Abstract
Residual depressive symptoms are commonly observed in adolescents with major depressive disorder (MDD) following treatment with selective serotonin reuptake inhibitors (SSRIs). This study combined a case-control analysis and an open-label fish oil (FO) trial to investigate the relationship between long-chain omega-3 (LCn-3) fatty acid status and residual depressive symptoms in SSRI-resistant adolescent MDD patients. Baseline erythrocyte docosahexaenoic acid (DHA)(-28%, p=0.0003), but not eicosapentaenoic acid (EPA)(-18%, p=0.2), was significantly lower in patients (n=20) compared with healthy controls (n=20). Patients receiving 10-week low-dose (2.4 g/d, n=7) and high-dose (16.2 g/d, n=7) FO exhibited significant increases in erythrocyte EPA and DHA composition. In the intent-to-treat sample, depressive symptoms decreased significantly in the high-dose group (n=7, -40%, p<0.0001), and there was a trend in the low-dose group (n=10, −20%, p=0.06). Symptom remission was observed in 40% of patients in the low-dose group and 100% of patients in the high-dose group. There were no significant changes in vital signs and adverse events were rated as mild or moderate in severity. These preliminary findings demonstrate that adolescents with SSRI-resistant depression exhibit robust DHA deficits, and suggest that adjunctive FO supplementation is well-tolerated and effective for increasing LCn-3 fatty acid status and augmenting SSRI antidepressant effects.
Keywords: Omega-3 fatty acids, Docosahexaenoic acid, Eicosapentaenoic acid, Adolescents, Erythrocyte, Major depressive disorder (MDD)
1. INTRODUCTION
Converging evidence suggests that a deficiency in long-chain omega-3 (LCn-3) fatty acids, including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), during brain development may represent a modifiable risk factor for mood disorders including major depressive disorder (MDD)[1]. Cross-national epidemiological data suggest that greater per capita intake of fish, a principal dietary source of preformed EPA+DHA, is associated with reduced lifetime prevalence rates of MDD [2,3]. Independent meta-analyses of controlled trials suggest that fish oil (FO) supplementation is superior to placebo for reducing depressive symptoms in adult MDD patients [4-8]. The initial onset of MDD frequently occurs during adolescence [9,10], and a large percentage of adolescents residing in western countries consume low quantities of LCn-3 fatty acids in their diet [11-13]. Preliminary FO supplementation trials have observed reductions in depressive symptoms in children and adolescents with established mood disorders [14-16]. These and other data suggest that greater dietary fish intake may be protective against the development of depressive symptoms, and that FO supplementation has antidepressant effects in patients with MDD.
A valid technique for determining an individuals LCn-3 fatty acid ‘status’ is gas chromatographic analysis of erythrocyte (red blood cell) fatty acid composition [17,18]. Erythrocyte EPA+DHA levels are positively correlated with fish intake frequency [19,20], and increase in a dose-dependent manner following FO supplementation [22,23]. Additionally, normative population data are beginning to emerge [20,21], and low erythrocyte EPA+DHA composition (termed the ‘omega-3 index’) has been proposed as a risk biomarker for coronary heart disease mortality [24]. Specifically, erythrocyte EPA+DHA composition of ≤4% is considered a high risk zone whereas >8% is a low risk zone [24]. Although this index has not been systematically evaluated in the context of MDD, a meta-analysis of fourteen cross-sectional studies found significantly lower erythrocyte EPA and DHA levels in patients with MDD [25]. More recent case-control studies found that erythrocyte EPA+DHA composition of ≤4.0% is significantly more prevalent among adolescent and adult MDD patients [26,27]. These and other data suggest that erythrocyte EPA+DHA composition may serve as a biomarker relevant to the pathophysiology and potentially etiology of MDD [28].
Multiple lines of evidence suggest that a dysregulation in serotonin neurotransmission is central to the pathophysiology and treatment of MDD [29,30]. Emerging translational evidence suggests that LCn-3 fatty acid status influences the development of central serotonin systems. Specifically, dietary n-3 fatty acid insufficiency during perinatal development is associated with systemic LCn-3 fatty acid deficits and impaired serotonin release [31], as well as elevated behavioral indices of depression and aggression [32], in young adulthood. In contrast, FO supplementation during development increases serotonin concentrations in rat frontal cortex [33], attenuates reductions in frontal cortex serotonin content in response to chronic stress [34], and decreases behavioral indices of depression [35]. Furthermore, FO supplementation augments the antidepressant-like effects of selective serotonin reuptake inhibitors (SSRIs) in rodents [36,37]. Similarly, controlled trials have observed greater reductions in depressive symptoms by combining FO with SSRIs compared with either treatment alone [38,39]. Together these data suggest that LCn-3 fatty acid status influences the maturation and resilience of serotonin neurotransmitter systems which may impact SSRI antidepressant effects.
Although SSRI medications have become the primary treatment for adolescent depression, approximately 30-40 percent of adolescent MDD patients exhibit residual symptoms following standard SSRI treatment [40,41]. Because untreated depression is associated with poor outcomes and increased risk of suicide [42,43], there is an urgent need to identify risk biomarkers and adjunctive treatments for adolescents with SSRI-resistant MDD to inform clinical practice [28]. Based on the translational evidence reviewed above, low LCn-3 fatty acid status may represent a modifiable risk factor for SSRI-resistance. To evaluate this, the present study first investigated erythrocyte LCn-3 fatty acid status of adolescents with SSRI-resistant MDD in a case-control analysis, and then determined the effects of open-label FO supplementation on erythrocyte LCn-3 fatty acid composition and residual depressive symptoms. Our specific prediction was that adolescents with SSRI-resistant MDD would exhibit erythrocyte EPA+DHA deficits compared with healthy adolescent controls, and that FO supplementation would dose-dependently increase erythrocyte EPA+DHA levels and decrease residual depressive symptoms.
