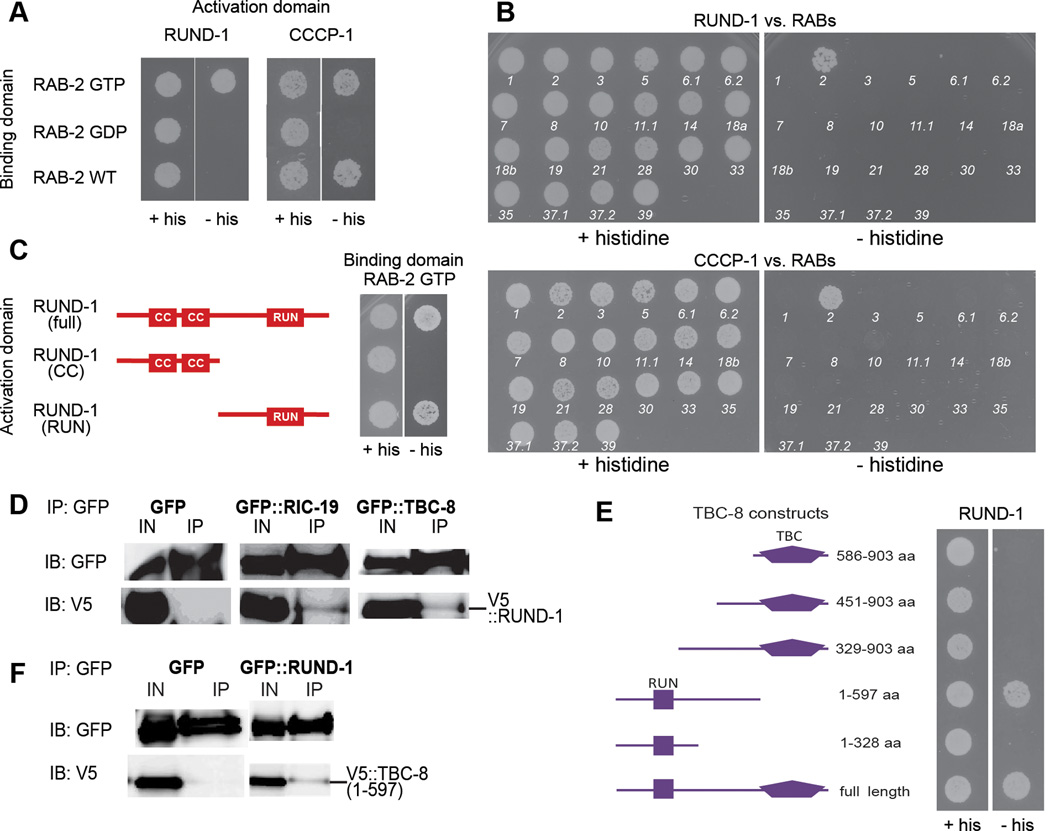
Figure 6. RUND-1 and CCCP-1 interact physically with activated RAB-2.
(A) RUND-1 interacts specifically with GTP-bound RAB-2 (RAB-2 GTP) in a yeast two-hybrid assay. RUND-1 did not show an interaction with wild-type RAB-2 (RAB-2 WT) or inactive GDP-bound RAB-2 (RAB-2 GDP). CCCP-1 interacts with RAB-2 GTP and RAB-2 WT, but not with RAB-2 GDP. Growth without histidine (- his) indicates a physical interaction.
(B) The RUND-1 and CCCP-1 interactions with RAB-2 are specific. The interactions of RUND-1 and CCCP-1 with C. elegans RAB proteins were examined by yeast two-hybrid. Numbers indicate the number of the RAB gene in C. elegans (e.g. 1 = RAB-1). RUND-1 and CCCP-1 interact only with RAB-2. RAB-27 could not be tested because of self-activation.
(C) RUND-1 interacts with RAB-2 via the RUN domain. Two truncations of RUND-1 were used: RUND-1 (CC) consists of amino acids 1–261. RUND-1 (RUN) consists of amino acids 262–549.
(D) RUND-1 interacts with RIC-19 and TBC-8. V5-tagged RUND-1 was coexpressed with GFP, GFP::RIC-19 or GFP::TBC-8 in HEK293 cells. Immunoprecipitation of GFP::RIC-19 or GFP::TBC-8 pulled down RUND-1. Immunoprecipitation of untagged GFP did not pull down RUND-1. IN: input, IP: immunoprecipitation, IB: immunoblotting.
(E) RUND-1 interacts with TBC-8 outside of its TBC domain. Truncations of TBC-8 were examined for interactions with RUND-1 by yeast two-hybrid.
(F) RUND-1 interacts with TBC-8 outside of its TBC domain. V5-tagged TBC-8 (1–597 aa) was coexpressed with GFP or GFP::RUND-1 in HEK293 cells. Immunoprecipitation of GFP::RUND-1 pulled down TBC-8 (1–597). IN: input, IP: immunoprecipitation, IB: immunoblotting.
To test for specificity of these interactions, we performed yeast two-hybrid assays with RUND-1 or CCCP-1 and GTP-bound versions of other members of the Rab, Rap, Ras, and Ral families of small GTPasess. RUND-1 and CCCP-1 showed an interaction only with RAB-2 (Figure 6B; data not shown). Thus, RUND-1 and CCCP-1 are specific RAB-2 interactors.
