Abstract
Background
The Amazon molly, Poecilia formosa (Teleostei: Poeciliinae) is an unisexual, all-female species. It evolved through the hybridisation of two closely related sexual species and exhibits clonal reproduction by sperm dependent parthenogenesis (or gynogenesis) where the sperm of a parental species is only used to activate embryogenesis of the apomictic, diploid eggs but does not contribute genetic material to the offspring.
Here we provide and describe the first de novo assembled transcriptome of the Amazon molly in comparison with its maternal ancestor, the Atlantic molly Poecilia mexicana. The transcriptome data were produced through sequencing of single end libraries (100 bp) with the Illumina sequencing technique.
Results
83,504,382 reads for the Amazon molly and 81,625,840 for the Atlantic molly were assembled into 127,283 and 78,961 contigs for the Amazon molly and the Atlantic molly, respectively. 63% resp. 57% of the contigs could be annotated with gene ontology terms after sequence similarity comparisons. Furthermore, we were able to identify genes normally involved in reproduction and especially in meiosis also in the transcriptome dataset of the apomictic reproducing Amazon molly.
Conclusions
We assembled and annotated the transcriptome of a non-model organism, the Amazon molly, without a reference genome (de novo). The obtained dataset is a fundamental resource for future research in functional and expression analysis. Also, the presence of 30 meiosis-specific genes within a species where no meiosis is known to take place is remarkable and raises new questions for future research.
Background
The evolution as well as the costs and benefits of sexual reproduction are central topics in evolutionary biology [1]. Despite theoretically well-defined costs of sexual reproduction, most prominently the twofold cost of producing males [2,3] that leads to a lower population growth rate for any sexual species, only about 0.1% of animal species have an asexual reproduction strategy [4]. Why this distribution prevails is an important puzzle in evolutionary biology. One consequence of asexual reproduction is the absence of full recombination. This probably impedes adaptation to changing environmental conditions and can lead in the long term to the accumulation of negative mutations, a phenomenon known as Muller’s Ratchet [5].
An especially interesting mode of reproduction is gynogenesis or sperm-dependent parthenogenesis [6,7]. Gynogenetic species are unisexual and therefore reproduce clonally, but they need sperm of males of closely related species to initiate embryogenesis. Usually, there is no contribution of the paternal genome to the next generation. Gynogenesis hence combines disadvantages of sexual and asexual reproduction.
Gynogenetic species typically originate through hybridisation of two sexually reproducing species [8] and can be thus considered as “frozen F1’s” [9], as no recombination occurs in subsequent generations after the hybridisation event.
An example for an asexual/sexual species complex is the gynogenetic Amazon molly, Poecilia formosa (Teleostei: Poeciliinae) and its parental species. The Amazon molly was the first unisexual vertebrate to be described [10]. It evolved presumably at least 120,000 years ago through a single hybridisation event [11] between a female Atlantic molly, P. mexicana[12], and a male sailfin molly, P. latipinna[13]. So far all experiments to artificially create Amazon molly like unisexuals through hybridisation of the parental species did not succeed [10,14,15], but always yield sexual F1 hybrids.
Amazon mollies occur in sympatry with at least one host species along the coastal versant of northern Mexico [16-18]. The Amazon molly produces diploid eggs without meiosis [19]. Embryogenesis is triggered by sperm of closely related males of three species, both parental species, P. latipinna and P. mexicana[10,17], and the Tamesí molly, P. latipunctata[20,21]. This pseudo-fertilization is internal and males of the sperm donor species have to copulate with the females of the Amazon molly for insemination, which has been described as a parasitic relationship of the Amazon molly with its sperm donor species [7]. Except in the rare case of paternal introgression where the complete [22] or parts of (microchromosomes [23]) the genetic material of the paternal species are passed on to the next generation, the reproduction of the Amazon molly is strictly clonal. There is no meiosis during the development of the gametes, such that new genetic variation only originates from mutations [19].
The Amazon molly is an excellent model for investigating the mechanisms of parthenogenetic, in particular gynogenetic reproduction, especially in comparison with its bisexual ancestors, as all these species are biologically very similar and mainly differ in their mode of reproduction (gynogenetic vs. bisexual) [7].
In the present study we compared the gonadal transcriptome of the Amazon molly, P. formosa and its maternal ancestor, the Atlantic molly, P. mexicana. Transcriptomic data were produced by Illumina sequencing and the obtained dataset will contribute significantly to unravel the evolution and mechanisms of the gynogenetic reproduction mode.
Furthermore we identified genes relevant for recombination and meiosis through comparative analysis. These genes will be functionally characterized in the future, in order to better understand mechanisms of gynogenesis and the genomic consequences of sexual vs. asexual reproduction.
Methods
Library construction and transcriptome sequencing
Samples were taken for each species (P. formosa and P. mexicana) from two laboratory born, fully mature females of same age and constitution (kept at the University of Potsdam). The founder fishes of the P. mexicana stock were collected in 1994 from the Laguna de Champaxan (Altamira, Tamaulipas, Mexico) and for P. formosa in 1993 from the Rio Purification (Barretal, Tamaulipas, Mexico), respectively. The fish were sacrificed on ice and the gonads were quickly excised, pooled into one sample for each species and frozen in liquid nitrogen. With regard to animal welfare, we followed internationally recognized guidelines and applicable national legislation. We received ethical approval from the deputy of animal welfare of the University of Potsdam.
The frozen tissues were moved to 600 μl RLT buffer (Qiagen), 6 μl β-mercaptoethanol (48.7%; Promega), and glass beads (0.75 - 1.0 mm, BioSpec Products) soaked in RNase away (Thermo Scientific). After the homogenization of the samples with a bead beater, the total RNA was extracted with the RNeasy® Fibrous Tissue Mini Kit (Qiagen) following the manufacturer’s protocol. Quality and concentration of the isolated RNA were measured with a NanoDrop (ND-1000, Thermo Scientific) spectrophotometer. A MINT-Universal cDNA synthesis kit (Protocol I, Evrogen) was used for construction of the cDNA and subsequently the cDNA was purified with a NucleoSpin® Gel and PCR Clean-up kit (Machery-Nagel) according to the manufacturer’s protocols.
