SYNOPSIS
Brain 18F-FDG PET allows the in vivo study of cerebral glucose metabolism, reflecting neuronal and synaptic activity.
18F-FDG PET has been extensively used to detect metabolic alterations in several neurological diseases vs. normal aging. However, healthy subjects exhibit variants of 18F-FDG distribution, especially as associated with aging. 18F-FDG uptake is usually homogeneous and symmetrical, but areas of slightly higher activity are observed in basal ganglia, frontal eye fields, posterior cingulate cortex and visual cortex. On the contrary, relatively lower metabolic activity is observed in medial temporal cortex.
This review will focus on 18F-FDG PET findings in so-called normal brain aging, and in particular on metabolic differences occurring with aging and as a function of people’s gender. The effect of different substances, medications and therapy procedures will be discussed, as well as common artifacts.
Keywords: 18F-FDG PET, normal brain, normal variants, aging brain
INTRODUCTION
Glucose is the main metabolic substrate of the brain and its oxidation produces the amount of energy that is necessary for an adequate cerebral activity.
The positron emission tomography (PET) tracer 18F-fluorodeoxyglucose (18F-FDG) allows the in vivo study of glucose metabolism, and since its introduction in 1976, it is the most widely used PET tracer in both clinical and research settings 1.
The local glucose consumption, and thus 18F-FDG cerebral uptake, correlates strictly with local neuronal activity, and proportionally increases with stimulus intensity or frequency 2 or decreases in conditions of sensory deprivation 3. Such metabolic variations take place at the level of synaptic connections 4. As such, neurotransmission and signal transduction are the processes with the highest energetic requirements. It has been estimated that the energetic demand of neurotransmission and related events exceeds 80% of total cerebral energetic consumption 5.
Connections between neurons are carried out mainly by excitatory glutametergic synapses, which account for the great majority of all cortical synapses, yielding an energetic consumption of around 80% of total cortical consumption 5.
A large body of literature demonstrated that 18F-FDG PET adds value to diagnostic evaluation of several neurological diseases. In particular, 18F-FDG PET substantially improves diagnostic accuracy and differential diagnosis, and enables earlier and better treatment planning of neurodegenerative disorders6,7.
While the majority of studies have focused on detection of abnormal, disease-specific 18F-FDG distribution patterns, relatively little is known about brain glucose metabolism in clinically and cognitively normal, healthy individuals, and about “normal variants” of FDG uptake in this population.
Thorough knowledge of the normal variants of brain function, occurring in healthy aging or related to gender, is critical for detection of abnormal findings and for investigating neurological diseases.
This review will focus on 18F-FDG PET findings in so-called normal brain aging, and in particular on metabolic differences occurring with aging and as a function of people’s gender. The effect of different substances, medications and therapy procedures will be discussed, as well as common artifacts.
IMAGING TECHNIQUE
Updated procedure guidelines for 18F-FDG PET brain imaging have been published by the Society of Nuclear Medicine in 2009 8 and include: all relevant information to be collected during the procedure, instructions for patient’s management and preparation for PET scanning, and a summary of the standardized acquisition protocol. These are summarized in Box 1, 2 and 3, respectively.
Box 1. Relevant patient history and data.
| Focused history: head trauma, known neurological or psychiatric disorders, brain tumors, prior brain operations |
| Clinical history: patient complaints, neurological/psychiatric examination, mental status exam (mini-mental state examination, neuropsychological tests), cognitive impairment |
| Recent morphologic imaging studies (e.g. computed tomography, magnetic resonance imaging or prior PET or single photon computed tomography brain studies) |
| Current medications |
Box 2. Patient preparation before PET scanning.
| Fasting for at least 4–6 hours |
| Oral hydration with water should be encouraged |
| Avoid caffeine, alcohol, or drugs that may affect cerebral glucose metabolism |
| Check blood glucose level (ideally not greater than 150–200 mg/dL) |
| Environmental conditions: “resting state”, patient with eyes open and ears unoccluded in a quiet, dimly-lit room, with minimal background noise |
| Start intravenous line for 18F-FDG administration (at least 10 minutes prior to tracer injection) |
Box 3. Acquisition protocol.
| Administered dose | 185–370 MBq |
| Acquisition starting time | 30 minutes post-injection 60 minutes post-injection (oncology) |
| During acquisition | Well-tolerated head immobilization procedures should be implemented, to minimize head movements |
| Acquisition duration | 20 minutes (depending on PET equipment and patient compliance) |
| Reconstruction | Transaxial matrix size: 128 × 128 or 256 × 256 Typical pixel size: 2–4 mm |
18F-FDG PET images should be reconstructed in transaxial, coronal and sagittal planes.
For a comprehensive evaluation of brain 18F-FDG PET images, all three projection planes should be used. The transaxial plane is recommended for evaluation of all cortical and subcortical structures. Transaxial images should be reoriented both along anterior commissure-posterior commissure (AC-PC) line and temporal long axis (for better assessment of temporal lobe) (Figure 1). The coronal plane is recommended for inspection of posterior cingulate, angular gyri, and medial temporal lobes (including hippocampal areas). The sagittal plane is valuable in evaluations of the frontal and temporal poles.
Figure 1.