2. Materials and Methods
2.1. Subjects
Male or female adolescents (8-24 years of age) diagnosed with MDD (DSM-IV-TR criteria) were recruited by advertisement, word of mouth, and referral from existing pediatric/adolescent recruitment infrastructure within the Department of Psychiatry, University of Cincinnati College of Medicine. The MDD diagnosis was confirmed with the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS)[44]. All adolescents were assessed by a board-certified child and adolescent psychiatrist. Written informed consent and assent were provided by a legal guardian and the subject, respectively. Participants were required to have a baseline score of >28 and <40 on the Children Depression Rating Scale-Revised (CDRS-R) despite being administered a standard therapeutic dose of an SSRI for a minimum of 6 weeks (i.e., SSRI-resistant). Patients were maintained on their baseline SSRI dose over the course of the trial. Patients were excluded by a positive urine pregnancy test, a history of seizures, traumatic brain injury, or major medical illness, having a urine drug screen which was positive for illicit substance use, were greater than 1 year outside appropriate age/grade level, required treatment with any psychotropic drug which might obscure the action of the study treatment, or had a seafood allergy. A dietary omega-3 intake questionnaire was administered at baseline to estimate current habitual dietary fish/seafood intake and to exclude patients currently taking omega-3 fatty acid supplements. A nested group of healthy controls with no personal history of a DSM-IV-TR Axis I disorder were recruited from the greater Cincinnati area. This trial was approved by the University of Cincinnati Institutional Review Board, and was registered at clinicaltrials.gov as NCT00511810.
2.2. Gas chromatography
Whole venous blood (10 ml) was collected into EDTA-coated BD Vacutainer tubes, and centrifuged at 4°C for 20 min (1,500 ×g). Plasma and buffy coat were removed and erythrocytes washed 3 times with 0.9% NaCl and stored at −80°C. Total erythrocyte membrane fatty acid composition was determined with a Shimadzu GC-2010 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD), as previously described [26]. The column was a DB-23 (123-2332): 30m (length), I.D. (mm) 0.32 wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). The carrier gas was helium with a column flow rate of 2.5 ml/min. Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters was based on areas calculated with Shimadzu Class VP 4.3 software. Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). Based on studies which added a known mass of heptadecanoic acid (17:0, 99%, Matreya LLC Inc., Pleasant Gap PA) to samples, the lower limit of detection with a threshold area of 500 and a 1 μl injection volume is approximately 200 ng of an individual fatty acid. All samples were processed by a technician blinded to group and treatment assignment. The primary measures of interest were EPA and/or DHA composition and the ratio of arachidonic acid to EPA and/or DHA.
2.3. Fish oil supplementation
Patients were randomized (stratified by gender) to open-label FO at a fixed EPA+DHA dose of either 2.4 g/day (Low-Dose: EPA 1.6 g + DHA 0.8 g; 4 capsules/d) or 16.2 g/day (High-Dose: EPA, 10.8 g + DHA 5.4 g; 2 tablespoons/day) for 10 weeks. In order to achieve high-dose FO while avoiding the burden of taking the equivalent of 27 capsules, high-dose FO was administered as a liquid. The FO is an ethyl ester formulation, derived from anchovies and sardines, and was generously supplied by The Inflammation Research Foundation. Compliance was evaluated by determining capsule counts (low-dose) or bottle volumes (high-dose) and self-reports at weekly visits. The fatty acid composition of the FO was independently confirmed by gas chromatography as described above. The low dose (2.4 g/d) was selected based on previous studies findings that similar doses were safe and efficacious in pediatric and adolescent patients with mood disorders [14-16], and the high dose (16.2 g/d) based on efficacy and safety data in pediatric and adolescent ADHD patients [45]. Patients were requested to maintain their current dietary habits over the course of the trial. To minimize gastrointestinal adverse events associated with FO supplementation, patients were instructed to take their supplements with meals. Based on effect sizes observed in prior FO intervention trials in pediatric and adolescent patients with mood disorders [14-16], the target sample size of n=10-15/dose group was estimated to have 80% power to detect large effect sizes.
2.4. Depression symptom ratings
At weekly visits depression symptom severity was determined with the Children’s Depression Rating Scale-Revised (CDRS-R), a 17-item observer-rated questionnaire [46,47]. All patients were rated by a board-certified child and adolescent psychiatrist with established inter-rater reliabilities (kappa > 0.9). Remission was defined as an endpoint CDRS-R score of ≤28 [41]. Response was defined as a ≥50% baseline-endpoint decrease in CDRS-R total score. If a patient’s depressive symptoms worsened over the course of the trial (defined as 30% worsening relative to baseline on two consecutive visits using CDRS-R total score), they were withdrawn from study participation and referred for alternate treatment.
2.5. Safety and tolerability assessments
Body weight (kg) and height (cm), a complete blood count (white blood cells, WBC; red blood cells, RBC; platelets), thyroid stimulating hormone (TSH) concentrations, and vital signs (pulse, blood pressure, and temperature) were obtained at baseline and endpoint. Sex- and age-adjusted body mass index (BMI, kg/m2) percentile and z scores were calculated. The frequency and severity of adverse events were assessed at each visit using the Side Effects Form for Children and Adolescents (SEFCA)[48]. In view of the potential risk for developing hypomanic symptoms following FO supplementation [49], manic symptoms were evaluated biweekly with the Young Mania Rating Scale (YMRS), an 11-item observer-rated questionnaire [50].
2.6. Statistical analyses
Statistical analyses were performed using Statistical Analysis System (SAS) software, version 9.0 (SAS Institute, Cary, NC, USA). Differences between patients and controls in demographic measures were evaluated using unpaired t-tests (2-tail, α=0.05) for continuous variables and Chi-square tests (2-tailed, α=0.05) for dichotomous variables. For the case-control fatty acid analysis, we employed Bonferroni correction for multiple comparisons (α=0.05/17 fatty acids and ratios = 0.003). Categorical assessments were used to determine the percentage of subjects with EPA+DHA levels ≤4.0 percent of total fatty acids (2-tailed Chi-square test, α=0.05). Baseline-endpoint changes in vital signs and labs were evaluated with a two-way ANOVA, with dose (low-dose, high-dose) and study time point (baseline, endpoint) as the main factors. Efficacy and tolerability analyses were performed on the intent-to-treat (ITT) sample, which included all patients who received at least one dose of study medication and also had at least one post-baseline efficacy and tolerability assessment. For mood symptom scores obtained at weekly (CDRS-R) or biweekly (YMRS) visits, a mixed-effects regression model (PROC MIXED) that included terms for dose, time, and dose-by-time interaction was used. AIC was used to select the variance-covariance structure for this model (inclusion or exclusion of subject-level random intercepts and slopes and autoregressive structure for the residual covariances). Pearson correlation coefficients were used to evaluate relationships between primary outcome measures (2-tail, α=0.05). For primary outcome measures, effect sizes were calculated using Cohen’s d, with small, medium, and large effect sizes being equivalent to d-values of 0.30, 0.50, and 0.80, respectively.