We split RUND-1 into two pieces and assayed binding to RAB-2. The coiled-coil domain fragment did not interact with GTP-bound RAB-2. The RUN domain fragment interacted robustly with GTP-bound RAB-2 (Figure 6C). This construct carries the entire RUN domain and a putative upstream α-helix that is needed for proper folding of other RUN domains (Kukimoto-Niino et al., 2006; Recacha et al., 2009). A shorter construct lacking the upstream α-helix and the beginning of the conserved A block of the RUN domain did not show an interaction with RAB-2 (data not shown). Thus, it seems likely that the RUN domain is required for the interaction of RUND-1 with RAB-2.
RUND-1 binds RIC-19 and TBC-8
RAB-2 binds TBC-8, a putative RAB-2 GAP, and RIC-19, a BAR domain protein (Hannemann et al., 2012; Sumakovic et al., 2009). RUND-1 also interacts with TBC-8 in yeast two-hybrid assays and with both TBC-8 and RIC-19 when coexpressed in HEK293 cells (Figure 6D–E). In contrast, CCCP-1 did not interact with TBC-8 or RIC-19 in two-hybrid assays (data not shown). Thus, RUND-1, but not CCCP-1, independently binds RAB-2 and other RAB-2 partners including RIC-19 and TBC-8.
TBC-8 consists of an N-terminal RUN domain, and a C-terminal TBC domain responsible for the GAP activity. A fragment of TBC-8 carrying its RUN domain binds RUND-1, but fragments carrying only the TBC GAP domain do not bind (Figures 6E and 6F). The TBC-8 RUN domain also binds RIC-19 (Hannemann et al., 2012). The RUND-1 coiled-coil domains do not interact with TBC-8, but the RUND-1 RUN domain exhibited a weak interaction with TBC-8 (data not shown). Thus, RUND-1 and TBC-8 interact via regions containing their RUN domains.
To determine whether ric-19 and tbc-8 exhibit genetic interactions with rund-1, we built double mutants and assayed locomotion and DCV cargo trafficking. Although ric-19 had little effect on locomotion on its own (Sumakovic et al., 2009), it significantly enhanced the locomotion defect of rund-1 (Figure 2G). Similarly, ric-19 enhanced the NLP-21::Venus trafficking defect of rund-1 (Figure S6B). These data indicate that RIC-19 acts in parallel to RUND-1, possibly as two distinct effectors of RAB-2. tbc-8 mutants also have defects in sorting vesicle cargo (Hannemann et al., 2012). However the sorting defects in rund-1 mutants were not enhanced in rund-1 tbc-8 double mutants (Figure S6B), indicating that tbc-8 and rund-1 act in the same pathway.
Discussion
We performed a genetic screen for mutants defective in dense-core vesicle function and identified two novel proteins, RUND-1 and CCCP-1. RUND-1 and CCCP-1 are required for proper sorting of DCV cargo, but are not required for DCV morphology or transport. Genetic and biochemical data show that these proteins are likely Rab2 interactors at the trans-Golgi. RUND-1 also interacts with the BAR domain protein RIC-19 and the RAB-2 GAP TBC-8, both of which individually interact with RAB-2. These results identify a set of interacting proteins that function in the trafficking of DCV cargos.
RUND-1 and CCCP-1 Interact with RAB-2 at the Golgi
The function of a Rab protein is defined by its localization to a specific cellular compartment and by its effector proteins. Rab2 was originally localized to the ER-Golgi intermediate compartment (Chavrier et al., 1990) and was thought to function in ER to Golgi anterograde trafficking (Tisdale et al., 1992), but Rab2 is distributed more broadly across the Golgi and may have additional roles (Chun et al., 2008; Sumakovic et al., 2009). Known effectors of Rab2 include several coiled-coil proteins associated with the Golgi (Hayes et al., 2009; Short et al., 2001; Sinka et al., 2008) and the BAR domain protein ICA69/RIC-19 (Buffa et al., 2008; Sumakovic et al., 2009).
Here we demonstrate that the RUN domain protein RUND-1 and the conserved coiled-coil protein CCCP-1 are possible novel effectors of RAB-2. Five lines of evidence support this conclusion. First, rund-1, cccp-1 and rab-2 mutants exhibit a similar locomotory phenotype. Second, rund-1, cccp-1 and rab-2 mutants exhibit similar defects in trafficking DCV cargo. Third, rund-1, cccp-1 and rab-2 act in the same genetic pathway. Fourth, RUND-1, CCCP-1 and RAB-2 colocalize at the trans-Golgi network. Fifth, RUND-1 and CCCP-1 bind to the active GTP-bound form of RAB-2, but not the inactive GDP-bound form.
Classical Rab effectors are recruited to a compartment by binding to the active GTP-bound Rab. For example, RIC-19 is recruited to the Golgi by GTP-RAB-2 and dispersed into the cytoplasm by GDP-RAB-2 (Sumakovic et al., 2009). However, neither RUND-1 nor CCCP-1 localization is dependent on RAB-2. RUND-1 localization is mediated by its RUN domain, but independently of RAB-2 binding. Recently, the RAB-10 effector EHBP-1 has been shown instead to mediate localization of its cognate Rab (Shi et al., 2010). However, RAB-2 localization does not depend on either RUND-1 or CCCP-1. Nor are RUND-1 and CCCP-1 required for each other’s localization. Thus, RUND-1, CCCP-1 and RAB-2 all appear to localize independently or have multiple binding partners at the Golgi.