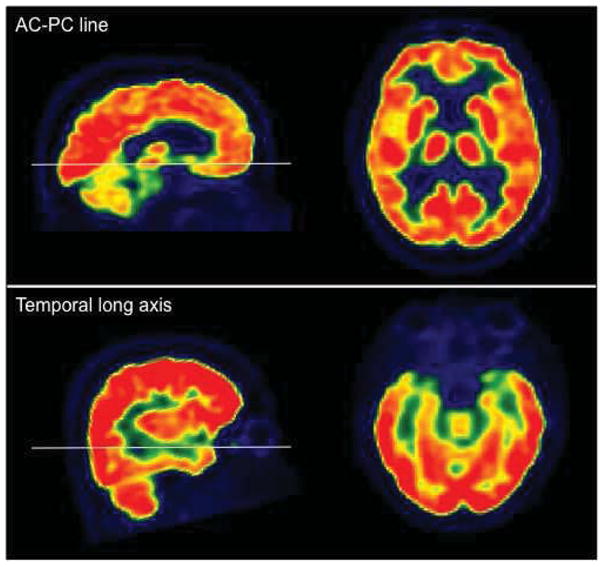
18F-FDG PET images oriented along AC-PC line and temporal long axis.
NORMAL ANATOMY
Glucose is the only source of energy of the brain, which is known to account for as much as 20% of total-body glucose metabolism in the fasting state. For these reasons, brain exhibits an intense 18F-FDG uptake, with higher activity in the grey matter as compared to the white matter.
In resting conditions, cerebral metabolic rate values for glucose are approximately 15 micromol/min/100g for the white matter, and 40–60 micromol/min/100g for the grey matter. In the normal brain, the grey to white matter activity ratio is 2.5 to 4.1.
Differences of 18F-FDG distribution have been observed among different brain regions. 18F-FDG uptake is usually higher in the frontal, parietal and occipital areas than in temporal cortex, and the basal ganglia have slightly higher activity than the cortex 9. Moreover, focal areas of increased uptake were observed in frontal eye fields, posterior cingulate cortex and visual cortex of normal subjects. Metabolic activity is lower in medial temporal cortex, including hippocampal areas, than in neocortical regions (Box 4 and Figure 2)9,10.
Box 4. Normal pattern of uptake.
Areas of higher uptake
|
Areas of lower metabolic activity
|
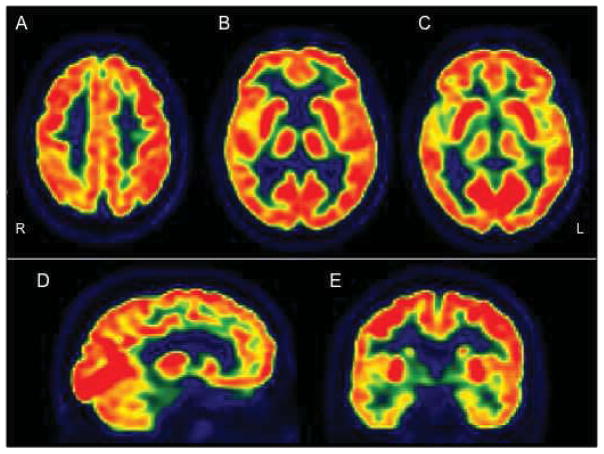
Figure 2.
18F-FDG PET of a normal subject. A, B, C: transaxial plane, showing higher 18F-FDG uptake in basal ganglia, frontal eye fields, posterior cingulate and visual cortices. D: sagittal plane. E: coronal plane, showing lower 18F-FDG activity in temporal lobes, particularly in medial temporal cortex, as compared to the other regions.
Brain 18F-FDG uptake is usually homogeneous and symmetrical. Slight asymmetries in 18F-FDG uptake have been observed in the Wernicke area, the frontal eye fields and the angular gyrus, with a prevalence of generally less than 10%. 11.
IMAGING FINDINGS
Several 18F-FDG PET studies investigated the normal variants of brain metabolic activity. Subtle differences in cerebral metabolic activity have been observed among cognitively normal, healthy individuals, mostly as related to the effects of scanning time, age and gender, but also to the effect of medications and therapy procedures.
Scanning time
Regional 18F-FDG uptake differences were observed in the normal human brain depending on scanning starting time after intravenous tracer injection.
Some studies compared 18F-FDG images obtained at 60 minutes post injection (p.i.) to earlier time frames (30 minutes p.i.) in the same individuals, and showed relatively higher 18F-FDG uptake in bilateral posterior cingulate gyrus, parietal and frontal association cortices, and subcallosal cortices, and relatively lower uptake in cerebellum and orbito-frontal areas in 60 minutes p.i. scans as compared to earlier images) 12. While the cause of such effects remains unclear, this might be due to regional differences in 18F-FDG transportation from plasma to tissue, or in glucose phosphorylation or dephosphorylation kinetics in different regions over time 13.
Age
Since its early phases of development, the human brain experiences several structural and functional changes.
According to the few 18F-FDG PET studies on brain maturation in children, global cerebral metabolic rate of glucose is low in the newborn period (13–25 micromol/min/100g), and rapidly rises to adult normal values (19–33 micromol/min/100g) at 2 years of age 14. Global cerebral metabolic activity continues to rise to values of 49–65 micromol/min/100g by age 3–4 years, and remains stable until approximately 9 years, when it begins to decline, reaching characteristic adult values by the end of the second decade of life 14. This time-course of global cerebral metabolic rate of glucose during childhood has been matched to the process of initial overproduction and subsequent elimination of excessive neurons and synapses occurring in the developing brain.
In addition to changes in global cerebral metabolic activity, normal brain development is associated to changes in metabolic patterns. In neonates, local cerebral glucose metabolism is highest in primitive areas, such as brainstem, cerebellar vermis, thalamus and primary sensorimotor cortex14,15. Glucose metabolism in parietal, temporal (including mesial temporal region), occipital cortices and basal ganglia increases at 3–5 months of age14,15.
Finally, the frontal cortex becomes metabolically active between 6 and 8 months 14,15.
While the window into brain metabolic changes in childhood is mainly derived from a few studies, more numerous 18F-FDG PET studies examined the effect of age on normal adult cerebral metabolic rate of glucose.