Sequencing of both non-normalized cDNA libraries as single read libraries (100 bp) was performed on one lane of an Illumina HiSeq 2000 sequencing system by a commercial provider (LGC Genomics GmbH, Berlin). This company also provided initial processing of the raw sequencing data with the analysis pipeline Casava (v1.8, Illumina Inc.) and the software FastQC (v0.9.2, Babraham Bioinformatics). Through quality reporting with the control tool FastQC, most of the sequencing errors appearing in any Illumina sequencing run (especially at the 3′-end) could be removed from the dataset, including the removal of all reads containing an unknown base character (‘N’), the trimming of the reads at the 3′ end to obtain reads with an average Phred quality score of at least 20 over a ten base window and the removal of reads shorter than 35 bp after trimming.
De novo transcriptome assembly and detection of contamination
The assembly of the transcriptome was accomplished using the software packages Velvet (v1.2.03) [24] and Oases (v0.2.06) [25], which are suitable to assemble a transcriptome with short reads in absence of a reference genome (de novo). For the assembly of both transcriptomes, the following settings were used: minimum k = 61, maximum k = 69, k steps = 4.
To detect potential contamination, all contigs were compared with protein sequence databases from different taxa using the software BLAST (v2.2.26+) [26]. The protein sequences for archaea, bacteria, fungi, and invertebrates were downloaded (May 2012) from the universal protein knowledgebase UniProtKB (Swiss-Prot & TrEMBL) which contains manually annotated entries (UniProtKB/Swiss-Prot) and furthermore entries which are computationally annotated (UniProtKB/TrEMBL) [27]. The content of each database was reduced by removing redundant sequences with 95% identity using the software CD-Hit (v4.5.4) [28]. All contigs of both species were compared with the clustered databases via the blastx algorithm, which translates the query sequences in all six possible frames. Contigs that had a match were subsequently blasted against a protein database of the zebrafish, Danio rerio, downloaded from NCBI (July 2012) [29]. The cut-off for each taxon was defined according to jumps within the distribution of the E-values. Transcripts with hits above the taxon-specific E-value cut-off were identified as contamination and excluded from further analyses.
Transcriptome annotation and comparative analysis
A challenge in genomics and transcriptomics investigations is to make the large amount of data accessible for further - mainly bioinformatic – analysis. A mandatory step is to annotate the data, i.e., to provide information about the biological background of the sequences based on the nucleotide, protein, and process level [30]. Sequences were therefore assigned to gene ontology (GO) terms that describe the biological process, cellular components, and molecular functions associated with a given gene product. The annotation of the transcriptomes was done with the software package GOblet (standalone local installation, November 2012) [31,32]. GOblet utilizes sequence similarities to already annotated and characterized proteins of other species based on BLAST comparisons and annotates the sequences with terms from the Gene Ontology project [33]. The assembled transcripts of the Amazon molly and the Atlantic molly were compared via BLAST against UniProt/Swiss-Prot protein databases of vertebrates, rodents, mammals, human, and invertebrates (November 2012) using only records with GO-annotations which are not inferred from electronical annotation (IEA) and an E-value cut-off of 10−10 was chosen.
The occurrences of the 148 generic GO terms (http://www.geneontology.org/GO_slims/goslim_generic.obo, February 2013) within the annotated data were computed and for each of the GO terms a Fisher’s exact test (α = 0.05) with false discovery rate (FDR) correction of the p-value was carried out to detect GO terms, which are over- or underrepresented in one of the two transcriptomes.
Furthermore, both transcriptomes were compared with protein and cDNA datasets downloaded from NCBI (March 2012) of Danio rerio (zebrafish), Oryzias latipes (medaka), Oreochromis niloticus (nile tilapia), the NCBI Unigene entries of Gasterosteus aceleatus (three-spined stickleback), and the Uniprot/Swiss-Prot (March 2012) database. The sequence similarity comparisons against the protein databases were conducted with the blastx algorithm. The tblastx algorithm used for the cDNA databases translates the query and furthermore the database nucleotide sequences in all six possible frames. For the NCBI databases the E-value cut-off was 10−50 and for the UniProt/Swiss-Prot 10−5. The best hits for each contig were scanned for specific expressions within the meiosis and reproduction related GO terms and already described genes involved in meiosis [34-37] and thus detected genes were afterwards tested for the occurrence of open reading frames (ORFs) on the OrfPredictor server [38] as well as the complete transcript data for each species.
Additional to the mentioned sequence similarity comparisons with the different databases the assembled transcripts were also compared with the available datasets of the transcriptomes of Atlantic molly specimens from southern Mexico [unique loci in the assembled transcriptome, [39] and the guppy, Poecilia reticulata, a congeneric species [assembled 454 contigs, [40].
Results
Transcriptome sequencing
The sequencing of the cDNA libraries generated 117,702,546 reads for the Amazon molly and 114,683,463 for the Atlantic molly (Table 1). Through quality control and trimming, the raw dataset was reduced to 83,504,382 (70.95%) reads with 7,854,663,223 bp for P. formosa and 81,625,840 (71.17%) reads with 7,698,453,781 bp for P. mexicana and the average length of the reads was 94 bp for both species. The error probability that a base was incorrectly called in a sequence read is specified by the Phred quality. For the datasets of both species, the average Phred value was about 35, corresponding to an error probability of 0.00035.
Table 1.