RUND-1 is a Member of the RUN Domain Protein Family
RUN domain proteins were originally proposed to be potential Rab and Rap effectors (Callebaut et al., 2001). Clear physical interactions with Rab and Rap proteins have been shown for some of these proteins (Bayer et al., 2005; Cormont et al., 2001; Fouraux et al., 2004; Janoueix-Lerosey et al., 1995, 1998; Kukimoto-Niino et al., 2006; Miserey-Lenkei et al., 2007; Recacha et al., 2009). Moreover, the crystal structure of the RUN domain of Rab6 IP1 in complex with Rab6 provides evidence that the RUN domain is sufficient to mediate binding to Rabs in at least some cases (Recacha et al., 2009). However, there are few functional studies that tie RUN domains to Rab function. Our data provide in vivo support to the conclusion that RUN domains mediate Rab protein functions.
We demonstrate that the RUN domain of RUND-1 is sufficient to interact with RAB-2. Like for other RUN domains, this interaction is specific to GTP-bound RAB-2. Moreover, RAB-2 is probably the only small GTPase that interacts with RUND-1. Most RUN domain proteins studied interact with at most a single Rab protein, although this has not been exhaustively analyzed (Bayer et al., 2005; Fukuda et al., 2011; Miserey-Lenkei et al., 2007; Recacha et al., 2009). A recent genome-wide analysis detected Rab interactors for only six of nineteen mammalian RUN domain proteins by yeast two-hybrid assays (Fukuda et al., 2011). However, this study did not detect an interaction between the mammalian orthologs of RUND-1 and RAB-2, so it is possible that weaker interactions were missed.
In addition to the RUN domain, most RUN domain proteins have other interaction domains (Callebaut et al., 2001). RUND-1 has two N-terminal coiled-coil domains. Coiled-coil domains typically mediate protein-protein interactions and are especially common in RUN domain proteins (Cormont et al., 2001; Fouraux et al., 2004; Janoueix-Lerosey et al., 1998; Lan et al., 2005; Matsunaga et al., 2009; Mori et al., 2007; Yang et al., 2002; Zhong et al., 2009). Unlike the coiled-coil domains of some Rab effectors such as the golgins (Burguete et al., 2008; Hayes et al., 2009; Short et al., 2001; Sinka et al., 2008), the coiled-coil domains of RUND-1 are not required for binding to RAB-2. However, the coiled-coil domains of RUND-1 are conserved, and the ox328 missense mutation in the second coiled-coil domain is a severe allele. It seems likely that the coiled-coil domains of RUND-1 mediate important interactions with other proteins acting in the RAB-2 pathway.
Function of RAB-2 and its Interactors
RAB-2 appears to have at least three interacting proteins in the DCV maturation pathway: RUND-1, CCCP-1 and RIC-19. These proteins could act separately as independent interactors of RAB-2, or they could act together in a single complex. The data are consistent with the possibility that RAB-2 forms a single complex with RUND-1, RIC-19, and TBC-8 since these proteins exhibit direct pairwise interactions and are colocalized at the Golgi (Figure 7A). In contrast, CCCP-1 interacts only with RAB-2 and may act as an independent effector. Interestingly, the locomotion and DCV cargo trafficking phenotypes of rab-2, rund-1 and cccp-1 are similar but not identical, indicating that there are distinct functions for each protein. The locomotion and trafficking defects of rund-1 and cccp-1 mutants are generally weaker than the rab-2 mutant defects, indicating that RUND-1 and CCCP-1 may participate in only certain aspects of RAB-2 function or to only a partial degree. Also, rund-1 mutants do not impair cargo secretion as assayed by coelomocyte uptake, suggesting a different final destination for missorted cargos in rund-1 mutants versus cccp-1 and rab-2 mutants (Sumakovic et al., 2009). However, the overt phenotypic similarities of these mutants as well as the genetic interactions suggest that these proteins act in the same process during cargo sorting into DCVs.
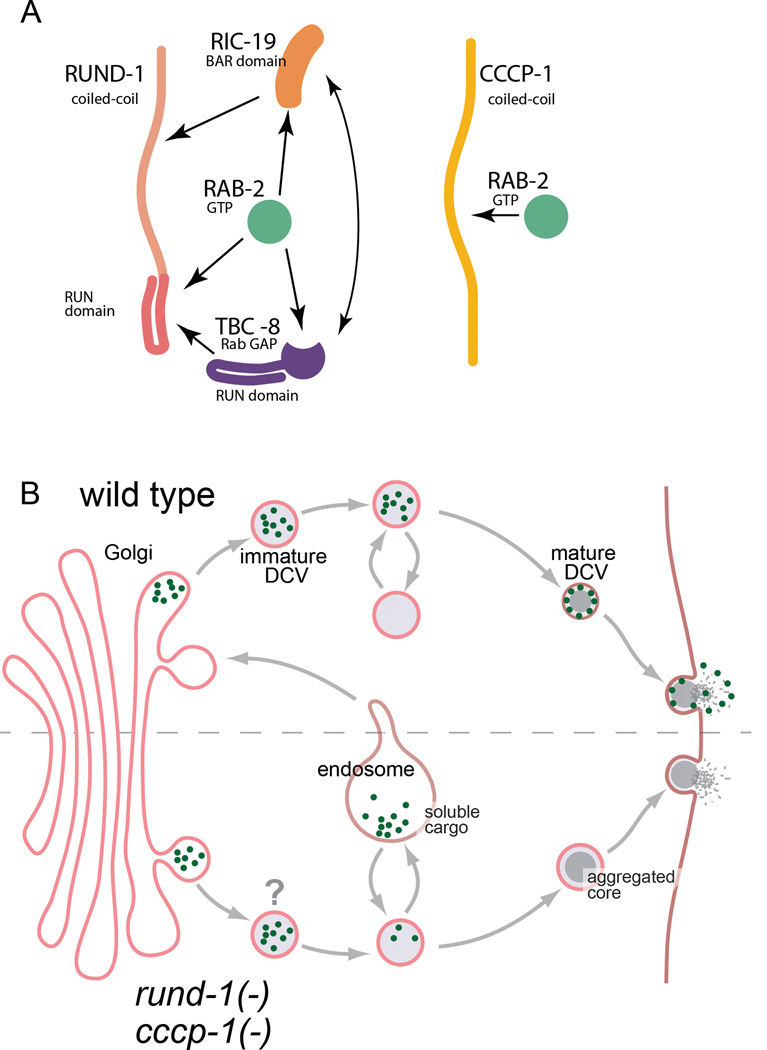
Figure 7. Model for RUND-1 and CCCP-1 action in DCV maturation.