During aging, the brain undergoes both structural and functional changes, whose regional distribution mirrors the reported cognitive decline usually observed in the normal elderly population16.
Enlargement of the ventricles and cortical sulci, due to brain atrophic alterations, is one of the major changes that may occur in middle aged to old healthy individuals. This age-related enlargement of cerebrospinal fluid spaces is reflected in brain metabolic reduction in the vicinity of ventricles, in frontobasal and perisylvian structures on 18F-FDG PET 17. Since these structures are mostly surrounded by white matter, their age-related hypometabolism is most likely due to white matter atrophy occurring in healthy aging.
Besides white matter changes, healthy aging is associated with grey matter alterations.
Global cerebral metabolic rates of glucose inversely correlate with age, with a reported decline of 12–13% between ages 20 and >70 years18–20.
In addition to the global decrease in brain metabolism, a number of studies have reported specific patterns of age-related regional metabolic declines.
The most common metabolic reductions with advancing age have been observed in the frontal lobes (Figure 3). In particular, the reduction of 18F-FDG uptake involves anterior cingulate cortex, dorsolateral and medial prefrontal cortices, and orbitofrontal cortex, bilaterally 17,19–28. The metabolic reduction in medial prefrontal cortices is correlated with age-associated cognitive decline in healthy subjects28.
Figure 3.
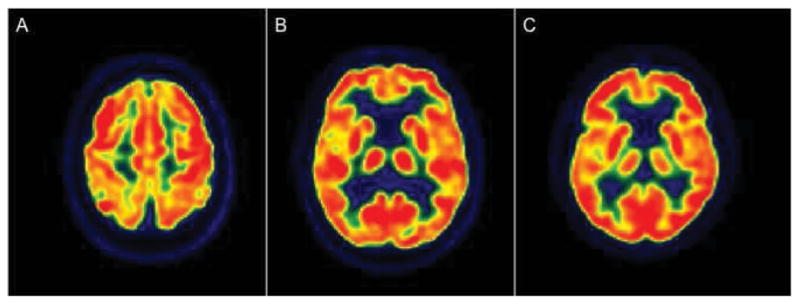
Age-related metabolic reductions in 3 cognitively normal subjects. A: 80 year-old subject, demonstrating prefrontal and superior parietal hypometabolism with sparing of primary sensory-motor cortices. B: 76 year-old subject, showing mild hypometabolism in anterior cingulate cortex, medial frontal regions and insula. C: 82 year-old subject, demonstrating hypometabolism involving anterior cingulate cortex, medial frontal regions and insula.
Age-related metabolic decreases have also been observed in neocortical regions other than frontal cortex, such as the insula20,24, the temporal lobes (particularly the temporal pole and lateral temporal cortex)19,22,24 and the parietal lobes (including supramarginal, superior and inferior parietal cortices)19,20,24.
In contrast, several cortical and subcortical areas have been reported to be relatively unaffected during aging, including primary motor cortices, occipital cortices (particularly visual areas and posterior cingulate cortex), precuneus, mesial temporal lobes (hippocampus, amygdala and parahippocampal gyrus), thalamus, putamen, pallidum and cerebellum17,19,20,24,27,29. Age-related metabolic changes in healthy individuals are summarized in Box 5.
Box 5. Age-related metabolic changes in healthy individuals.
|
Age-related hypometabolism
|
Frontal lobes
|
| Insula |
Temporal lobes
|
Parietal lobes
|
|
Least altered regions during aging
|
| Primary motor cortices |
Occipital cortices
|
| Precuneus |
Mesial temporal lobes
|
| Thalamus Putamen, pallidum Cerebellum |
Brain metabolic changes observed in normal aging support the developmental theory, according to which age-related brain changes follow the phylogenetic and ontogenetic axes30. The topographic pattern of metabolic decline in normal aging matches in a reverse sense the metabolic functional changes observed in developing human brain. The first structures to neurally develop are the same areas that are spared from metabolic deterioration occurring with age (i.e., brainstem, thalamus, cerebellum, sensorimotor cortex, hippocampus), whereas the frontal regions, which become metabolically active during the third and last levels of development of the central nervous system, are the most consistently affected by the aging process.
The regional distribution of age-related hypometabolism, which involves primarily the frontal lobes, is substantially different from the patterns of brain metabolic impairment typical of Alzheimer’s disease (AD) and other dementias. AD is characterized by hypometabolism in precuneus and posterior cingulate cortex, parieto-temporal regions and more variably, medial temporal regions, while the frontal cortex becomes affected at late disease stages6,31. As many aging individuals experience memory loss, such metabolic differences make 18F-FDG PET particularly useful in the diagnosis of AD vs. normal aging, and in the understanding of age-related vs. AD-related memory impairment. While memory impairment due to AD is strictly linked to posterior cingulate and hippocampal hypometabolism, memory deficits observed during normal aging may reflect mainly a failure of encoding and retrieval processes of episodic memory, which depends on frontal cortex integrity 32.
The identification of brain metabolic abnormalities typical of AD in 18F-FDG PET of cognitively normal subjects has a strong predictive value for future development of AD. Longitudinal 18F-FDG PET studies in normal individuals who later declined to AD demonstrated that AD-related metabolic reductions precede the onset of clinical symptoms by many years and correlate with diagnosis of AD 33. Hippocampal metabolic impairment usually precedes that in the cortical regions in cognitively normal individuals declining to dementia due to AD, while the cortical hypometabolism becomes evident later 33.
Gender
Several 18F-FDG PET studies focused on the effect of gender on brain metabolism to highlight possible metabolic differences and corresponding behavioral differences between men and women.