3. Results
3.1. Case-control analysis
For demographic variables there were no significant differences between MDD patients and healthy controls (Table 1). For patients, mean daily SSRI doses were: fluoxetine 26±16.5 mg; citalopram 26.7±28.9 mg; sertraline 70.8±43.1 mg; escitalopram 20±0 mg. The case-control comparison of total erythrocyte fatty acid composition is presented in Table 2. Erythrocyte DHA composition (−28%, p=0.0003, d = 1.3), but not EPA (−18%, p=0.2) or DPA (−8%, 22:5n-3)(p=0.07), was significantly lower in patients compared with controls. EPA+DHA composition (−28%, p=0.0001, d = 1.4) and the sum of LCn-3 fatty acids (EPA+DPA+DHA, −21%, p=0.0001) were significantly lower in patients. A significantly greater proportion of patients (90%) exhibited EPA+DHA composition ≤4.0% compared with controls (40%)(Chi-Square: p=0.002). Erythrocyte AA composition did not differ between patients and controls, and the AA/DHA (+27%, p=0.0001) and AA/EPA+DHA (+25%, p=0.0001) ratios, but not the AA/EPA ratio (+13%, p=0.48), were significantly greater in patients. Among all MDD patients (n=20), EPA+DHA (r = +0.36, p=0.11), DHA (r = +0.35, p=0.13), and the AA/EPA+DHA (r = −0.35, p=0.12) and AA/EPA (r = −0.24, p=0.29) ratios, were not significantly correlated with baseline CDRS-R total scores. Other major fatty acids did not differ between patients and controls after correcting for multiple comparisons.
Table 1. Demographic variables for healthy controls and MDD patients.
| Controls (n=20) |
MDD (n=20) |
P- alue2 |
|
|---|---|---|---|
| Age (yrs) | 15.5 ± 2.4 | 15.6 ± 3.2 | 0.91 |
| Gender (% male) | 45 | 40 | 0.98 |
| Race (n) | |||
| Caucacian | 17 | 19 | 0.61 |
| African American | 2 | 1 | |
| Other | 1 | 0 | |
| Height (cm) | 165.2 ± 10.1 | 163.2 ± 13.8 | 0.60 |
| Weight (kg) | 64.4 ± 19.7 | 62.3 ± 19.1 | 0.73 |
| BMI (kg/m2) | 22.6 ± 5.5 | 24.2 ± 7.2 | 0.49 |
| BMI z score | 0.3 ± 1.2 | 0.6 ± 1.1 | 0.44 |
| BMI percentile | 59.0 ± 34.1 | 65.1 ± 32.4 | 0.56 |
| Smoking status (current) (n) | 1 | 2 | 1.0 |
| Age at Onset (years) | – | 13.1 ± 3.1 | – |
| Duration of Illness (yrs) | – | 2.4 ± 2.7 | – |
| CDRS-R Total Score | – | 34.7 ± 4.7 | – |
| SSRI (n) | |||
| Fluoxetine | – | 10 | – |
| Citalopram | – | 3 | – |
| Escitalopram | – | 1 | – |
| Sertraline | – | 6 | – |
Values are group mean ± S.D.
Two-tailed t-test or Chi-square test
Table 2. Comparison of erythrocyte fatty acid composition in healthy controls and MDD patients.
| Fatty Acid1 | Controls(n=20) | MDD (n=20) |
P- value2 |
|---|---|---|---|
| Palmitic acid (16:0) |
17.29 ± 1.26 | 17.86 ± 0.98 | 0.12 |
| Stearic acid (18:0) |
17.49 ± 1.11 | 17.15 ± 0.45 | 0.21 |
| Oleic acid (18:1 n-9) |
11.81 ± 0.95 | 12.56 ± 0.85 | 0.01 |
| Vaccenic acid (18:1 n-7) |
1.09 ± 0.36 | 1.21 ± 0.15 | 0.17 |
| Linoleic acid LA, 18:2n-6)( |
11.78 ± 1.31 | 11.35 ± 0.81 | 0.23 |
| Homo-γ-linolenic (HGLA, 20:3n-6) |
1.69 ± 0.27 | 1.89 ± 0.31 | 0.03 |
| Arachidonic acid (AA, 20:4n-6) |
17.80 ± 1.40 | 18.10 ± 1.21 | 0.52 |
| Docosatetraenoic acid (22:4n-6) |
4.48 ± 0.53 | 4.75 ± 0.46 | 0.10 |
| Eicosapentaenoic acid (EPA, 20:5n- 3) |
0.34 ± 0.16 | 0.28 ± 0.10 | 0.20 |
| Docosapenaenoic acid (22:5n-3) |
2.40 ± 0.43 | 2.20 ± 0.28 | 0.07 |
| Docosahexaenoic acid (DHA, 22:6n- 3) |
3.90 ± 1.01 | 2.80 ± 0.59 | 0.0003 |
| EPA±DHA | 4.24 ± 1.03 | 3.10 ± 0.58 | 0.0002 |
| AA:DHA | 4.80 ± 1.20 | 6.60 ± 1.20 | 0.0001 |
| AA:EPA | 67.9 ± 42.3 | 77.8 ± 45.4 | 0.48 |
| AA:EPA±DHA | 4.40 ± 1.02 | 5.90 ± 0.93 | 0.0001 |
Values are group mean fatty acid composition (wt % total fatty acids) ± S.D.
Two-tailed t-test.