(A) RUND-1 may be in a complex with activated RAB-2, RIC-19 and TBC-8 since all of these molecules bind each other in pairwise combinations. CCCP-1 binds activated RAB-2, but does not bind RUND-1, RIC-19 or TBC-8, so it may be in a separate complex.
(B) RUND-1 and CCCP-1 localize near the trans-Golgi and are involved in regulating cargo sorting during the formation of mature DCVs. Soluble cargo (green dots) are retained in the mature vesicle and released at the plasma membrane. In the absence of rund-1 and cccp-1, immature DCVs may have an improper identity (denoted by ‘?’), causing them to lose soluble cargo to the endolysosomal system. However, insoluble cargo is not lost, including peptides that aggregate and form the characteristic dense-core seen by EM. The process of aggregation is depicted as a gradual graying and condensation of the vesicle center during the maturation process. Though axonally localized DCVs carry reduced amounts of certain cargos in rund-1 mutants, overall release is normal as assayed by coelomocyte uptake, suggesting that the “lost” cargos are still secreted, perhaps as a result of their missorting to the constitutive secretory pathway. In cccp-1 and rab-2 mutants, release of such cargos is reduced, suggesting that they may be misdirected to the lysosome and degraded.
The primary cellular defect we observe in these mutants is the reduced trafficking of fluorescent proteins targeted to DCVs, with no major effects on the morphology or transport of vesicles themselves. It is not clear which class of cargo accounts for the behavioral defects. The fact that the locomotion defects of rab-2, rund-1 and cccp-1 mutants are enhanced by mutations in the proprotein convertase egl-3 indicates that the behaviorally relevant missorted cargos are not processed by EGL-3, but rather may be peptides processed by other convertases or cargos that do not require processing at all.
What are the functions of RAB-2, RUND-1 and CCCP-1 at the Golgi during maturation of DCVs? There are several possibilities, not all mutually exclusive, such as specifying vesicle identity, tethering, budding or cargo sorting. First, vesicle identity is often conveyed by Rab proteins (Stenmark, 2009), and RAB-2 and its interactors could provide identity to maturing DCVs. In the absence of markers of identity, the vesicles may become endosomal in character and fuse with the endolysosomal system, thereby losing some of their cargos (Figure 7B). Second, RUND-1 and CCCP-1 may tether vesicles via the ‘tentacular matrix’ that moves vesicles processively through the Golgi (Munro, 2011; Sinka et al., 2008; Yu and Hughson, 2010). The tentacular matrix is posited to be formed by the golgins, long coiled-coil proteins that localize to the Golgi and capture vesicles by binding Rab proteins. Third, RUND-1 may act directly in the formation of vesicles. RUND-1 and RAB-2 interact with RIC-19, which contains a crescent-shaped BAR domain that may induce membrane curvature (Frost et al., 2009). Finally, RAB-2 and its interactors could be involved more directly in sorting. Since none of the proteins have a transmembrane domain, they are unlikely to serve as a sorting receptor per se, but may be involved in the retrograde retrieval of a sorting receptor.
The possible RUND-1 complex may consist not only of the RAB-2 interactors RIC-19 and RUND-1, but the RAB-2 GAP protein TBC-8, which should turn off the Rab. The combination of positive and negative factors suggests that the complex may be quite dynamic. The binding of interactors to RAB-2 may simultaneously recruit the GAP that will hydrolyze GTP and cause dissolution of the complex. Such fine temporal control of RAB-2 activation may help deliver vesicles to their trans-Golgi acceptor and then release them from the acceptor complex as maturation proceeds.
The human ortholog of RUND-1 is RUNDC1, which is highly conserved in both the coiled-coil and RUN domains. When RUNDC1 is expressed in rund-1 mutants, the human protein is localized to the Golgi and rescues the mutant phenotype, indicating a strong conservation of function. Human RUNDC1 has been identified as an inhibitor of the tumor suppressor p53 (Llanos et al., 2006), and RUNDC1 expression is used as a prognostic marker of metastasis in breast cancer tumors (van ’t Veer et al., 2002). Thus, RUNDC1 is a possible oncogene. RUNDC1 has not been reported to have any function in the nervous system, but maps to chromosomal position 17q21, in the same region of a likely autism gene (Cantor et al., 2005). An individual with autism has also been found to carry a de novo nonsense mutation in Rab2A, the human ortholog of RAB-2 (Sanders et al., 2012). Because Rundc1 and Rab2A are expressed in the mouse brain (Allen Brain Atlas), it is possible that RUND-1 and its interactors function in DCV cargo trafficking in the vertebrate brain as well as in nematodes.