Brain volume is reportedly greater in men than women, with a higher percentage of grey matter in female and a higher percentage of white matter in male subjects 34. However, 18F-FDG findings have been controversial, as summarized in Table 1.
Table 1.
Summary of literature on the effect of gender on cerebral glucose metabolism in normal healthy subjects.
| Study | Men/Women | Mean age (years)(M/W) | 18F-FDG PET findings |
|---|---|---|---|
| Hsieh et al. 2012 | 50/50 | 58.58/57.56 | M>W in bilateral visual cortices and cerebellum |
| Kim et al. 2009 | 32/46 | 46.6/40.6 | No differences. M: gender-specific age-related hypometabolism in insula W: gender-specific age-related hypometabolism in caudate nucleus |
| Brickman et al. 2003 | 35/35 | 54.0 (all subjects) | M>W in caudate nucleus |
| Kawachi et al. 2002 | 22/22 | 63.0/63.1 | M>W in insula, middle temporal gyrus, medial frontal lobe; W>M in hypothalamus |
| Willis et al. 2002 | 38/28 | 38.1/41.0 | W>M (global) W: maximal age-related hypometabolism in mid-superior temporal gyrus; M: maximal age-related hypometabolism in a more superior temporal gyrus; |
| Volkow et al. 1997 | 15/13 | 44/44 | W>M in temporal poles and cerebellum |
| Murphy et al. 1996 | 55/65 | 53 (all subjects) | W>M in thalamus M>W in hippocampus |
| Gur et al. 1995 | 37/24 | 27 (all subjects) | M>W in orbitofrontal lobes, temporal poles, temporo-occipital areas, hippocampus and amigdala |
| Andreason et al. 1994 | 21/18 | 26 (all subjects) | W>M in orbitofrontal and medial frontal cortices, posterior cingulate cortex and caudate nucleus |
| Miura et al. 1990 | 17/15 | 30 (all subjects) | No differences. |
| Yoshii et al. 1988 | 39/37 | 54 (all subjects) | W>M (global) |
| Baxter et al. 1987 | 7/7 | 32 (all subjects) | W>M (global) |
The inconsistencies among studies are likely due to differences in sample size, subjects’ age, and image analysis procedures. Hormones (i.e. estrogen) are another potential source of variationon cerebral metabolism of female subjects 35. Correction, or lack of, for differences in brain size and skull thickness between gender groups is another potential confounding factor which should be taken into account 36.
Substances/Medications
Several substances and medications may influence the cerebral metabolic rate of glucose, predominantly altering global metabolism but also with possible effects on regional distribution (Box 6).
Box 6. Effects of several substances and medications on cerebral glucose metabolism in normal individuals.
| Substance/Medication | Effect on brain glucose metabolism |
|---|---|
| Caffeine | Global mean reduction (−18%), most prominent in anterior cingulare cortex |
| Alcohol | Global mean reduction (from −9% to −25%), most prominent in occipital cortex and cerebellum |
| Amphetamines | Low-doses: metabolic decrease in cortical and subcortical areas High-doses: global metabolic increase, in particular in caudate nucleus, thalamus and anterior cingulate cortex Lon-term effect: metabolic reduction in cingulare cortex, striata, amygdala and hippocampus |
| Cocaine | Acute: increased metabolic activity in medial prefrontal cortex, expanding in prefrontal, sensorimotor, anterior cingulate cortices and in striata Long-term: reduced metabolic activity in striata and orbito-frontal cortex |
| Anaestetics | Global mean reduction (−46%) |
| Benzodiazepines | Global metabolic reduction, most prominent in occipito-cerebellar regions |
| Neuroleptics | Metabolic increase in striata and thalamus Metabolic decrease in frontal and anterior cingulate cortices |
| Corticosteroids | Generalized reduction of glucose metabolic activity |
| Chemotherapy | Reduction in cortical metabolic activity (−17%) Metabolic decrease in inferior frontal cortex |
Among the substances capable of altering cerebral metabolism, caffeine is one of the most commonly used. Caffeine is part of the methylxantine family and plays a vasoconstrictive effect. It has been shown that caffeine assumption prior PET examination decreases global cerebral glucose metabolism as measured with 18F-FDG with a mean change of −18%; the reduction is particularly prominent in the anterior cingulate cortex 44.
Alcohol has a broad range of actions on many neurotransmitter systems. The brain metabolic response to acute administration of ethanol in healthy subjects has been investigated in several studies, and it has been consistently shown a global reduction of brain glucose metabolism after ethanol administration, although with various degrees across different studies (from −9% to −25%) 45–47. Discrepancies in the amount of metabolic reduction could be due to differences in: amount of ethanol admnistered, PET scanners, composition of sample and data analyses. The greatest metabolic decrease in absolute metabolism usually occurs in the occipital cortex and in cerebellum. Moderate hypometabolism has been observed in the lymbic system, parietal cortex, frontal cortex, cingulate gyrus, temporal cortex, thalamus and midbrain. The smallest decrease could be observed in the basal ganglia 45–47.
Abuse substances such as amphetamines and cocaine significantly alter glucose brain metabolism. Low doses of amphetamines affect cerebral metabolism by decreasing metabolic rates of glucose of cortical and subcortical areas 48. On the contrary, at high doses amphetamines increase whole-brain glucose metabolism, particularly in striata, thalami and anterior cingulate cortex 49. Amphetamine abuse exerts also long-term effects on the brain: 18F-FDG PET of extasy abusers, even after detoxification, demonstrates hypometabolism in striata, amygdala, hippocampus and cingulate cortex 50.