3.2. Open-label intervention
3.2.1. Subject recruitment and attrition
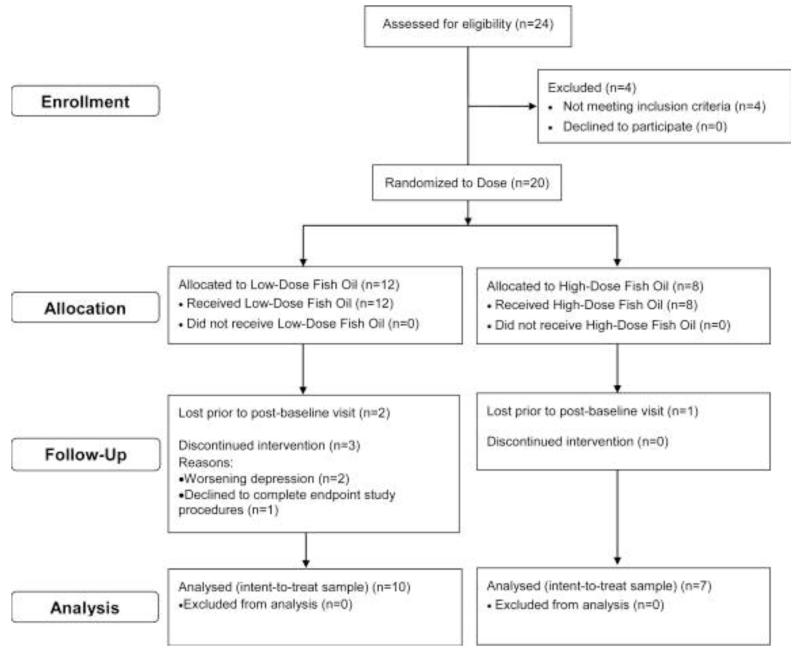
A flow diagram illustrating the sequence of subject recruitment and attrition is illustrated in Figure 1. A total of 14 patients completed the 10 week open-label intervention (low-dose, n=7; high-dose, n=7). A total of 3 patients were lost to follow-up post-randomization, and there were 3 patients randomized to low-dose that terminated study participation early (2 patients (weeks 3 and 6) due to a worsening of depressive symptoms, and 1 patient (week 9) declined to complete endpoint study procedures). At baseline, low-dose and high-dose groups did not differ in age (p=0.30), gender (p=1.0), race (p=0.99), BMI (p=0.94), or age at onset of MDD (p=0.92). At baseline, the majority of patients consumed fish ≤1 times/month (70%), and monthly fish intake frequency did not differ between low-dose and high-dose groups (p=0.29).
Figure 1.
Flow diagram illustrating the sequence of subject recruitment and attrition.
3.2.2. Erythrocyte fatty acid composition
Based on capsule counts (low-dose) or bottle volumes (high-dose) determined at weekly visits, there was a compliance rate of 92% for the low-dose group and 97% for the high-dose group. At baseline, erythrocyte EPA+DHA composition did not differ between low-dose and high-dose groups (p=0.75), and among all patients was positively correlated with fish intake frequency but this did not reach significance (r = +0.28, p=0.12). Change in erythrocyte fatty acid compositions for patients with baseline and endpoint values are presented in Table 3. For each of the primary measures, the main effect of Time was significant, and the main effect of Dose and the Time × Dose interaction were not significant. EPA+DHA composition increased significantly in low-dose (+57%, p=0.0001) and high-dose (+62%, p=0.003) groups. Endpoint EPA+DHA composition was 7.3%±0.6% for low-dose and 7.9%±1.3% for high-dose (p=0.70), and all patients exhibited EPA+DHA composition ≥4.0%. Endpoint EPA+DHA composition in low-dose (+42%, p<0.0001) and high-dose (+46%, p=0.0002) groups were significantly greater than healthy controls. The AA/DHA ratio decreased significantly in low-dose (−57%, p<0.0001) and high-dose (−53%, p=0.0002) groups, and the AA/EPA+DHA ratio decreased significantly in low-dose (−64%, p<0.0001) and high-dose (−58%, p=0.0002) groups.
Table 3. Effects of 10 week fish oil supplementation on erythrocyte fatty acid composition in MDD patients.
| Fatty Acid1 | Baseline | Week 10 |
P-value2 |
|---|---|---|---|
| Low-Dose (n=7) | |||
| Palmitic acid (16:0) | 18.23 ± 1.03 | 18.45 ± 0.72 | 0.64 |
| Stearic acid (18:0) | 17.11 ± 0.62 | 17.04 ± 0.67 | 0.83 |
| Oleic acid (18:1 n-9) | 12.24 ± 0.50 | 12.11 ± 0.49 | 0.63 |
| Vaccenic acid (18:1 n- 7) |
1.28 ± 0.15 | 1.23 ± 0.25 | 0.67 |
| Linoleic acid (LA, 18:2n-6) |
11.31 ± 0.69 | 10.57 ± 0.95 | 0.12 |
| Homo-γ-linolenic (HGLA, 20:3n-6) |
1.75 ± 0.16 | 1.55 ± 0.13 | 0.02 |
| Arachidonic acid (AA, 20:4n-6) |
18.04 ± 0.79 | 15.29 ± 1.74 | 0.002 |
| Docosatetraenoic acid (22:4n-6) |
4.64 ± 0.50 | 3.55 ± 0.55 | 0.01 |
| Eicosapentaenoic acid (EPA, 20:5n-3) |
0.28 ± 0.16 | 1.95 ± 0.76 | 0.0003 |
| Docosapenaenoic acid (22:5n-3) |
2.28 ± 0.33 | 3.45 ± 0.71 | 0.003 |
| Docosahexaenoic acid (DHA, 22:6n-3) |
2.81 ± 0.66 | 5.36 ± 0.83 | 0.0001 |
| EPA+DHA | 3.09 ± 0.63 | 7.32 ± 1.47 | 0.0001 |
| AA:DHA | 6.73 ± 1.45 | 2.93 ± 0.67 | 0.0001 |
| AA:EPA | 95.4 ± 71.4 | 9.63 ± 5.86 | 0.009 |
| AA:EPA±DHA | 6.04 ± 1.06 | 2.20 ± 0.67 | 0.0001 |
| High-Dose (n=7) | |||
| Palmitic acid (16:0) | 17.55 ± 0.80 | 17.41 ± 0.54 | 0.71 |
| Stearic acid (18:0) | 17.26 ± 0.37 | 17.18 ± 0.43 | 0.72 |
| Oleic acid (18:1 n-9) | 12.61 ± 1.15 | 12.27 ± 0.70 | 0.52 |
| Vaccenic acid (18:1 n- 7) |
1.23 ± 0.20 | 1.11 ± 0.22 | 0.31 |
| Linoleic acid (LA, 18:2n-6) |
11.50 ± 0.84 | 10.37 ± 0.88 | 0.03 |
| Homo-γ-linolenic (HGLA, 20:3n-6) |
1.86 ± 0.22 | 1.41 ± 0.42 | 0.03 |
| Arachidonic acid (AA, 20:4n-6) |
18.75 ± 0.85 | 16.12 ± 2.43 | 0.02 |
| Docosatetraenoic acid (22:4n-6) |
4.54 ± 0.34 | 3.55 ± 0.66 | 0.005 |
| Eicosapentaenoic acid (EPA, 20:5n-3) |
0.29 ± 0.06 | 2.27 ± 2.05 | 0.03 |
| Docosapenaenoic acid (22:5n-3) |
2.11 ± 0.22 | 3.20 ± 0.79 | 0.004 |
| Docosahexaenoic acid (DHA, 22:6n-3) |
2.70 ± 0.46 | 5.61 ± 1.79 | 0.001 |
| EPA±DHA | 2.99 ± 0.47 | 7.88 ± 3.51 | 0.003 |
| AA:DHA | 7.08 ± 1.00 | 3.30 ± 1.62 | 0.0002 |
| AA:EPA | 66.7 ± 15.1 | 22.6 ± 31.2 | 0.006 |
| AA:EPA±DHA | 6.37 ± 0.84 | 2.65 ± 1.66 | 0.0002 |
Values are group mean fatty acid composition (wt % total fatty acids) ± S.D.