Transcriptome sequencing results (100 bp, single end)
| Species | Poecilia formosa | Poecilia mexicana |
|---|---|---|
| Reads (n) |
117,702,546 |
114,683,463 |
| Filtered reads (n) |
89,267,205 |
87,221,251 |
| Trimmed reads (n) |
83,504,382 |
81,625,840 |
| Total bases (bp)T |
7,854,663,223 |
7,698,453,781 |
| Average read lengthT |
94 |
94 |
| Average Phred qualityT | 34.5 | 34.6 |
T: After trimming.
The processed reads for both species are available at the Sequence Read Archive (SRA, http://www.ncbi.nlm.nih.gov/sra) with the ID number PRJNA200586 for the Amazon molly and PRJNA200587 for the Atlantic molly.
Assembly and contamination load
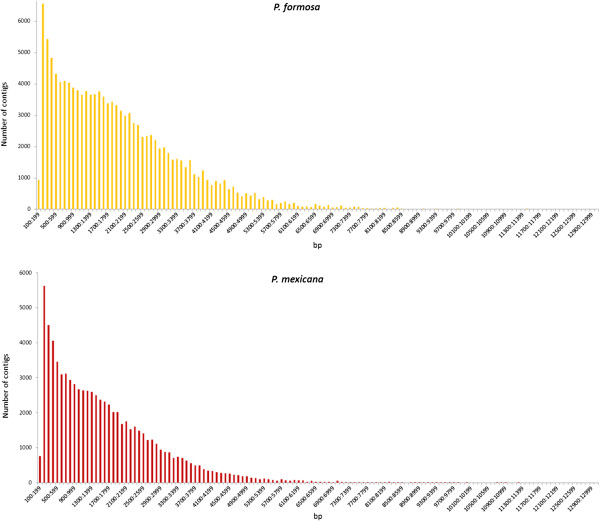
The de novo assembly with the Velvet/Oases software package yielded 127,283 contigs for the Amazon molly and 78,961 contigs for the Atlantic molly (Table 2) with an average length of 1,990 bp (range: 102 – 13,078 bp) and 1,638 bp (range: 100–11,242 bp), respectively. The N50 value reflects the quality of an assembly and is described by the weighted median of the contigs, which was 2,809 bp for P. formosa and 2,363 bp for P. mexicana. The length distribution of the assembled contigs for both species is shown in Figure 1.
Table 2.
Statistics for the de novo assembly
| Species | Poecilia formosa | Poecilia mexicana |
|---|---|---|
| Total bases (bp) |
253,322,491 |
129,352,105 |
| Total contigs* (n) |
125,991 |
78,223 |
| N50 (bp) |
2,809 |
2,363 |
| GC Content (%) | 46.63 | 45.60 |
*Without contigs presumably originating from contamination (see text for details).
Figure 1.
Length distribution of the contigs for the Amazon molly ( P. formosa ) and for the Atlantic molly ( P. mexicana ).
The sequence similarity comparisons with the UniProt/Swiss-Prot databases identified 1,291 contigs (1.02%) for the Amazon molly and 738 contigs (0.93%) for the Atlantic molly that were likely contaminations. These contigs were therefore removed from the transcriptome datasets.
Transcriptome annotation and identification of candidate genes
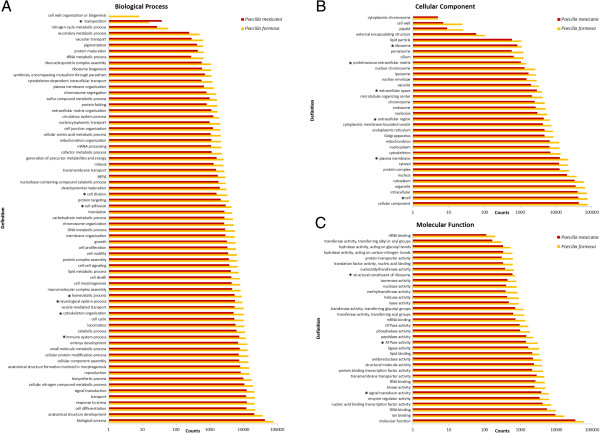
79,656 contigs (63.23%) for the Amazon molly and 44,785 (57.26%) for the Atlantic molly were assigned to a total of 12,328 different GO terms. 331 of these were unique to P. formosa (673 contigs) and 341 to P. mexicana (430 contigs). From the 148 generic GOslim terms 137 occurred within each transcriptome. The distribution of the GO term counts was very similar in both species, but 16 GO terms showed significant differences between the two species (Figure 2).
Figure 2.
Occurrence of the represented GO terms within the annotated transcriptomes of the Amazon molly (P. formosa) and the Atlantic molly (P. mexicana). The classification of the GO terms are shown for the main categories “Biological process” (A), “Cellular component” (B) and “Molecular function” (C). Significant differences between the two species are labelled with an asterisk.
In comparisons with genomic resources of different taxa, the lowest number of hits was obtained with the three-spined stickleback (Table 3). The BLAST searches against the cDNA or protein databases of the zebrafish gave similar results for the Amazon molly and the Atlantic molly. This also applies for the comparisons with the databases of the medaka and the nile tilapia. Also the comparison of our data with published transcriptomes of the genus Poecilia[39,40] gave approximately equivalent results for P. formosa and P. mexicana (Table 4). The number of unique matches for the UniProt/Swiss-Prot database was 14,537 (16.10%) for P. formosa and 13,749 (26.53%) for P. mexicana. Of these matches, 10,929 were shared across the two species, while 3,608 (3.99%) and 2,820 (5.44%) hits were unique for the Amazon molly and the Atlantic molly, respectively. The best hits for each contig of these BLAST searches were screened for genes involved in reproduction, recombination, and especially in meiosis. In total, 75 such genes could be detected within the transcriptomes of the Amazon molly and Atlantic molly (Table 5), corresponding to 940 contigs, i.e., 630 contigs (67.02%) for the Amazon molly and 310 contigs (32.98%) for the Atlantic molly. All these contigs have open reading frames. The subset of the 75 genes contained seven genes only found in P. formosa (Ago4, Cdk14, Cdk16, Rad1, Smarca2, Smarca4, Xrcc3), while six were only found in P. mexicana (C15orf60, Mcm4, Zmcm3, Msh5, Rad9B, Rmi2). Smarca2 and Smarca4 - both only found in the transcriptome of the Amazon molly, represented by 11 and 10 contigs, respectively - are members of the SWI/SNF protein family, which are involved in transcription regulation [41,42].