Experimental Procedures
Strains
Worm strains were cultured and maintained using standard methods (Brenner, 1974). A complete list of strains and mutations used is provided in the Extended Experimental Procedures.
Screen for suppressors of activated Gq
egl-30(tg26) encodes an activating mutation in Gαq. We mutagenized egl-30(tg26) worms with 0.5 mM ENU (N-ethyl-N-nitrosourea) for four hours as described (De Stasio and Dorman, 2001). We screened the F2 generation for suppression of the hyperactive locomotion phenotype of egl-30(tg26). From screens of ~18,000 mutagenized haploid genomes, we isolated 43 mutants. Most of these mutants suppressed not only the hyperactivity of egl-30(tg26), but also the small size and slower growth rate, indicating that they specifically suppress Gq and do not affect locomotory function indirectly. To more generally test for the specificity of suppression, we built double mutants between egl-30(tg26) and a number of mutations that impair movement by affecting function of the neuromuscular junction: the N-type calcium channel unc-2, the synaptic vesicle docking/priming protein unc-18, the synaptic vesicle kinesin unc-104, the ryanodine receptor unc-68, the proprotein convertase egl-3, and the acetylcholine receptor assembly factors unc-50 and unc-74. In all cases, the double mutants exhibited phenotypes that resembled a combination of the two individual mutants, indicating additive effects with egl-30(tg26). Locomotion rates were reduced, but double mutants were uncoordinated, small and slower growing than either of the parents. Thus, general disruptions of neuronal or muscular function do not nonspecifically suppress egl-30(tg26).
We mapped 39 of the suppressors and assigned them to 22 different complementation groups (Table 1). Several were known to act in the Gq pathway, including loss-of-function mutations in egl-30/Gqα, ric-8/Gα GEF, unc-73/Trio, egl-8/phospholipase Cβ, and the DAG-binding synaptic protein UNC-13. Others included genes thought to be involved in DCV secretion, including unc-31/CAPS, and pkc-1/protein kinase C (Sieburth et al., 2007). The remaining 18 unidentified mutants comprised 15 complementation groups with one or two alleles each. Mutants in six genes had a strong unmotivated locomotion phenotype. We characterized two of these in detail, rund-1 and cccp-1.
Table 1.
Mutants isolated as Gq suppressors.
| Gene | Protein | Function | # alleles |
|---|---|---|---|
| egl-30 | Gq alpha | Gq-alpha GTPase | 6 |
| ric-8 | synembryn | G-protein GEF | 1 |
| egl-8 | PLCbeta | Gq effector, DAG synthesis | 4 |
| unc-13 | Munc13 | DAG binding, MUN domain, SV + DCV exocytosis | 1 |
| unc-31 | CAPS | MUN domain, DCV exocytosis | 6 |
| pkc-1 | protein kinase C | DAG binding DCV exocytosis | 1 |
| unc-73 | Trio | Gq-alpha effector Rho GEF | 2 |
| rund-1 | Rundc1 | Golgi Rab2 effector | 2 |
| cccp-1 | C10orf118 | Golgi Rab2 effector | 1 |
| 13 others | 15 |
Mapping and cloning rund-1 and cccp-1
The rund-1(ox281) mutation was mapped to the left arm of chromosome X using single nucleotide polymorphisms (SNPs) in the Hawaiian strain CB4856 (Davis et al., 2005). Fine mapping, facilitated by picking recombinants between rund-1(ox281) and dpy-3, narrowed the rund-1 interval to a 60 kb region with nine predicted genes, most on cosmid T19D7. Injection of cosmid T19D7 into rund-1(ox281) rescued the mutant phenotype. RNAi of the gene T19D7.4 caused a defecation defect reminiscent of rund-1 mutants. We sequenced the T19D7.4 gene in the rund-1 mutants ox281 and ox328. rund-1(ox281) is a G to A transition in the splice acceptor of the sixth intron. rund-1(ox328) is an A to C transversion that causes a T237P missense mutation in the second coiled-coil domain. We rescued rund-1 mutants with a transgene containing only T19D7.4, confirming the gene identification.
The cccp-1(ox334) mutation was mapped to a 126 kb region on the left arm of chromosome III with 16 predicted genes. Because no C. elegans cosmids covered this region, we obtained two C. briggsae BACs (from CHORI) carrying orthologs of the genes in this region and injected them into cccp-1(ox334) mutants. The BAC RPCI94_09N13 rescued the cccp-1 locomotion defect, while the BAC RPCI94_26P21 did not. RPCI94_09N13 carries orthologs of two C. elegans genes in this region, Y49E10.23 and Y49E10.24. Both cccp-1 alleles introduce early stop mutations in Y49E10.23: cccp-1(ox334) carries a T to A transversion in exon 7 and cccp-1(e1122) carries a C to T transition in exon 9, leading to stop mutations at L343 and Q482 respectively. We subsequently rescued cccp-1(ox334) with a transgene containing only Y49E10.23.
Locomotion assays
We performed tracking assays by videotaping worms and processing the movies using a custom-made plugin for ImageJ (White et al., 2007). Assays were initiated by transferring three to five well-fed worms with a platinum worm pick to a tracking plate with a spot of food. Movies were taken for thirty minutes. For the rare periods in which a worm left the food, the data were omitted. For all experiments except the tissue-specific rescue of rund-1, data were collected on two different days for each strain and combined. There was no significant day-to-day variation.