Two-tailed t-tests.
3.2.3. Depression symptom ratings
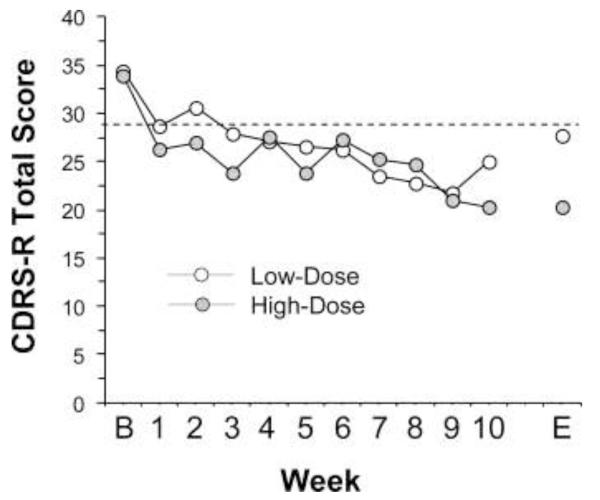
Baseline CDRS-R total scores in low-dose and high-dose groups did not differ significantly (p=0.94). Change in CDRS-R total scores over the 10 week trial are presented in Figure 2. For the CDRS-R mixed-effects model, the dose by time interaction was not significant (p=0.670). The baseline-last available CDRS-R total score declined significantly in the high-dose group (−40%, p=0.0001, d = 5.2) and there was a trend for a decrease in the low-dose group (−20%, p=0.063, d = 0.93). After removal of the n=3 patients in the low-dose group that did not complete the 10 week treatment trial, baseline CDRS-R total scores decreased significantly at endpoint (−27%, p=0.002, d = 2.2). At Week 10, 60% of patients in the low-dose group and 100% of patients in the high-dose group met criteria for symptom remission (CDRS-R total score ≤28), and 40% of patients in the low-dose group and 100% of patients in the high-dose group met criteria for response (≥50% baseline-endpoint decline in CDRS-R total score). Among all completers (n=14), the baseline-endpoint change in CDRS-R total scores was not significantly correlated with baseline erythrocyte EPA+DHA (r = +0.17, p=0.56) and DHA (r = −0.03, p=0.92), or AA/EPA+DHA (r = −0.03, p=0.89) and AA/EPA (r = −0.01, p=0.98) ratios. Moreover, the baseline-endpoint change in CDRS-R total scores were not significantly correlated with baseline-endpoint change in erythrocyte EPA+DHA (r = −0.04, p=0.88), DHA (r = −0.01, p=0.95), or AA/EPA+DHA (r = −0.13, p=0.66) and AA/EPA (r = −0.02, p=0.93) ratios.
Figure 2.
Mean depression symptom severity scores (CDRS-R total score) in MDD patients treated with low-dose (2.4 g/d, n=10) or high-dose (16.2 g/d, n=7) FO over the 10 week trial. B = baseline, E = mean endpoint score including the last available CDRS-R score carried forward for the n=3 patients in the low-dose group with early termination on weeks 3, 6, and 9. Dotted line demarks symptom remission (CDRS-R total score of 28).
3.2.4. Tolerability and safety assessments
Baseline-endpoint changes in vital signs and labs are presented in Table 4. The main effects of Time and Dose, and the Time x Dose interaction, were not significant for any measure. The most commonly reported adverse events at any point post-baseline over the course of the 10 week trial are presented in Table 5. Among all patients, the most frequently reported adverse events were headache, nasal congestion, decreased appetite, nausea, and lethargy which were rated as mild or moderate in severity. Headache, abdominal pain, and vomiting were more frequently reported (p<0.01) by subjects in the high-dose group than the low-dose group, and difficulty staying asleep, dizziness and dizziness when standing, increased appetite, and joint aches were more frequently reported by subjects in the low-dose group. Baseline YMRS total scores in low-dose and high-dose groups did not differ significantly (p=0.86). YMRS total scores declined significantly in the high-dose group (−77%, p=0.004) and there was a similar trend in the low-dose group (−23%, p=0.37).
Table 4. Effects of 10 week fish oil supplementation on vital signs and laboratory measures in MDD patients.