Table 3.
Summary of BLAST comparisons
| Taxa | Entries | BLAST algorithm | Poecilia formosa | Poecilia mexicana | |
|---|---|---|---|---|---|
|
Gasterosteus aculeatus |
cDNA |
16,728 |
tblastx |
45,098 (35.79%) |
24,653 (31.51%) |
|
Danio rerio |
cDNA |
28,037 |
tblastx |
72,993 (57.94%) |
39,005 (49.86%) |
| Protein |
27,014 |
blastx |
72,045 (57.18%) |
38,368 (49.05%) |
|
|
Oryzias latipes |
cDNA |
22,456 |
tblastx |
78,151 (62.03%) |
42,575 (54.43%) |
| Protein |
22,078 |
blastx |
76,539 (60.75%) |
41,422 (52.95%) |
|
|
Oreochromis niloticus |
cDNA |
23,339 |
tblastx |
78,231 (62.09%) |
42,999 (54.97%) |
| Protein |
23,162 |
blastx |
76,301 (60.56%) |
41,744 (53.34%) |
|
| Swiss-Prot | Protein | 534,695 | blastx | 90,288 (71.66%) | 51,821 (66.25%) |
Shown are for each taxon the number of the sequences of the cDNA and protein databases, the BLAST algorithm used, and the number of hits (percentage) for the Amazon molly (P. formosa) and the Atlantic molly (P. mexicana).
Table 4.
Comparison to published transcriptomes of the genus Poecilia
| Taxa | Entries | Poecilia formosa | Poecilia mexicana |
|---|---|---|---|
|
Poecilia mexicana[39] |
53,245 |
24,635 (46.27%) |
24,574 (46.15%) |
| Poecilia reticulata[40] | 54,987 | 11,843 (21.54%) | 12,039 (21.89%) |
Listed are the number of hits (percentage) of the available cDNA entries of the genus Poecilia compared through the tblastx algorithm (E-value cut-off was 10−50) with our Amazon molly (P. formosa) and Atlantic molly (P. mexicana) transcriptomes.
Table 5.
Identified genes, their UniProt accession IDs and the number of corresponding contigs for the Amazon molly (P. formosa; Pofo) and the Atlantic molly (P. mexicana; Pome)
|
Gene name |
Description |
UniProt accession ID |
Number of contigs |
|
|---|---|---|---|---|
| Pofo | Pome | |||
| Ago1 |
Argonaute 1, Eukaryotic translation initiation factor 2C 1 |
Q8CJG1 |
1 |
7 |
| Ago2 |
Argonaute 2, Eukaryotic translation initiation factor 2C 2 |
Q8CJG0 |
10 |
2 |
| Ago3 |
Argonaute 3, Eukaryotic translation initiation factor 2C 3 |
Q9H9G7 |
8 |
9 |
| Ago4 |
Argonaute 4, Eukaryotic translation initiation factor 2C 4 |
Q9HCK5 |
9 |
0 |
| C15orf60 |
Meiotic recombination protein REC114-like |
Q7Z4M0 |
0 |
2 |
| CcnA1 |
Cyclin-A1 |
Q92161 |
13 |
7 |
| CcnA2 |
Cyclin-A2 |
P30274 |
20 |
8 |
| CcnC |
Cyclin-C |
Q28F72 |
8 |
3 |
| Cdk1 |
Cell division protein kinase/Cyclin-dependent kinase 1 |
Q9DG98 |
3 |
3 |
| Cdk2 |
Cell division protein kinase/Cyclin-dependent kinase 2 |
P43450 |
1 |
1 |
| Cdk4 |
Cyclin-dependent kinase 4 |
Q91727 |
9 |
4 |
| Cdk7 |
Cyclin-dependent kinase 7 |
P51953 |
18 |
2 |
| Cdk10 |
Cell division protein kinase/Cyclin-dependent kinase 10 |
Q2TBL8 |
1 |
1 |
| Cdk14 |
Cell division protein kinase/Cyclin-dependent kinase 14 |
B0VXL7 |
3 |
0 |
| Cdk16 |
Cell division protein kinase/Cyclin-dependent kinase 16 |
Q00536 |
3 |
0 |
| Mcm2 |
DNA helicase MCM2, Minichromosome maintenance protein 2 |
Q6DIH3 |
10 |
1 |
| Mcm3 |
DNA helicase MCM3, Minichromosome maintenance protein 3 |
Q5ZMN2 |
1 |
3 |
| Mcm4 |
DNA helicase MCM4, Minichromosome maintenance protein 4 |
P33991 |
0 |
2 |
| Mcm4B |
DNA replication licensing factor MCM4-B, Minichromosome maintenance protein 4-B |
P30664 |
3 |
3 |
| Mcm5 |
DNA helicase MCM5, Minichromosome maintenance 5 |
Q561P5 |
18 |
2 |
| Mcm6 |
DNA helicase MCM6, Minichromosome maintenance 6 |
Q14566 |
3 |
4 |
| Mcm7 |
DNA helicase MCM7, Minichromosome maintenance 7 |
Q6NX31 |
18 |
3 |
| Mcm8 |
DNA helicase MCM8, Minichromosome maintenance 8 |
Q9UJA3 |
15 |
2 |
| Mcm9 |
DNA helicase MCM9, Minichromosome maintenance 9 |
Q6NRM6 |
19 |
1 |
| Zmcm3 |
Zygotic minichromosome maintenance protein 3 |
Q7ZXZ0 |
0 |
4 |
| Mei1* |
Meiosis inhibitor protein 1 |
Q5TIA1 |
11 |
11 |
| Mlh1* |
DNA mismatch repair protein Mlh1, MutL protein homolog 1 |
P40692 |
7 |
4 |
| Mlh3* |