Cocaine assumption before 18F-FDG PET examination induces a significant increase of brain metabolism in several cortical and subcortical areas, directly related to the substance exposure 51. Cocaine-induced increases in brain metabolism are localized in the medial prefrontal cortex in cases of limited exposure. As cocaine exposure increases, hypermetabolism could be observed in the prefrontal and anterior cingulate cortices and in regions of sensorimotor processing. Enhanced metabolic activity may be also found in the striatum 51. Besides, 18F-FDG PET scans of detoxified cocaine abusers may show alterations of glucose brain metabolism, with significant reduction of metabolic activity in striata and orbito-frontal cortex 52.
Among the medications that have been shown to alter brain glucose metabolism, there are several drugs acting on the central nervous system, such as anaesthetics, sedatives and neuroleptics, as well as corticosteroids and chemotherapy agents.
Anaesthetics, as propofol, isoflurane and barbiturates, produce a global, substantially uniform, metabolic reduction, at levels reaching −46% of normal metabolic activity 53,54.
Benzodiazepines as lorazepam induce a significant decrease in global cerebral metabolism,, with more prominent reduction in occipital cortex and cerebellum 55.
Neuroleptic drugs as haloperidol have been demonstrated to increase metabolic activity in striata and thalami and to reduce glucose metabolism in frontal lobes and anterior cingulate cortex 56.
Moreover, consumption of corticosteroids before 18F-FDG PET scan induces alterations of cerebral metabolism, with a generalized reduction of glucose metabolic activity 57.
Finally, brain 18F-FDG PET scan in cognitively normal subjects may show a lower brain metabolism (at levels around −17%) after chemotharapy than before the treatment 58. This condition, known as “chemo-brain”, could be observed also years after the chemotherapy, with reduction of cortical metabolism, particularly in inferior frontal gyrus 59,60.
Therapy procedures
Changes occurring after radiotherapy, especially external beam radiotherapy, may mimic hypometabolism due to neurodegeneration.
In the first weeks immediately after radiotherapy, radiation may induce elevated 18F-FDG uptake in normal brain tissues, due to inflammatory changes 63,64. These post-therapy changes could be visible also in the epithelial surfaces.
A long-term consequence after radiotherapy may be a reduction up to −5% of cerebral metabolic activity in the irradiated brain tissue, as compared to non-irradiated brain tissue 65.
ARTIFACTS
The main factors that could cause artifacts on 18F-FDG PET images, potentially leading to interpretation pitfalls, are the following:
Hyperglycemia. As 18F-FDG enters and is metabolized in cells using the same mechanisms as glucose, elevated plasma glucose levels result in decreased uptake of 18F-FDG in the normal brain tissue 66. In particular, hyperglycemia could induce a reduction of 18F-FDG uptake in all brain regions (including cortical and subcortical gray matter, white matter and cerebellum), at levels reaching −54% 67.
Patient motion. Patient motion during PET scanning introduces errors in the attenuation correction and image blurring leading to artifactual changes in activity concentrations and degradation of image resolution. It has been demonstrated that a mismatch as little as 5 mm may cause an error as much as 10% in measured 18F-FDG PET activity, and a 10-mm mismatch can cause a 25% error 68,69. Lateral movements of patient’s head during image acquisition cause a common motion artifact showing unilateral cerebral hypometabolism, involving also the ipsilateral scalp. In these cases, non attenuation corrected PET images should be observed, demonstrating the displacement of patient’s head. These artifacts could be corrected using computer realignment or calculating the attenuation correction with a standard attenuation coefficient.
Calculated attenuation. When a transmission image or a computed tomography (CT) scan is not available, attenuation correction could be calculated using a standard attenuation coefficient. However, this method does not take into account differences across subjects, in particular variations of skull thickness. For example, subjects with hyperostosis frontalis may show artifactual reduction of frontal metabolic activity.
Metallic artifacts. CT-based correction method overestimates the attenuation of metallic objects included in the field of view and results in artifactually increased 18F-FDG activity in PET images. When metallic artifacts are suspected, the absence of high activity in uncorrected images should be confirmed, in order to prevent potential misinterpretation.
SUMMARY
Brain 18F-FDG PET allows the in vivo study of cerebral glucose metabolism, reflecting neuronal and synaptic activity.
18F-FDG PET has been extensively used to detect metabolic alterations in several neurological diseases vs. normal aging. However, healthy subjects exhibit variants of 18F-FDG distribution, especially as associated with aging.
In normal healthy subjects, cerebral metabolic rate values for glucose are around 15 micromol/min/100g for the white matter, and around 40–60 micromol/min/100g for the grey matter. 18F-FDG uptake is usually homogeneous and symmetrical, but areas of slightly higher activity are observed in basal ganglia, frontal eye fields, posterior cingulate cortex and visual cortex. On the contrary, relatively lower metabolic activity is observed in medial temporal cortex.
During aging, global cerebral metabolic rate of glucose decreases with a reported decline of 12–13% between ages 20 and >70 years. Healthy aging is associated with mild cortical hypometabolism involving preferentially the frontal lobes, in particular anterior cingulate cortex, dorsolateral and medial prefrontal cortices, and orbitofrontal cortex.
Age-related metabolic reductions are also observed in the insula, the temporal lobes (particularly the temporal pole and lateral temporal cortex) and the parietal lobes.
primary motor cortices, occipital cortices (particularly visual areas and posterior cingulate cortex), precuneus, mesial temporal lobes (hippocampus, amygdala and parahippocampal gyrus), thalamus, putamen, pallidum and cerebellum are the least age-affected regions.
Gender-related differences have been reported although definitive results remain to be established.
18F-FDG uptake in the normal brain could be affected by several substances and medications including caffeine, alcohol, abuse drugs as amphetmines and cocaine, sedatives, neuroleptics, corticosteroids and chemotherapy agents. Besides, radiotherapy could have both short-term and long-term effects on brain metabolism, causing 18F-FDG uptake changes in irradiated brain tissue.