| Variable1 | Baseline | Week 10 |
P- value2 |
|---|---|---|---|
| Low-Dose (n=7) | |||
| Height (cm) | 164.8 ± 12.2 | 165.0 ± 12.7 | 0.97 |
| Weight (kg) | 54.6 ± 3.8 | 55.5 ± 2.3 | 0.43 |
| BMI (kg/m2) | 20.2 ± 2.3 | 20.8 ± 2.8 | 0.68 |
| BMI z score | −0.04 ± 1.0 | 0.20 ± 0.9 | 0.66 |
| BMI percentile | 47.7 ± 32.0 | 54.1 ± 28.8 | 0.72 |
| Heart rate (bpm) | 79.1 ± 10.4 | 74.3 ± 13.1 | 0.84 |
| Blood Pressure (mmHg) | □ | □ | |
| Systolic | 112.6 ± 9.4 | 118.1 ± 9.5 | 0.26 |
| Diastolic | 64.9 ± 12.5 | 72.1 ± 8.9 | 0.21 |
| Temperature (Celsius) | 36.8 ± 0.4 | 37.0 ± 0.3 | 0.37 |
| TSH (mIU/L) | 1.9 ± 0.7 | 1.7 ± 0.5 | 0.72 |
| WBC (K/uL) | 6.3 ± 0.6 | 6.2 ± 1.0 | 0.84 |
| RBC (M/uL) | 4.5 ± 0.7 | 4.5 ± 0.6 | 0.97 |
| Platelets (K/uL) | 227.5 ± 61.2 | 229.0 ± 70.5 | 0.96 |
| High-Dose (n=7) | |||
| Height (cm) | 162.7 ± 18.6 | 159.3 ± 15.3 | 0.72 |
| Weight (kg) | 67.1 ± 25.7 | 72.6 ± 26.3 | 0.67 |
| BMI (kg/m2) | 24.8 ± 8.1 | 25.9 ± 7.9 | 0.79 |
| BMI z score | 0.9 ± 1.2 | 1.3 ± 0.7 | 0.49 |
| BMI percentile | 72.9 ± 34.9 | 86.4 ± 14.3 | 0.36 |
| Heart rate (bpm) | 80.4 ± 8.7 | 81.1 ± 4.9 | 0.85 |
| Blood Pressure (mmHg) | □ | □ | |
| Systolic | 117.6 ± 12.8 | 110.6 ± 10.3 | 0.28 |
| Diastolic | 70.0 ± 8.2 | 66.6 ± 6.9 | 0.42 |
| Temperature (Celsius) | 36.3 ± 0.6 | 36.8 ± 0.5 | 0.12 |
| TSH (mlU/L) | 1.8 ± 0.9 | 2.1 ± 1.1 | 0.52 |
| WBC (K/uL) | 7.4 ± 3.4 | 7.4 ± 2.4 | 0.97 |
| RBC (M/uL) | 4.6 ± 0.4 | 4.7 ± 0.3 | 0.87 |
| Platelets (K/uL) | 291.4 ± 48.7 | 289.3 ± 51.1 | 0.94 |
Values are group mean ± S.D. (see Methods for abbreviations)
Two-tailed t-test.
Table 5. Incidence of common adverse events: during fish oil supplementation.
| Adverse Event1 | Low+High (n=17) |
Low- Dose (n=10) |
High- Dose (n=7) |
P- value2 |
|---|---|---|---|---|
| Headache | 86 | 100 | 71 | 0.001 |
| Nasal congestion | 75 | 78 | 71 | 0.33 |
| Appetite Decrease | 69 | 67 | 71 | 0.65 |
| Nausea | 63 | 56 | 71 | 0.04 |
| Lethargy | 63 | 56 | 71 | 0.04 |
| Drowsiness | 62 | 67 | 57 | 0.19 |
| Abdominal pain | 58 | 44 | 71 | 0.002 |
| Difficulty concentrating | 56 | 56 | 57 | 0.99 |
| Difficulty staying asleep | 55 | 67 | 43 | 0.001 |
| Difficulty falling asleep | 49 | 56 | 43 | 0.09 |
| Diarrhea | 37 | 44 | 29 | 0.04 |
| Difficulty waking up | 37 | 44 | 29 | 0.04 |
| Vomitting | 33 | 22 | 43 | 0.002 |
| Dizziness when standing | 29 | 44 | 14 | 0.001 |
| Appetite Increase | 29 | 44 | 14 | 0.001 |
| Dizziness | 29 | 44 | 14 | 0.001 |
| Joint aches | 29 | 44 | 14 | 0.001 |
Percentage of subjects in the intent-to-treat sample with any post-baseline instance of the event during the 10 week trial.
Two-tailed Chi-Square test (Low-dose vs. High-dose).
4. Discussion
This study investigated the relationship between LCn-3 fatty acids status and residual depressive symptoms in adolescents with SSRI-resistant MDD. We found that SSRI-resistant patients exhibited robust DHA deficits compared with healthy adolescents. Furthermore, 10-week adjunctive FO supplementation significantly increased erythrocyte EPA+DHA composition and decreased depressive symptom severity scores in the high-dose group, and there was a trend for a decrease in the low-dose group. In the intent-to-treat sample, symptom remission was observed in 40% of patients in the low-dose group and 100% of patients in the high-dose group. There were no significant baseline-endpoint changes in BMI, blood counts, thyroid stimulating hormone concentrations, or vital signs. Adverse events including headache and gastrointestinal symptoms were rated as mild to moderate and did not result in discontinuation of treatment. Neither dose was associated with the development of manic or hypomanic symptoms as assessed by YMRS total score. Together, these findings demonstrate that adolescents with SSRI-resistant MDD exhibit robust DHA deficits, and suggest that FO supplementation is safe and efficacious for increasing erythrocyte EPA+DHA levels and augmenting SSRI antidepressant effects.
This preliminary study has several important limitations. First, although we observed statistically significant baseline-endpoint changes with large effect sizes for our primary outcome measures, the relatively small number of subjects randomized to each treatment group may not be a representative sample of adolescent SSRI-resistant MDD patients. Second, the intervention trial design was open-label, and the observed baseline-endpoint changes in primary outcome measures should be viewed as preliminary given the potential for a placebo effect. However, the present findings are consistent with previous placebo-controlled trials finding that adjunctive LCn-3 fatty acid supplementation augmented SSRI efficacy in adult MDD patients [38,39]. Third, the duration of FO supplementation was relatively short (10 weeks), and greater changes in primary outcome measures may occur with a longer supplementation period. However, the 10 week duration was selected based in part on a non-human primate study finding that approximately 10 weeks of FO supplementation was required to normalize cortical DHA levels from a deficient state [52]. Nevertheless, a larger and longer placebo-controlled study is warranted to extend and confirm the present findings.
The mean erythrocyte EPA+DHA composition observed in healthy adolescents was 4.3%, which is similar to that observed in a large cohort of healthy subjects residing in the United States (4.5%)[21]. The mean erythrocyte EPA+DHA composition observed at baseline in adolescent MDD patients was 3.1%, and 90% of MDD patients exhibited an erythrocyte EPA+DHA composition of ≤4.0% compared with 40% of healthy controls. The latter finding is consistent with previous studies finding that erythrocyte EPA+DHA composition of ≤4.0% is more prevalent among adult and adolescent MDD patients than controls [26,27]. The observed EPA+DHA deficit was primarily attributable to robust DHA deficits, also consistent with previous observations [26,27]. This finding may take on additional significance because erythrocyte DHA composition is positively correlated with prefrontal cortex DHA composition [51,52], and lower DHA levels have been observed in the postmortem prefrontal cortex and anterior cingulate of adult MDD patients [53-55]. Moreover, imaging studies suggest that erythrocyte DHA levels are positively correlated with functional prefrontal activity [56] and neurometabolic integrity in the anterior cingulated [57] of healthy developing youth. Together these data suggest that the robust erythrocyte DHA deficits observed in SSRI-resistant MDD patients may be relevant to central neuropathological processes associated with MDD.