DNA mismatch repair protein Mlh3, MutL protein homolog 3 |
Q9UHC1 |
6 |
1 |
| Mnd1* |
Meiotic nuclear division protein 1 homolog |
Q32L19 |
11 |
3 |
| Mns1* |
Meiosis-specific nuclear structural protein 1 |
Q6PBA8 |
1 |
2 |
| Mre11 |
Double-strand break repair protein MRE11 |
Q9W6K1 |
2 |
1 |
| Msh2* |
DNA mismatch repair protein Msh2, MutS protein homolog 2 |
Q5XXB5 |
10 |
6 |
| Msh3* |
DNA mismatch repair protein Msh3, MutS protein homolog 3 |
P20585 |
8 |
4 |
| Msh4* |
DNA mismatch repair protein Msh4, MutS protein homolog 4 |
O15457 |
7 |
4 |
| Msh5* |
DNA mismatch repair protein Msh5, MutS protein homolog 5 |
O43196 |
0 |
4 |
| Msh6* |
DNA mismatch repair protein Msh6, MutS protein homolog 6 |
P52701 |
26 |
6 |
| Piwil1 |
Piwi-like protein 1 |
Q8UVX0 |
13 |
6 |
| Piwil2 |
Piwi-like protein 2 |
A6P7L8 |
7 |
2 |
| Pms2 |
DNA mismatch repair protein (endonuclease) PMS2 |
P54278 |
12 |
1 |
| Psmc3ip* |
Homologous-pairing protein 2 homolog (HOP2) |
Q63ZL2 |
3 |
2 |
| Rad1 |
Cell cycle checkpoint protein RAD1 |
Q5R7X9 |
4 |
0 |
| Rad21* |
Double-strand-break repair protein rad21 homolog |
O60216 |
9 |
12 |
| Rad50 |
DNA repair protein RAD50 |
P70388 |
2 |
2 |
| Rad51B* |
DNA repair protein RAD51 homolog B |
Q91917 |
23 |
3 |
| Rad51C* |
DNA repair protein RAD51 homolog 3 |
O43502 |
7 |
1 |
| Rad51D* |
DNA repair protein RAD51 homolog 4/D |
O75771 |
7 |
5 |
| Rad52 |
DNA repair protein RAD52 homolog |
P39022 |
10 |
11 |
| Rad54A* |
DNA repair and recombination protein RAD54-like |
Q92698 |
18 |
6 |
| Rad54B* |
DNA repair and recombination protein RAD54B |
Q9DG67 |
9 |
1 |
| Rad9A |
Cell cycle checkpoint control protein RAD9A |
Q99638 |
8 |
3 |
| Rad9B |
Cell cycle checkpoint control protein RAD9B |
Q6WBX8 |
0 |
1 |
| Rec8* |
Meiotic recombination protein REC8 homolog |
O95072 |
3 |
2 |
| RecQl1 |
ATP-dependent DNA helicase Q1 |
Q9Z129 |
3 |
3 |
| RecQl4 |
ATP-dependent DNA helicase Q4 |
O94761 |
3 |
6 |
| RecQl5 |
ATP-dependent DNA helicase Q5 |
O94762 |
5 |
2 |
| Rmi1 |
RecQ-mediated genome instability protein 1 |
A4IF98 |
15 |
1 |
| Rmi2 |
RecQ-mediated genome instability protein 2 |
Q5ZM20 |
0 |
2 |
| Sfr1* |
Swi5-dependent recombination DNA repair protein 1 homolog, Meiosis protein 5 homolog |
B7ZD04 |
2 |
1 |
| Smarca2 |
SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 2 |
Q6DIC0 |
11 |
0 |
| Smarca4 |
SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 4 |
A7Z019 |
10 |
0 |
| Smc1a* |
Structural maintenance of chromosomes protein 1A |
Q9CU62 |
10 |
2 |
| Smc1b* |
Structural maintenance of chromosomes protein 1B |
Q8NDV3 |
2 |
2 |
| Smc2* |
Structural maintenance of chromosomes protein 2 |
P50533 |
9 |
2 |
| Smc3* |
Structural maintenance of chromosomes protein 3 |
Q9CW03 |
2 |
3 |
| Smc4* |
Structural maintenance of chromosomes protein 4 |
P50532 |
7 |
8 |
| Smc5* |
Structural maintenance of chromosomes protein 5 |
Q802R9 |
4 |
1 |
| Smc6* |
Structural maintenance of chromosomes protein 6 |
Q6P9I7 |
24 |
3 |
| Smchd1 |
Structural maintenance of chromosomes flexible hinge domain-containing protein 1 |
A6NHR9 |
25 |
37 |
| Spo11* |
Meiotic recombination protein |
Q9Y5K1 |
4 |
11 |
| Stag1* |
Cohesin subunit SA-1 |
Q8WVM7 |
3 |
13 |
| Stag2* |
Cohesin subunit SA-2 |
Q8N3U4 |
7 |
14 |
| Xrcc1 |
DNA repair protein XRCC1 |
Q60596 |
21 |
3 |
| Xrcc2* |
DNA repair protein XRCC2 |
Q9CX47 |
19 |
12 |
| Xrcc3* |
DNA repair protein XRCC3 |
Q08DH8 |
2 |
0 |
| Xrcc4 | DNA repair protein XRCC4 | Q924T3 | 24 | 6 |
Among the 75 genes, 31 genes could be identified which are supposedly specific to meiosis. One example is the meiosis-specific Spo11 gene, which encodes for the SPO11 protein, a type II topoisomerase-like enzyme that is essential for the formation of double-strand breaks (DSBs) between homologous chromosomes during meiosis [43,44]. Four contigs of the Amazon molly and eleven of the Atlantic molly matched to the Spo11 gene.