Finally, several artifacts may influence 18F-FDG brain distribution and cause interpretation pitfalls.
Key Points.
Brain 18F-FDG PET allows the in vivo study of cerebral glucose metabolism, reflecting neuronal and synaptic activity.
18F-FDG PET has been extensively used to detect metabolic alterations in several neurological diseases vs. normal aging; however, healthy subjects exhibit variants of 18F-FDG distribution, especially as associated with aging.
Healthy aging is associated with mild cortical hypometabolism involving preferentially the frontal lobes, in particular anterior cingulate cortex, dorsolateral and medial prefrontal cortices, and orbitofrontal cortex.
18F-FDG uptake in the normal brain could be affected by several substances and medications including caffeine, alcohol, abuse drugs as amphetmines and cocaine, sedatives, neuroleptics, corticosteroids and chemotherapy agents.
Several artifacts may influence 18F-FDG brain distribution and cause interpretation pitfalls.
Acknowledgments
This work was supported in part by National Institute of Health/National Institute on Aging 5R01AG035137 and Alzheimer’s association IIRG-09-132030 (L.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Valentina Berti, Email: valentina.berti@unifi.it, Nuclear Medicine Unit, Department of Biomedical, Experimental and Clinical Sciences, University of Florence, Largo Brambilla, 3, 50134, Florence, Phone: +39 055 794 6502, Fax: +39 055 4224392.
Lisa Mosconi, Email: lisa.mosconi@med.nyu.edu, Center for Brain Health, New York University School of Medicine, 145 East 32nd Street, 5th Floor, Room 5122, New York, NY 10016, Phone: (212) 263-3255, Fax: (212) 263-3270.
Alberto Pupi, Email: a.pupi@dfc.unifi.it, Nuclear Medicine Unit, Department of Biomedical, Experimental and Clinical Sciences, University of Florence, Largo Brambilla, 3, 50134, Florence, Phone: +39 055 7947527, Fax: +39 055 4224392.
References
- 1.Sokoloff L, Reivich M, Kennedy C, et al. THE [14C]DEOXYGLUCOSE METHOD FOR THE MEASUREMENT OF LOCAL CEREBRAL GLUCOSE UTILIZATION: THEORY, PROCEDURE, AND NORMAL VALUES IN THE CONSCIOUS AND ANESTHETIZED ALBINO RAT. Journal of Neurochemistry. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 2.Sokoloff L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab. 1981;1(1):7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy C, Rosiers Des MH, Sakurada O, et al. Metabolic mapping of the primary visual system of the monkey by means of the autoradiographic [14C]deoxyglucose technique. Proc Natl Acad Sci USA. 1976;73(11):4230–4234. doi: 10.1073/pnas.73.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24(2):321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- 5.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27(8):489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Mosconi L, Tsui WH, Herholz K, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. Journal of Nuclear Medicine. 2008;49(3):390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 8.Waxman AD, Herholz K, Lewis DH, et al. Society of Nuclear Medicine Procedure Guideline for FDG PET Brain Imaging. Journal of Nuclear Medicine. 2009:1–12. [Google Scholar]
- 9.Kochunov P, Ramage AE, Lancaster JL, et al. Loss of cerebral white matter structural integrity tracks the gray matter metabolic decline in normal aging. Neuro Image. 2009;45(1):17–28. doi: 10.1016/j.neuroimage.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibáñez V, Pietrini P, Furey ML, et al. Resting state brain glucose metabolism is not reduced in normotensive healthy men during aging, after correction for brain atrophy. Brain Res Bull. 2004;63(2):147–154. doi: 10.1016/j.brainresbull.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ivançević V, Alavi A, Souder E, et al. Regional cerebral glucose metabolism in healthy volunteers determined by fluordeoxyglucose positron emission tomography: appearance and variance in the transaxial, coronal, and sagittal planes. Clin Nucl Med. 2000;25(8):596–602. doi: 10.1097/00003072-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Ishii K, Sakamoto S, Hosaka K, Mori T, Sasaki M. Variation in FDG uptakes in different regions in normal human brain as a function of the time (30 and 60 minutes) after injection of FDG. Annals of Nuclear Medicine. 2002;16(4):299–301. doi: 10.1007/BF03000112. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Kanno I, Murakami M, Shishido F, Uemura K. Tomographic Mapping of Kinetic Rate Constants in the Fluorodeoxyglucose Model Using Dynamic Positron Emission Tomography. Journal of Cerebral Blood Flow & Metabolism. 1986;6(4):447–454. doi: 10.1038/jcbfm.1986.78. [DOI] [PubMed] [Google Scholar]
- 14.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 15.Kinnala A, Suhonen-Polvi H, Aarimaa T, et al. Cerebral metabolic rate for glucose during the first six months of life: an FDG positron emission tomography study. Archives of Disease in Childhood - Fetal and Neonatal Edition. 1996;74(3):F153–F157. doi: 10.1136/fn.74.3.F153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron JC, Godeau C. Brain mapping: the systems. San Diego: 2000. Human aging. [Google Scholar]