While the etiology of the lower DHA levels observed in adolescent MDD patients may be multifactorial [19], this deficit could not be attributed to group differences in age, BMI, or smoking status. Moreover, we previously found that chronic treatment with fluoxetine, resulting in clinically-relevant plasma fluoxetine concentrations, did not alter rat erythrocyte EPA or DHA levels [58]. Here we demonstrate that 10-week dietary FO supplementation is sufficient to increase erythrocyte DHA and EPA to levels similar to those observed in healthy adults residing in Japan [20]. This finding suggests that increasing the intake of preformed EPA+DHA is sufficient to correct the low EPA+DHA status observed in adolescent MDD patients. It is notable that the baseline-endpoint increase in erythrocyte EPA+DHA composition was similar in both low- and high-dose FO groups. This finding is not consistent with prior studies finding that FO supplementation increases erythrocyte EPA+DHA levels in a dose-dependent manner [22,23]. Therefore, it is possible that compliance rates in the high-dose group were lower than indicated by self-report and bottle volumes. It is also notable that two patients in the low-dose group, and none in the high-dose group, experienced a worsening of depressive symptoms. Together these findings suggest that future FO supplementation trials in SSRI-resistant MDD patients should consider employing a flexible dose design with upward titration based on treatment response, as previously demonstrated in pediatric bipolar patients [16].
Consistent with some previous studies [26,59,60], but not others [61-63], DHA, EPA+DHA and the AA/EPA ratio were not significantly correlated with baseline CDRS-R total scores. However, this relationship may have been confounded by concomitant SSRI treatment and the fact that all MDD patients exhibited similar low EPA and DHA levels. Furthermore, baseline-endpoint change in CDRS-R total scores were not correlated with baseline or baseline-endpoint change in EPA+DHA, DHA, and the AA/EPA ratio. While these findings suggest that erythrocyte LCn-3 fatty acid status and depressive symptoms and treatment response are poorly correlated in SSRI-resistant MDD patients, larger studies are required to further evaluate this relationship.
In conclusion, this study found that adolescents with SSRI-resistant MDD exhibit robust erythrocyte DHA deficits compared with healthy adolescent controls. This study also demonstrated that FO supplementation is efficacious and well-tolerated for increasing erythrocyte EPA+DHA levels in adolescents with SSRI-resistant MDD, and is associated with reductions in residual depressive symptoms. Although this trial was open-label and employed a small sample size, the present findings suggest that low DHA status may represent a modifiable risk factor for SSRI-resistance in adolescent MDD patients. Larger placebo-controlled trials are warranted to further evaluate adjunctive FO as an option for treating residual depressive symptoms in SSRI-resistant MDD patients.
Highlights.
SSRI-resistant adolescent MDD patients exhibit robust erythrocyte long-chain omega-3 (LCn-3) fatty acid deficits.
Fish oil supplementation significantly increases erythrocyte LCn-3 fatty acid levels in MDD patients.
Fish oil supplementation is safe and well-tolerated and may augment SSRI efficacy.
Additional studies are warranted to further evaluate adjunctive fish oil supplmentation as an option for SSRI-resistant MDD patients.
Layman Summary.
An accumulating body of evidence has implicated dietary essential long-chain omega-3 (LCn-3) fatty acids in the pathophysiology and treatment of major depressive disorder (MDD). This study demonstrates that adolescents with SSRI-resistant MDD exhibit significant LCn-3 fatty acid deficits compared with healthy controls. It also demonstrates that fish oil supplementation is safe, well-tolerated, and effective for increasing LCn-3 fatty acid and is associated with reductions in depressive symptoms.
Acknowledgements
Supported in part by an investigator-initiated research grant from the Inflammation Research Foundation (IRF) to R.K.M., and DK59630 to P.T. The IRF and NIH did not have any role in the design, implementation, analysis or interpretation of the research. R.K.M. has received research support from Martek Biosciences Inc, Ortho-McNeil Janssen, NARSAD, and NIH, and was a member of the IRF scientific advisory board. M.P.D. has received research support from AstraZeneca, Eli Lilly, Johnson & Johnson, Shire, Ortho-McNeil Janssen, Pfizer, Otsuka, Shire, Amylin, Lundbeck, Novartis., Somerset, Sumitomo, Thrasher Foundation, GlaxoSmithKline, NARSAD, and NIMH, NIDA, NIAAA, and is a consultant for GlaxoSmithKline, Eli Lilly, Pfizer, and Merck. J.R.S. has received research support from Eli Lilly, Shire, Forest Research Laboratories, Lundbeck, and the American Academy of Child and Adolescent Psychiatry. The other authors do not have any conflicts to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].McNamara RK. Developmental long-chain omega-3 fatty acid deficiency and prefrontal cortex pathology in psychiatric disorders. In: Collins RO, Adams JL, editors. Prefrontal Cortex: Developmental Differences and Role in Neurological Disorders. Nova Science Publishers, Inc.; U.S.A.: 2013. pp. 1–38. [Google Scholar]
- [2].Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- [3].Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. British Journal of Psychiatry. 2004;184:404–408. doi: 10.1192/bjp.184.5.404. [DOI] [PubMed] [Google Scholar]
- [4].Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. American Journal of Clinical Nutrition. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- [5].Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. Journal of Clinical Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- [6].Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. Journal of Clinical Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- [7].Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. Journal of the American College of Nutrition. 2009;28:525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- [8].Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. Journal of Clinical Psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burke KC, Burke JD, Jr, Rae DS, Regier DA. Comparing age at onset of major depression and other psychiatric disorders by birth cohorts in five US community populations. Archives of General Psychiatry. 1991;48:789–795. doi: 10.1001/archpsyc.1991.01810330013002. [DOI] [PubMed] [Google Scholar]
- [10].Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- [11].Harel Z, Riggs S, Vaz R, White L, Menzies G. Omega-3 polyunsaturated fatty acids in adolescents: knowledge and consumption. Journal of Adolescent Health. 2001;28:10–15. doi: 10.1016/s1054-139x(00)00179-8. [DOI] [PubMed] [Google Scholar]
- [12].O’Sullivan TA, Ambrosini G, Beilin LJ, Mori TA, Oddy WH. Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition. 2011;27:153–159. doi: 10.1016/j.nut.2009.11.019. [DOI] [PubMed] [Google Scholar]
- [13].Sichert-Hellert W, Wicher M, Kersting M. Age and time trends in fish consumption pattern of children and adolescents, and consequences for the intake of long-chain n-3 polyunsaturated fatty acids. European Journal of Clinical Nutrition. 2009;63:1071–1075. doi: 10.1038/ejcn.2009.40. [DOI] [PubMed] [Google Scholar]
- [14].Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. European Journal of Clinical Nutrition. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- [15].Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. American Journal of Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- [16].Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. European Neuropsychopharmacology. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [17].Harris WS, von Schacky C, Park Y. Standardizing methods for assessing omega-3 fatty acid biostatus. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. Nova Science Publishers, Inc.; USA: 2013. pp. 385–398. [Google Scholar]