For each species, one unique meiosis-specific gene was detected. The Xrcc3 gene codes for a protein involved in DNA damage repair [45] and was only found in the transcriptome of the Amazon molly. The Msh5 gene, found only in the transcriptome of the Atlantic molly, is a eukaryotic homolog of the mutS genes and the protein MSH5 participates as heteroduplex together with the MSH4 protein in meiotic recombination [46,47].
Discussion
This study is the first description of the transcriptome of an unisexual vertebrate. Such organisms are models for studying the evolution and maintenance of sexual recombination. Most importantly, we use a comparative approach to study the gynogenetic Amazon molly, P. formosa relative to its maternal ancestor the Atlantic molly, P. mexicana. Our obtained datasets add to a small, yet growing, number of genomic data available for the paradigmatic subfamily Poeciliinae (phylogeny according to [48]) and provides relevant information for different future research topics.
About 115 million reads for each sample were obtained by Illumina sequencing of two unnormalized cDNA libraries on one lane. After trimming and quality control, ~70% (15.5 Gb) of the sequence reads were used for assembly and annotation.
A transcriptome of P. mexicana[39] and of the guppy, P. reticulata[40], have already been described, but neither the genome of the Amazon molly nor the genomes of the two ancestor species are currently available. So far, only the genome of another member of the subfamily Poeciliinae, the platyfish (Xiphophorus maculatus) has been studied extensively [49]. Consequently, the assembly of the reads was conducted without a reference genome (de novo), and therefore the sequencing coverage had to be higher than 30x [50]. In our study, the coverage was 52.11x for the Amazon molly and 57.89x for the Atlantic molly.
The unequal number of contigs for the Amazon molly and the Atlantic molly can be ascribed to a higher number of transcripts predicted for the Amazon molly during assembly, leading to a different number of base pairs that were utilized for assembly by the assembly software (~250 million bp for the Amazon molly and ~130 million bp for the Atlantic molly). This difference can be explained by the fact that the Amazon molly is a hybrid species and therefore has two different alleles at any locus, i.e., one from the maternal ancestor, P. mexicana and one of the paternal ancestor, P. latipinna. In all other aspects, i.e., coverage, average read length of the assembled contigs, and theN50 value, both species were quite similar. In addition, the contamination load of about 1% for each contig set was very low. Most of the contigs presumably originating from contamination were assigned to invertebrates, but some contaminations could be assigned to fungi and bacteria. Such contamination can occur at different steps of the library preparation or the sequencing.
The results of the BLAST searches against the different fish species showed similar results for both species. The percentage of database matches did not correlate with phylogenetic relationship to the mollies, but rather with the completeness of the genomic resources. The smaller number of matches with the three-spined stickleback is due to the fact that this database is smaller and presumably less complete than those of the other fish model species. The medaka, O. latipes, is the more closely related species to both molly species [51], but most matches were found with the more distantly related zebrafish, D. rerio, which is a well established model organism and thus has a well investigated and annotated genome and the highest number of entries in the database. The comparisons among transcriptomes within the genus Poecilia reveal a congruence between number of shared transcripts and phylogenetic relationship: As expected, the number of hits of our new assembled transcriptomes was higher to the unique loci of P. mexicana[39] than to those of P. reticulata[40]. The high concordance of both transcriptomes with the well-established UniProt/Swiss-Prot database was used for the identification of candidate genes. 75 genes associated with reproduction and meiosis were detected within the contig sets for both species. 31 genes of these are specific for meiosis, like the Spo11 genes or the Msh genes. Nonetheless, several genes that also play a central role during meiosis are not listed in our table of the candidate genes. One example is the Dmc1 gene, which is a member of the SMC protein family. The DMC1 protein is required for meiotic recombination, especially for the homologous pairing of chromosomes during meiosis [52]. In fact, we detected this gene within the transcriptomes of both species, but with a too high E-Value to consider the assignment reliable.
The identification of meiosis-specific genes within the transcriptome of the Amazon molly raises questions concerning the function of these genes and their encoded proteins, as the unisexual Amazon molly reproduces via gynogenesis, and the diploid eggs are produced by apomixis i.e., without meiotic reduction [19]. The meiosis-specific genes have thus lost their putative prime purpose. Their function – if any - in the gonads of an apomictic species remains enigmatic. It would be interesting to evaluate the abundance of the respective proteins, both in gonads and other tissue. This could provide some hint as to whether these genes and proteins maintain their original function or shifted to other functions. A first step would be to examine the tissue-specific expression patterns of these genes across various types of tissue via real-time polymerase chain reaction.
The differential occurrence of some meiosis-specific genes between the Amazon molly and the Atlantic molly as well as the differences in contig numbers can - as above mentioned – be interpreted by the hybrid nature of the Amazon molly. To corroborate this hypothesis, we will compare the transcriptomes of all three species, i.e., the Amazon molly and both parental species.
Conclusions
Here we describe the first transcriptome analysis of the all-female and hybrid species P. formosa, the Amazon molly, and the first transcriptome of any unisexual vertebrate of hybrid origin. The transcriptome was assembled and annotated without a reference genome, using short single end reads obtained through Illumina sequencing. Through comparisons with the transcriptome of the maternal ancestor P. mexicana and BLAST searches against other fish species and the UniProt database, a significant number of candidate genes relevant for reproduction and especially meiosis could be identified. The obtained dataset, and especially the identified meiosis-specific genes can act as starting point for further studies like gene-/tissue – specific expressions analysis. The transcriptomic data of the second ancestor species, P. latipinna, and a well annotated genome of the Amazon molly – when becoming available – will allow for a more comprehensive understanding of the genetic architecture of the Amazon molly.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IMS conducted the lab work, the bioinformatic analysis and drafted the manuscript. SH and DG contributed to the bioinformatics. IS and RT conceived the study, supervised the work, and contributed to the manuscript. All authors read and approved the manuscript.