To measure stimulated locomotion on food, body bends were counted in the first two minutes after placing animals on thin lawns of bacteria. A body bend was defined as the movement of the tail from maximum to minimum amplitude of the sine wave (Miller et al., 1999). For heatshock expression, animals were exposed to 34° for 1 hr and assayed 24 hours later as adults. To measure locomotion in liquid, thrashing was assayed as described (Hobson et al., 2011). We placed animals in a drop of M9 on an unseeded plate, waited for one minute, and then counted thrashes for 90 seconds. A thrash was defined as a change in the direction of the bend in the middle of the animal. All body bending and thrashing assays were performed on at least two different days for each set of strains, but each graph shows the data from a single representative experiment.
Imaging and image analysis
Worms were mounted on 2% agarose pads and anesthetized with 50 mM sodium azide. Images were obtained using a Zeiss Pascal confocal microscope, a Leica SP2 confocal microscope or a Nikon 80i wide-field compound microscope. To image the dorsal or ventral nerve cords, young adult animals were oriented with dorsal or ventral side up by exposure to the anesthetic for ten minutes on the slide before placing the cover slip. For quantitative imaging of dorsal cord fluorescence, all strains in a given experiment were imaged on the same days and all microscope settings were kept constant. The same section of the dorsal cord posterior of the vulva was imaged in all worms. Maximum intensity projections were quantified using ImageJ software, measuring the total fluorescence in a region of interest encapsulating the cord and subtracting the background fluorescence of a region of identical size adjacent to the cord. For colocalization images, Pearson’s correlation coefficients were calculated using Nikon Elements software by selecting a circular region of interest around individual cell bodies in the ventral cord.
Statistics
P values were determined using InStat 3.1a and GraphPad Prism 5.0d (GraphPad Software). Data sets were analyzed by a one-way ANOVA to test for differences among the set followed by a Bonferroni posthoc test to examine selected comparisons, or by the Kruskal-Wallis nonparametric ANOVA to test for differences among the set followed by Dunn’s test to examine selected comparisons. For categorical data, we calculated a two-sided P value using Fisher’s exact test.
For other methods see Extended Experimental Procedures.
Supplementary Material
Acknowledgments
We thank K. Iwasaki, K. Miller, J. Kaplan, Z. Zhou, S. Mitani, B. Grant, C. Frøkjær-Jensen, G. Hollopeter, and P. McEachern for strains and plasmids; Y. Kohara for cDNA clones; the Sanger Center for cosmids; R. Rawson for help with confocal microscopy; D. Hobson for data analysis algorithms; and I. Topalidou for comments on the manuscript. Strains were provided by the CGC (P40 OD010440). MA was supported by Helen Hay Whitney and by NIH R00MH082109. MH was supported by the Univ Göttingen GGNB program (DFG grant GSC 226/1). SE was supported by German Research Foundation CMPB and the European Neuroscience Institute. This work was supported by NIH NS034307 and NSF IOS-0920069 to EMJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahras M, Otto GP, Tooze SA. Synaptotagmin IV is necessary for the maturation of secretory granules in PC12 cells. J. Cell Biol. 2006;173:241–251. doi: 10.1083/jcb.200506163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailion M, Inoue T, Weaver CI, Holdcraft RW, Thomas JH. Neurosecretory control of aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7394–7397. doi: 10.1073/pnas.96.13.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J. Biol. Chem. 1997;272:19637–19640. doi: 10.1074/jbc.272.32.19637. [DOI] [PubMed] [Google Scholar]
- Avery L, Bargmann CI, Horvitz HR. The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics. 1993;134:455–464. doi: 10.1093/genetics/134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani CA, Gharib S, Simon MI, Sternberg PW. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165:1805–1822. doi: 10.1093/genetics/165.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M, Fischer J, Kremerskothen J, Ossendorf E, Matanis T, Konczal M, Weide T, Barnekow A. Identification and characterization of Iporin as a novel interaction partner for rab1. BMC Cell Biol. 2005;6:15. doi: 10.1186/1471-2121-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol. Biol. Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgonovo B, Ouwendijk J, Solimena M. Biogenesis of secretory granules. Curr. Opin. Cell Biol. 2006;18:365–370. doi: 10.1016/j.ceb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage L, Avery L, Katz A, Kim UJ, Mendel JE, Sternberg PW, Simon MI. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron. 1996;16:999–1009. doi: 10.1016/s0896-6273(00)80123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa L, Fuchs E, Pietropaolo M, Barr F, Solimena M. ICA69 is a novel Rab2 effector regulating ER-Golgi trafficking in insulinoma cells. Eur. J. Cell Biol. 2008;87:197–209. doi: 10.1016/j.ejcb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Burguete AS, Fenn TD, Brunger AT, Pfeffer SR. Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185. Cell. 2008;132:286–298. doi: 10.1016/j.cell.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Fukushige T, Notkins AL, Krause M. Insulinoma-Associated Protein IA-2, a Vesicle Transmembrane Protein, Genetically Interacts with UNC-31/CAPS and Affects Neurosecretion in Caenorhabditis elegans. J. Neurosci. 2004;24:3115–3124. doi: 10.1523/JNEUROSCI.0101-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I, de Gunzburg J, Goud B, Mornon JP. RUN domains: a new family of domains involved in Ras-like GTPase signaling. Trends Biochem. Sci. 2001;26:79–83. doi: 10.1016/s0968-0004(00)01730-8. [DOI] [PubMed] [Google Scholar]