- [18].Fekete K, Marosvölgyi T, Jakobik V, Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. American Journal of Clinical Nutrition. 2009;89:2070S–2084S. doi: 10.3945/ajcn.2009.27230I. [DOI] [PubMed] [Google Scholar]
- [19].Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- [20].Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–135. [PubMed] [Google Scholar]
- [21].Harris WS, Pottala JV, Varvel SA, Borowski JJ, Ward JN, McConnell JP. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins. Leukotrienes and Essential Fatty Acids. 2013;88:257–263. doi: 10.1016/j.plefa.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [22].Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. American Journal of Clinical Nutrition. 2008;88:801–809. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]
- [23].Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. Journal of the American Heart Association. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive Medicine. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- [25].Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biological Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- [26].McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. Journal of Affective Disorders. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pottala JV, Talley JA, Churchill SW, Lynch DA, von Schacky C, Harris WS. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins. Leukotrienes and Essential Fatty Acids. 2012;86:161–165. doi: 10.1016/j.plefa.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [28].McNamara RK, Strawn JR. Role of long-chain omega-3 fatty acids in psychiatric practice. PharmaNutrition. 2013;1:41–49. doi: 10.1016/j.phanu.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Progress in Brain Research. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- [30].Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- [31].Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. Journal of Neurochemistry. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- [32].DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. Journal of Lipid Research. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- [33].Chalon S, Delion-Vancassel S, Belzung C, Guilloteau D, Leguisquet AM, Besnard JC, Durand G. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. Journal of Nutrition. 1998;128:2512–2519. doi: 10.1093/jn/128.12.2512. [DOI] [PubMed] [Google Scholar]
- [34].Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, Bodard S, Kousignian I, Belzung C, Chalon S. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. Journal of Lipid Research. 2008;49:340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- [35].Carlezon WA, Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biological Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- [36].Laino CH, Fonseca C, Sterin-Speziale N, Slobodianik N, Reinés A. Potentiation of omega-3 fatty acid antidepressant-like effects with low non-antidepressant doses of fluoxetine and mirtazapine. European Journal of Pharmacology. 2010;648:117–126. doi: 10.1016/j.ejphar.2010.08.047. [DOI] [PubMed] [Google Scholar]
- [37].Lakhwani L, Tongia SK, Pal VS, Agrawal RP, Nyati P, Phadnis P. Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Poloniae Pharmaceutica. 2007;64:271–276. [PubMed] [Google Scholar]
- [38].Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. Journal of Clinical Psychopharmacology. 2012;32:61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Australian & New Zealand Journal of Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- [40].Kennard B, Silva S, Vitiello B, Curry J, Kratochvil C, Simons A, Hughes J, Feeny N, Weller E, Sweeney M, Reinecke M, Pathak S, Ginsburg G, Emslie G, March J, TADS Team Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS) Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- [41].Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J. A doubleblind, randomized placebo-controlled trial of fluoxetine in depressed children and adolescents with depression. Archives of General Psychiatry. 1997;54:1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- [42].Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Archives of General Psychiatry. 2002;59:225–231. doi: 10.1001/archpsyc.59.3.225. [DOI] [PubMed] [Google Scholar]
- [43].Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. Journal of Child Psychology and Psychiatry. 2006;47:372–394. doi: 10.1111/j.1469-7610.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- [44].Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- [45].Sorgi PJ, Hallowell EM, Hutchins HL, Sears B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutrition Journal. 2007;6:16. doi: 10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. Journal of Child and Adolescent Psychopharmacology. 2010;20:513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Poznanski EO, Cook SC, Carroll BJ, Corzo H. Use of the Children’s Depression Rating Scale in an inpatient psychiatric population. Journal of Clinical Psychiatry. 1983;44:200–203. [PubMed] [Google Scholar]
- [48].Klein RG, Abikoff H, Barkley RA, et al. Clinical trials in children and adolescents. In: Prien RF, Robinson DS, editors. Clinical Evaluation of Psychotropic Drugs: Principles and Guidelines. Raven; New York: 1994. [Google Scholar]
- [49].Kinrys G. Hypomania associated with omega3 fatty acids. Archives of General Psychiatry. 2000;57:715–716. doi: 10.1001/archpsyc.57.7.715-a. [DOI] [PubMed] [Google Scholar]
- [50].Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- [51].Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Research Bulletin. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- [52].Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. The Journal of Lipid Research. 1990;31:237–247. [PubMed] [Google Scholar]
- [53].Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2010;82:111–119. doi: 10.1016/j.plefa.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biological Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- [55].McNamara RK, Jandacek R, Tso P, Dwivedi Y, Ren X, Pandey GN. Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. Journal of Psychiatric Research. 2013;47:1187–1191. doi: 10.1016/j.jpsychires.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McNamara RK, Able JA, Jandacek R, Rider T, Tso P, Eliassen JC, Alfieri D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. American Journal of Clinical Nutrition. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].McNamara RK, Jandacek R, Rider T, Tso P, Weber W, Chu W-J, Strakowski SM, Adler CM, DelBello MP. Low docosahexaenoic acid status is associated with reduced indices of cortical integrity in the anterior cingulate of healthy male children: A 1H MRS study. Nutritional Neuroscience. 2013;16:183–190. doi: 10.1179/1476830512Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McNamara RK, Able JA, Rider T, Jandacek R, Tso P. Effect of chronic fluoxetine treatment on male and female rat erythrocyte and prefrontal cortex fatty acid composition. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:1317–1321. doi: 10.1016/j.pnpbp.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One. 2010;5:e10635. doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu JJ, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, Mann JJ, Sublette ME. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. Journal of Clinical Psychiatry. 2013;74:732–738. doi: 10.4088/JCP.12m07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- [62].Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- [63].Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. Journal of Affective Disorders. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]