Contributor Information
Ina Maria Schedina, Email: schedina@uni-potsdam.de.
Stefanie Hartmann, Email: stefanie.hartmann@uni-potsdam.de.
Detlef Groth, Email: dgroth@uni-potsdam.de.
Ingo Schlupp, Email: schlupp@ou.edu.
Ralph Tiedemann, Email: tiedeman@uni-potsdam.de.
Acknowledgements
We thank Francesco Lamanna for support with the bioinformatics analysis. Funding was provided by the University of Potsdam.
References
- Otto SP, Lenormand T. Resolving the paradox of sex and recombination. Nat Rev Genet. 2002;3:252–261. doi: 10.1038/nrg761. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. The Evolution of Sex. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Lehtonen J, Jennions MD, Kokko H. The many costs of sex. Trends Ecol Evol. 2012;27:172–178. doi: 10.1016/j.tree.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Vrijenhoek RC. Animal clones and diversity. Bioscience. 1998;48:617–628. doi: 10.2307/1313421. [DOI] [Google Scholar]
- Muller HJ. The relation of recombination to mutational advance. Mutat Res. 1964;1:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Beukeboom LW, Vrijenhoek RC. Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J Evol Biol. 1998;11:755–782. doi: 10.1007/s000360050117. [DOI] [Google Scholar]
- Schlupp I. The evolutionary ecology of gynogenesis. Annu Rev Ecol Evol Syst. 2005;36:399–417. doi: 10.1146/annurev.ecolsys.36.102003.152629. [DOI] [Google Scholar]
- Simon J-C, Delmotte F, Rispe C, Crease T. Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol J Linn Soc. 2003;79:151–163. doi: 10.1046/j.1095-8312.2003.00175.x. [DOI] [Google Scholar]
- Vrijenhoek RC. Factors affecting clonal diversity and coexistence. Am Zool. 1979;19:787–797. [Google Scholar]
- Hubbs CL, Hubbs LC. Apparent parthenogenesis in nature, in a form of fish of hybrid origin. Science. 1932;76:628–630. doi: 10.1126/science.76.1983.628. [DOI] [PubMed] [Google Scholar]
- Stöck M, Lampert K, Möller D, Schlupp I, Schartl M. Monophyletic origin of multiple clonal lineages in an asexual fish (Poecilia formosa) Mol Ecol. 2010;19:5204–5215. doi: 10.1111/j.1365-294X.2010.04869.x. [DOI] [PubMed] [Google Scholar]
- Avise JC, Trexler JC, Travis J, Nelson WS. Poecilia mexicana is the recent female parent of the unisexual fish Poecilia formosa. Evolution. 1991;45:1530–1533. doi: 10.2307/2409901. [DOI] [PubMed] [Google Scholar]
- Schartl M, Wilde B, Schlupp I, Parzefall J. Evolutionary origin of a parthenoform, the Amazon molly Poecilia formosa, on the basis of a molecular genealogy. Evolution. 1995;49:827–835. doi: 10.2307/2410406. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Brett BLH, Miller RR. Interspecific hybridization and the evolutionary origin of a gynogenetic fish, Poecilia formosa. Evolution. 1980;34:917–922. doi: 10.2307/2407997. [DOI] [PubMed] [Google Scholar]
- Lampert KP, Lamatsch DK, Fischer P, Epplen JT, Nanda I, Schmid M, Schartl M. Automictic reproduction in interspecific hybrids of poeciliid fish. Curr Biol. 2007;17:1948–1953. doi: 10.1016/j.cub.2007.09.064. [DOI] [PubMed] [Google Scholar]
- Gabor CR, Ryan MJ. Geographical variation in reproductive character displacement in mate choice by male sailfin mollies. Proc Biol Sci. 2001;268:1063–1070. doi: 10.1098/rspb.2001.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlupp I, Parzefall J, Schartl M. Biogeography of the Amazon molly, Poecilia formosa. J Biogeogr. 2002;29:1–6. doi: 10.1046/j.1365-2699.2002.00651.x. [DOI] [Google Scholar]
- Costa GC, Schlupp I. Biogeography of the Amazon molly: ecological niche and range limits of an asexual hybrid species. Global Ecol Biogeogr. 2010;19:442–451. [Google Scholar]
- Rasch EM, Monaco PJ, Balsano JS. Cytophotometric and autoradiographic evidence for functional apomixis in a gynogenetic fish, Poecilia formosa and its related, triploid unisexuals. Histochemistry. 1982;73:515–533. doi: 10.1007/BF00493366. [DOI] [PubMed] [Google Scholar]
- Niemeitz A, Kreutzfeldt R, Schartl M, Parzefall J, Schlupp I. Male mating behaviour of a molly, Poecilia latipunctata: a third host for the sperm-dependent Amazon molly, Poecilia formosa. Acta Ethologica. 2002;5:45–49. doi: 10.1007/s10211-002-0065-2. [DOI] [Google Scholar]
- Ptacek MB, Childress MJ, Kittell MM. Characterizing the mating behaviours of the Tamesí molly, Poecilia latipunctata, a sailfin with shortfin morphology. Anim Behav. 2005;70:1339–1348. doi: 10.1016/j.anbehav.2005.03.019. [DOI] [Google Scholar]
- Lamatsch DK, Nanda I, Epplen JT, Schmid M, Schartl M. Unusual triploid males in a microchromosome-carrying clone of the Amazon molly, Poecilia formosa. Cytogenet Cell Genet. 2000;91:148–156. doi: 10.1159/000056836. [DOI] [PubMed] [Google Scholar]
- Schartl M, Nanda I, Schlupp I, Wilde B, Epplen JT, Schmid M, Parzefall J. Incorporation of subgenomic amounts of DNA as compensation for mutational load in a gynogenetic fish. Nature. 1995;373:68–71. doi: 10.1038/373068a0. [DOI] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28:1086–1092. doi: 10.1093/bioinformatics/bts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- The UniProt Consortium. Reorganizing the protein space at the Universal Protein Resource (UniProt) Nucl Acids Res. 2012;40:D71–D75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Tao T, Madden T, Camacho C, Szilagyiet L. BLAST® Help [Internet] Bethesda (MD): National Center for Biotechnology Information (US); 2008. BLAST FTP Site. 2011 May 29. http://www.ncbi.nlm.nih.gov/books/NBK62345/ [Google Scholar]