- Cantor RM, Kono N, Duvall JA, Alvarez-Retuerto A, Stone JL, Alarcón M, Nelson SF, Geschwind DH. Replication of autism linkage: fine-mapping peak at 17q21. Am. J. Hum. Genet. 2005;76:1050–1056. doi: 10.1086/430278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlie NK, Schade MA, Thomure AM, Miller KG. Presynaptic UNC-31 (CAPS) is required to activate the G alpha(s) pathway of the Caenorhabditis elegans synaptic signaling network. Genetics. 2006;172:943–961. doi: 10.1534/genetics.105.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chun DK, McEwen JM, Burbea M, Kaplan JM. UNC-108/Rab2 regulates postendocytic trafficking in Caenorhabditis elegans. Mol. Biol. Cell. 2008;19:2682–2695. doi: 10.1091/mbc.E07-11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormont M, Mari M, Galmiche A, Hofman P, Le Marchand-Brustel Y. A FYVE-finger-containing protein, Rabip4, is a Rab4 effector involved in early endosomal traffic. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1637–1642. doi: 10.1073/pnas.031586998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MW, Hammarlund M, Harrach T, Hullett P, Olsen S, Jorgensen EM. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics. 2005;6:118. doi: 10.1186/1471-2164-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stasio EA, Dorman S. Optimization of ENU mutagenesis of Caenorhabditis elegans. Mutat. Res. 2001;495:81–88. doi: 10.1016/s1383-5718(01)00198-x. [DOI] [PubMed] [Google Scholar]
- Dikeakos JD, Reudelhuber TL. Sending proteins to dense core secretory granules: still a lot to sort out. J. Cell Biol. 2007;177:191–196. doi: 10.1083/jcb.200701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, Omichinski JG. Functional and structural characterization of a dense core secretory granule sorting domain from the PC1/3 protease. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7408–7413. doi: 10.1073/pnas.0809576106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittie AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J. Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Iwasaki K. Regulation of retrograde signaling at neuromuscular junctions by the novel C2 domain protein AEX-1. Neuron. 2002;33:249–259. doi: 10.1016/s0896-6273(01)00587-6. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Charlie NK, Richmond JE, Hegermann J, Eimer S, Miller KG. Impaired dense core vesicle maturation in Caenorhabditis elegans mutants lacking Rab2. J. Cell Biol. 2009;186:881–895. doi: 10.1083/jcb.200902095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouraux MA, Deneka M, Ivan V, van der Heijden A, Raymackers J, van Suylekom D, van Venrooij WJ, van der Sluijs P, Pruijn GJM. Rabip4’ is an effector of rab5 and rab4 and regulates transport through early endosomes. Mol. Biol. Cell. 2004;15:611–624. doi: 10.1091/mbc.E03-05-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kobayashi H, Ishibashi K, Ohbayashi N. Genome-wide investigation of the Rab binding activity of RUN domains: development of a novel tool that specifically traps GTP-Rab35. Cell Struct. Funct. 2011;36:155–170. doi: 10.1247/csf.11001. [DOI] [PubMed] [Google Scholar]
- Gaglia MM, Kenyon C. Stimulation of movement in a quiescent, hibernation-like form of Caenorhabditis elegans by dopamine signaling. J. Neurosci. 2009;29:7302–7314. doi: 10.1523/JNEUROSCI.3429-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud B, Zahraoui A, Tavitian A, Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990;345:553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Burdina AO, Touroutine D, Berthelot-Grosjean M, Parekh H, Richmond JE. Tomosyn negatively regulates CAPS-dependent peptide release at Caenorhabditis elegans synapses. J. Neurosci. 2007;27:10176–10184. doi: 10.1523/JNEUROSCI.2339-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev. Cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Hannemann M, Sasidharan N, Hegermann J, Kutscher LM, Koenig S, Eimer S. TBC-8, a putative RAB-2 GAP, regulates dense core vesicle maturation in Caenorhabditis elegans. PLoS Genet. 2012;8:e1002722. doi: 10.1371/journal.pgen.1002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol. Biol. Cell. 2009;20:209–217. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RJ, Liu Q, Watanabe S, Jorgensen EM. Complexin maintains vesicles in the primed state in C. elegans. Curr. Biol. 2011;21:106–113. doi: 10.1016/j.cub.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson SJ, Clynen E, Baggerman G, Janssen T, Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J. Neurochem. 2006;98:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- Janoueix-Lerosey I, Jollivet F, Camonis J, Marche PN, Goud B. Two-hybrid system screen with the small GTP-binding protein Rab6. Identification of a novel mouse GDP dissociation inhibitor isoform and two other potential partners of Rab6. J. Biol. Chem. 1995;270:14801–14808. doi: 10.1074/jbc.270.24.14801. [DOI] [PubMed] [Google Scholar]
- Janoueix-Lerosey I, Pasheva E, de Tand MF, Tavitian A, de Gunzburg J. Identification of a specific effector of the small GTP-binding protein Rap2. Eur. J. Biochem. 1998;252:290–298. doi: 10.1046/j.1432-1327.1998.2520290.x. [DOI] [PubMed] [Google Scholar]
- Jockusch WJ, Speidel D, Sigler A, Sørensen JB, Varoqueaux F, Rhee J-S, Brose N. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Kakhlon O, Sakya P, Larijani B, Watson R, Tooze SA. GGA function is required for maturation of neuroendocrine secretory granules. EMBO J. 2006;25:1590–1602. doi: 10.1038/sj.emboj.7601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J. Neurosci. 2001;21:9265–9272. doi: 10.1523/JNEUROSCI.21-23-09265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology (Bethesda) 2006;21:124–133. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J. Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukimoto-Niino M, Takagi T, Akasaka R, Murayama K, Uchikubo-Kamo T, Terada T, Inoue M, Watanabe S, Tanaka A, Hayashizaki Y, et al. Crystal structure of the RUN domain of the RAP2-interacting protein x. J. Biol. Chem. 2006;281:31843–31853. doi: 10.1074/jbc.M604960200. [DOI] [PubMed] [Google Scholar]