- 17.Zuendorf G, Kerrouche N, Herholz K, Baron J-C. Efficient principal component analysis for multivariate 3D voxel-based mapping of brain functional imaging data sets as applied to FDG-PET and normal aging. Hum Brain Mapp. 2002;18(1):13–21. doi: 10.1002/hbm.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentourkia M, Bol A, Ivanoiu A, et al. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. Journal of the Neurological Sciences. 2000;181(1–2):19–28. doi: 10.1016/S0022-510X(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 19.Moeller JR, Ishikawa T, Dhawan V, et al. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16(3):385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Willis MW, Ketter TA, Kimbrell TA, et al. Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res. 2002;114(1):23–37. doi: 10.1016/s0925-4927(01)00126-3. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh T-C, Lin W-Y, Ding H-J, et al. Sex- and age-related differences in brain FDG metabolism of healthy adults: an SPM analysis. J Neuroimaging. 2012;22(1):21–27. doi: 10.1111/j.1552-6569.2010.00543.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim I-J, Kim S-J, Kim Y-K. Age- and Sex-Associated Changes in Cerebral Glucose Metabolism in Normal Healthy Subjects: Statistical Parametric Mapping Analysis of F-18 Fluorodeoxyglucose Brain Positron Emission Tomography. Acta Radiol. 2009;50(10):1169–1174. doi: 10.3109/02841850903258058. [DOI] [PubMed] [Google Scholar]
- 23.Garraux G, Salmon E, Degueldre C, Lemaire C, Laureys S, Franck G. Comparison of impaired subcortico-frontal metabolic networks in normal aging, subcortico-frontal dementia, and cortical frontal dementia. Neuro Image. 1999;10(2):149–162. doi: 10.1006/nimg.1999.0463. [DOI] [PubMed] [Google Scholar]
- 24.Kalpouzos G, Chételat G, Baron J-C, et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30(1):112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Yanase D, Matsunari I, Yajima K, et al. Brain FDG PET study of normal aging in Japanese: effect of atrophy correction. Eur J Nucl Med Mol Imaging. 2005;32(7):794–805. doi: 10.1007/s00259-005-1767-2. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto T, Matsumoto T, Fujita S, et al. Changes in glucose metabolism due to aging and gender-related differences in the healthy human brain. Psychiatry Res. 2008;164(1):58–72. doi: 10.1016/j.pscychresns.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuro Image. 2002;17(1):302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 28.Pardo JV, Lee JT, Sheikh SA, et al. Where the brain grows old: Decline in anterior cingulate and medial prefrontal function with normal aging. Neuro Image. 2007;35(3):1231–1237. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brickman AM, Buchsbaum MS, Shihabuddin L, Hazlett EA, Borod JC, Mohs RC. Striatal size, glucose metabolic rate, and verbal learning in normal aging. Cognitive Brain Research. 2003;17(1):106–116. doi: 10.1016/S0926-6410(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 30.Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosconi L, Brys M, Glodzik-Sobanska L, De Santi S, Rusinek H, De Leon MJ. Early detection of Alzheimer’s disease using neuroimaging. Experimental Gerontology. 2007;42(1–2):129–138. doi: 10.1016/j.exger.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age- related memory changes: a PET study. Neuro Report. 1996;7(6):1165. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- 33.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36(5):811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosgrove KP, Mazure CM, Staley JK. ScienceDirect.com - Biological Psychiatry - Evolving Knowledge of Sex Differences in Brain Structure, Function, and Chemistry. Biological psychiatry. 2007 doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiman EM, Armstrong SM, Matt KS, Mattox JH. The application of positron emission tomography to the study of the normal menstrual cycle. Hum Reprod. 1996;11(12):2799–2805. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- 36.Miura SA, Schapiro MB, Grady CL, et al. Effect of gender on glucose utilization rates in healthy humans: a positron emission tomography study. J Neurosci Res. 1990;27(4):500–504. doi: 10.1002/jnr.490270410. [DOI] [PubMed] [Google Scholar]
- 37.Kawachi T, Ishii K, Sakamoto S, Matsui M, Mori T, Sasaki M. Gender differences in cerebral glucose metabolism: a PET study. Journal of the Neurological Sciences. 2002;199(1–2):79–83. doi: 10.1016/s0022-510x(02)00112-0. [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, Wang GJ, Fowler JS, et al. Gender differences in cerebellar metabolism: test-retest reproducibility. Am J Psychiatry. 1997;154(1):119–121. doi: 10.1176/ajp.154.1.119. [DOI] [PubMed] [Google Scholar]
- 39.Murphy DGM. Sex Differences in Human Brain Morphometry and Metabolism: An In Vivo Quantitative Magnetic Resonance Imaging and Positron Emission Tomography Study on the Effect of Aging. Arch Gen Psychiatry. 1996;53(7):585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 40.Gur R, Mozley L, Mozley P, et al. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267(5197):528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- 41.Andreason PJ, Zametkin AJ, Guo AC, Baldwin P, Cohen RM. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res. 1994;51(2):175–183. doi: 10.1016/0165-1781(94)90037-X. [DOI] [PubMed] [Google Scholar]
- 42.Yoshii F, Barker WW, Chang JY, et al. Sensitivity of cerebral glucose metabolism to age, gender, brain volume, brain atrophy, and cerebrovascular risk factors. J Cereb Blood Flow Metab. 1988;8(5):654–661. doi: 10.1038/jcbfm.1988.112. [DOI] [PubMed] [Google Scholar]
- 43.Baxter LR, Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21(3):237–245. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Newberg AB, Wang J, et al. Caffeine’s effects on resting-state oxygen and glucose metabolism: A combined MR and PET study. Proc Intl Soc Mag Reson Med. 2009:17. [Google Scholar]