- Stein L. Genome annotation: from sequence to biology. Nat Rev Genet. 2001;2:493–503. doi: 10.1038/35080529. [DOI] [PubMed] [Google Scholar]
- Hennig S, Groth D, Lehrach H. Automated gene ontology annotation for anonymous sequence data. Nucleic Acids Res. 2003;31:3712–3715. doi: 10.1093/nar/gkg582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth D, Hartmann S, Panopoulou G, Poustka AJ, Hennig S. GOblet: annotation of anonymous sequence data with gene ontology and pathway terms. J Integr Bioinform. 2008;5:104. doi: 10.2390/biecoll-jib-2008-104. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium. Gene Ontology: tool for the unification of biology. Nat Genet. 2005;25:25–19. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh MA, Malik SB, Logsdon JM Jr. A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr Biol. 2005;15:185–191. doi: 10.1016/j.cub.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Malik SB, Pightling AW, Stefaniak LM, Schurko AM, Logsdon JM Jr. An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis. PLoS One. 2007;3:e2879. doi: 10.1371/journal.pone.0002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurko AM, Logsdon JM Jr, Eads BD. Meiosis genes in Daphnia pulex and the role of parthenogenesis in genome evolution. BMC Evol Biol. 2009;9:78. doi: 10.1186/1471-2148-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby BJ, Reinholdt LG, Schimenti JC. Positional cloning and characterization of Mei1, a vertebrate-specific gene required for normal meiotic chromosome synapsis in mice. Proc Natl Acad Sci U S A. 2003;100:15706–15711. doi: 10.1073/pnas.2432067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min XJ, Butler G, Storms R, Tsang A. OrfPredictor: predicting protein-coding regions in EST-derived sequences. Nucleic Acids Res. 2005;33:W677–W680. doi: 10.1093/nar/gki394. Web Server: http://proteomics.ysu.edu/tools/OrfPredictor.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JL, Passow CN, Plath M, Arias Rodriguez L, Yee MC, Tobler M. Genomic resources for a model in adaptation and speciation research: characterization of the Poecilia mexicana transcriptome. BMC Genomics. 2012;13:652. doi: 10.1186/1471-2164-13-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser BA, Weadick CJ, Janowitz I, Rodd FH, Hughes KA. Sequencing and characterization of the guppy (Poecilia reticulata) transcriptome. BMC Genomics. 2011;12:202. doi: 10.1186/1471-2164-12-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H, Muramatsu M, Nomoto A, Kato H. Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoic acid receptor. Nucl Acids Res. 1994;22:1815–1820. doi: 10.1093/nar/22.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsanam P, Winston F. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 2000;16:345–351. doi: 10.1016/S0168-9525(00)02060-6. [DOI] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/S0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- Celerin M, Merino ST, Stone JE, Menzie AM, Zolan ME. Multiple roles of Spo11 in meiotic chromosome behavior. EMBO J. 2000;19:2739–2750. doi: 10.1093/emboj/19.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbs RS, Zhao Y, Tucker JD, Scheerer JB, Siciliano MJ, Hwang M, Liu N, Legerski RJ, Thompson LH. Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene. Proc Natl Acad Sci U S A. 1995;92:6354–6358. doi: 10.1073/pnas.92.14.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan KM, Meyer-Gauen G, Lyons-Weiler J, Hays JB. Evolutionary origin, diversification and specialization of eukaryotic MutS homolog mismatch repair proteins. Nucl Acids Res. 2000;28:463–471. doi: 10.1093/nar/28.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden T, Acharya S, Butz C, Berardini M, Fishel R. hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell. 2004;15:437–451. doi: 10.1016/j.molcel.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Hrbek T, Seckinger J, Meyer A. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol Phylogenet Evol. 2007;43:986–998. doi: 10.1016/j.ympev.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Schartl M, Walter RB, Shen Y, Garcia T, Catchen J, Amores A, Braasch I, Chalopin D, Volff JN, Lesch KP, Bisazza A, Minx P, Hillier L, Wilson RK, Fuerstenberg S, Boore J, Searle S, Postlethwait JH, Warren WC. The genome of the platyfish, Xiphophorus maculatus, provides insights into evolutionary adaptation and several complex traits. Nat Genet. 2013;45:567–572. doi: 10.1038/ng.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Bruno VM, Fang Z, Meng X, Blow M, Zhang T, Sherlock G, Snyder M, Wanget Z. Rnnotator: an automated de novo transcriptome assembly pipeline from stranded RNA-seq reads. BMC Genomics. 2010;11:663. doi: 10.1186/1471-2164-11-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiamarga DH, Miya M, Yamanoue Y, Mabuchi K, Satoh TP, Inoue JG, Nishida M. Interrelationships of Atherinomorpha (medakas, flyingfishes, killifishes, silversides, and their relatives): The first evidence based on whole mitogenome sequences. Mol Phylogenet Evol. 2008;49:598–605. doi: 10.1016/j.ympev.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Bishop D, Park D, Xu L, Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;60:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]