- Lan Z, Kurata WE, Martyn KD, Jin C, Lau AF. Novel rab GAP-like protein, CIP85, interacts with connexin43 and induces its degradation. Biochemistry. 2005;44:2385–2396. doi: 10.1021/bi048306w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinwand SG, Chalasani SH. Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat. Neurosci. 2013;16:1461–1467. doi: 10.1038/nn.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos S, Efeyan A, Monsech J, Dominguez O, Serrano M. A high-throughput loss-of-function screening identifies novel p53 regulators. Cell Cycle. 2006;5:1880–1885. doi: 10.4161/cc.5.16.3140. [DOI] [PubMed] [Google Scholar]
- Lundquist EA. Small GTPases. WormBook. 2006:1–18. doi: 10.1895/wormbook.1.67.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Waharte F, Boulet A, Cuif M-H, Tenza D, El Marjou A, Raposo G, Salamero J, Héliot L, Goud B, et al. Rab6-interacting protein 1 links Rab6 and Rab11 function. Traffic. 2007;8:1385–1403. doi: 10.1111/j.1600-0854.2007.00612.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Wada T, Suzuki T, Kubota Y, Inagaki N. Singar1, a novel RUN domain-containing protein, suppresses formation of surplus axons for neuronal polarity. J. Biol. Chem. 2007;282:19884–19893. doi: 10.1074/jbc.M700770200. [DOI] [PubMed] [Google Scholar]
- Munro S. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Loh YP. How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol. Endocrinol. 2008;22:2583–2595. doi: 10.1210/me.2008-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recacha R, Boulet A, Jollivet F, Monier S, Houdusse A, Goud B, Khan AR. Structural basis for recruitment of Rab6-interacting protein 1 to Golgi via a RUN domain. Structure. 2009;17:21–30. doi: 10.1016/j.str.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi A, Chen CC-H, Banerjee R, Glodowski D, Audhya A, Rongo C, Grant BD. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol. Biol. Cell. 2010;21:2930–2943. doi: 10.1091/mbc.E10-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B, Preisinger C, Körner R, Kopajtich R, Byron O, Barr FA. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J. Cell Biol. 2001;155:877–883. doi: 10.1083/jcb.200108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat. Neurosci. 2007;10:49–57. doi: 10.1038/nn1810. [DOI] [PubMed] [Google Scholar]
- Singh K, Chao MY, Somers GA, Komatsu H, Corkins ME, Larkins-Ford J, Tucey T, Dionne HM, Walsh MB, Beaumont EK, et al. C. elegans Notch Signaling Regulates Adult Chemosensory Response and Larval Molting Quiescence. Curr. Biol. 2011;21:825–834. doi: 10.1016/j.cub.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka R, Gillingham AK, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J. Cell Biol. 2008;183:607–615. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese S, Petrie M, Schuske K, Ailion M, Ann K, Iwasaki K, Jorgensen EM, Martin TFJ. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 2007;27:6150–6162. doi: 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Sumakovic M, Hegermann J, Luo L, Husson SJ, Schwarze K, Olendrowitz C, Schoofs L, Richmond J, Eimer S. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J. Cell Biol. 2009;186:897–914. doi: 10.1083/jcb.200902096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner WB. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J. Cell Biol. 1991;115:1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Martens GJ, Huttner WB. Secretory granule biogenesis: rafting to the SNARE. Trends Cell Biol. 2001;11:116–122. doi: 10.1016/s0962-8924(00)01907-3. [DOI] [PubMed] [Google Scholar]
- Urbé S, Page LJ, Tooze SA. Homotypic fusion of immature secretory granules during maturation in a cell-free assay. J. Cell Biol. 1998;143:1831–1844. doi: 10.1083/jcb.143.7.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk C, Sternberg PW. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat. Neurosci. 2007;10:1300–1307. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- Van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AAM, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Walent JH, Porter BW, Martin TF. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Punge A, Hollopeter G, Willig KI, Hobson RJ, Davis MW, Hell SW, Jorgensen EM. Protein localization in electron micrographs using fluorescence nanoscopy. Nat. Methods. 2011;8:80–84. doi: 10.1038/nmeth.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler F, Page L, Urbé S, Tooze SA. Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol. Biol. Cell. 2001;12:1699–1709. doi: 10.1091/mbc.12.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The sensory circuitry for sexual attraction in C. elegans males. Curr. Biol. 2007;17:1847–1857. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Yang J, Kim O, Wu J, Qiu Y. Interaction between tyrosine kinase Etk and a RUN domain- and FYVE domain-containing protein RUFY1. A possible role of ETK in regulation of vesicle trafficking. J. Biol. Chem. 2002;277:30219–30226. doi: 10.1074/jbc.M111933200. [DOI] [PubMed] [Google Scholar]
- You Y, Kim J, Raizen DM, Avery L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 2008;7:249–257. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I-M, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu. Rev. Cell Dev. Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K-M, Dong Y-M, Ge Q, Zhu D, Zhou W, Lin X-G, Liang T, Wu Z-X, Xu T. PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron. 2007;56:657–669. doi: 10.1016/j.neuron.2007.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.