- 45.Volkow ND, Hitzemann R, Wolf AP, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Research: Neuroimaging. 1990;35(1):39–48. doi: 10.1016/0925-4927(90)90007-S. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Franceschi D, Fowler JS. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuro Image. 2006 doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Zhu W, Volkow ND, Ma Y, Fowler JS, Wang G-J. RELATIONSHIP BETWEEN ETHANOL-INDUCED CHANGES IN BRAIN REGIONAL METABOLISM AND ITS MOTOR, BEHAVIOURAL AND COGNITIVE EFFECTS. Alcohol and …. 2004 doi: 10.1093/alcalc/agh023. [DOI] [PubMed] [Google Scholar]
- 48.Wolkin A, Angrist B, Wolf A, et al. Effects of amphetamine on local cerebral metabolism in normal and schizophrenic subjects as determined by positron emission tomography. Psychopharmacology. 1987;92(2) doi: 10.1007/BF00177923. [DOI] [PubMed] [Google Scholar]
- 49.Vollenweider FX, Maguire RP, Leenders KL, Mathys K, Angst J. Effects of high amphetamine dose on mood and cerebral glucose metabolism in normal volunteers using positron emission tomography (PET) Psychiatry Research: Neuroimaging. 1998;83(3):149–162. doi: 10.1016/S0925-4927(98)00033-X. [DOI] [PubMed] [Google Scholar]
- 50.BUCHERT R, OBROCKI J, THOMASIUS R, et al. Long-term effects of ‘ecstasy’ abuse on the human brain studied by FDG PET. Nuclear Medicine Communications. 2001;22(8):1–9. doi: 10.1097/00006231-200108000-00007. Available at: http://journals.lww.com/nuclearmedicinecomm/Fulltext/2001/08000/Long_term_effects_of__ecstasy__abuse_on_the_human.7.aspx. [DOI] [PubMed] [Google Scholar]
- 51.Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging and Behavior. 2010;4(3–4):212–219. doi: 10.1007/s11682-010-9100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkow ND, Hitzemann R, Wang G-J, et al. Long-Term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 53.Alkire MT, Haier RJ, Barker SJ, Shah NK, Wu JC, Kao JY. Cerebral Metabolism during Propofol Anesthesia in Humans Studied with Positron Emission Tomography. Anesthesiology. 1995;82(2):393. doi: 10.1097/00000542-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Alkire MTM, Haier RJP, Shah NKM, Anderson CTM. Positron Emission Tomography Study of Regional Cerebral Metabolism in Humans during Isoflurane Anesthesia. Anesthesiology. 1997;86(3):549. doi: 10.1097/00000542-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Wang GJ, Volkow ND, Levy AV, et al. Measuring reproducibility of regional brain metabolic responses to lorazepam using statistical parametric maps. Journal of Nuclear Medicine. 1999;40(5):715–720. [PubMed] [Google Scholar]
- 56.Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry. 1996;153(1):41–49. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- 57.Fulham Michael J, Brunetti Arturo, Aloj Luigi, Raman Ramesh, Dwyer Andrew J, Chiro Giovanni Di. Decreased cerebral glucose metabolism in patients with brain tumors: an effect of corticosteroids. 1995 doi: 10.3171/jns.1995.83.4.0657. http://dxdoiorg/103171/jns19958340657. [DOI] [PubMed]
- 58.Sorokin J, Saboury B, Ahn JA, Moghbel M, Basu S, Alavi A. Adverse Functional Effects of Chemotherapy on Whole-Brain Metabolism: A PET/CT Quantitative Analysis of FDG Metabolic Pattern of the “Chemo-Brain”. Clin Nucl Med. 2013:1. doi: 10.1097/RLU.0b013e318292aa81. [DOI] [PubMed] [Google Scholar]
- 59.Silverman DHS, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 60.Simó M, Rifà-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2013;37(8):1311–1321. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 61.Volkow ND, Kim S, Wang GJ, Alexoff D, Logan J. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuro Image. 2012 doi: 10.1016/j.neuroimage.2012.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang G-J, Volkow ND, Franceschi D, et al. Regional Brain Metabolism During Alcohol intoxication. Alcoholism: Clinical and Experimental Research. 2000;24(6):822–829. [PubMed] [Google Scholar]
- 63.Hautzel H, Müller-Gärtner HW. Early changes in fluorine-18-FDG uptake during radiotherapy. Journal of Nuclear Medicine. 1997;38(9):1384–1386. [PubMed] [Google Scholar]
- 64.Metser U, Even-Sapir E. Increased 18F-Fluorodeoxyglucose Uptake in Benign, Nonphysiologic Lesions Found on Whole-Body Positron Emission Tomography/Computed Tomography (PET/CT): Accumulated Data From Four Years of Experience With PET/CT. Seminars in Nuclear Medicine. 2007;37(3):206–222. doi: 10.1053/j.semnuclmed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Kesner AL, Lau VK, Speiser M, et al. Time-course of effects of external beam radiation on [18F]FDG uptake in healthy tissue and bone marrow. Journal of Applied Clinical Medical Physics. 2008;9(3) doi: 10.1120/jacmp.v9i3.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishimori T, Watanabe Y, Sakata C, et al. Effect of blood glucose level on physiological FDG uptake in the brain. J Nucl Med. 2009;50(Supplement 2):134. [Google Scholar]
- 67.Ishizu K, Nishizawa S, Yonekura Y, et al. Effects of hyperglycemia on FDG uptake in human brain and glioma. Journal of Nuclear Medicine. 1994;35(7):1104–1109. [PubMed] [Google Scholar]
- 68.Andersson JL, Vagnhammar BE, Schneider H. Accurate attenuation correction despite movement during PET imaging. Journal of Nuclear Medicine. 1995;36(4):670–678. [PubMed] [Google Scholar]
- 69.Huang S-C, Hoffman EJ, Phelps ME, Kuhl DE. Quantitation in Positron Emission Computed Tomography: 2. Effects of Inaccurate Attenuation Correction. Journal of Computer Assisted Tomography. 1979;3(6):804–814. [PubMed] [Google Scholar]