Abstract
Associations between brain cortical tissue volume and cognitive function in old age are frequently interpreted as suggesting that preservation of cortical tissue is the foundation of successful cognitive aging. However, this association could also, in part, reflect a lifelong association between cognitive ability and cortical tissue. We analyzed data on 588 subjects from the Lothian Birth Cohort 1936 who had intelligence quotient (IQ) scores from the same cognitive test available at both 11 and 70 years of age as well as high-resolution brain magnetic resonance imaging data obtained at approximately 73 years of age. Cortical thickness was estimated at 81 924 sampling points across the cortex for each subject using an automated pipeline. Multiple regression was used to assess associations between cortical thickness and the IQ measures at 11 and 70 years. Childhood IQ accounted for more than two-third of the association between IQ at 70 years and cortical thickness measured at age 73 years. This warns against ascribing a causal interpretation to the association between cognitive ability and cortical tissue in old age based on assumptions about, and exclusive reference to, the aging process and any associated disease. Without early-life measures of cognitive ability, it would have been tempting to conclude that preservation of cortical thickness in old age is a foundation for successful cognitive aging when, instead, it is a lifelong association. This being said, results should not be construed as meaning that all studies on aging require direct measures of childhood IQ, but as suggesting that proxy measures of prior cognitive function can be useful to take into consideration.
Keywords: cognitive ability, cognitive aging, cortical thickness, intelligence, IQ
Introduction
Understanding human cognitive aging is one of the greatest scientific challenges to society today. A better understanding of why people age differently with respect to cognitive function requires research on the aging brain. Various structural and functional aspects of the brain have been associated with cognitive function at different stages in life,1 and prominent among these is cortical thickness.2, 3, 4, 5, 6 However, especially in old age, it is difficult to establish a clear causal connection between cortical thickness and cognitive function. It is certainly possible that retaining cortical thickness is a foundation for successful cognitive aging. Indeed, many researchers showing brain–cognitive ability associations in old age will offer this interpretation of their results.7 Nevertheless, there are other possibilities, including the major confound of a long-standing trait association between cognitive ability and cortical thickness across the lifespan.8
One way to disambiguate these possibilities is to test the association between brain cortical thickness and cognitive ability in old age with either variable also available from a much younger age. Obviously, magnetic resonance imaging (MRI) data from youth is not available for elderly subjects. We therefore examined the association between cortical thickness and cognitive ability in a large sample of community-dwelling older people. This is the Lothian Birth Cohort 1936 (LBC1936), in whom data from the same well-validated Moray House Test (MHT) of general cognitive ability (intelligence) are available in both youth (∼11 years) and older age (∼70 years).9 In addition, because these subjects were all born in the same year and were MRI scanned at almost the same age (∼73 years), the data does not suffer from the confounding effect that large differences in chronological age can have on the association between brain structure and cognitive ability.10, 11
To examine the hypothesis that the lifetime-stable trait of intelligence12 may be related to later life brain structure, we first tested for any cross-sectional association between cortical thickness and intelligence in old age. We then tested for associations between cortical thickness in old age and the same subject's intelligence measured in youth. Next, we examined the cross-sectional association between cortical thickness and intelligence in old age after covarying for intelligence from youth. Any cross-sectional cortical thickness–intelligence association in old age remaining after adjusting for intelligence in youth would provide rarely available evidence for a contribution of cortical thickness to age-related cognitive changes. On the other hand, if the cross-sectional cortical thickness–intelligence association is substantially attenuated or disappears after adjusting for childhood intelligence, it could indicate that: the brain–intelligence association in old age reflects a lifelong impact of cortical thickness on cognitive functions; intelligence in childhood influences neurodevelopmental processes, including into old age (reverse causation); or lifelong associations behind cortical thickness and intelligence are due to shared causes.
Materials and methods
Sample
Participants were all born in 1936 and are members of the LBC1936. At approximately 11 years of age, participants were tested on the MHT No. 12 of general cognitive ability in the Scottish Mental Survey of 1947 (SMS1947).13, 14 Recruitment and retesting, including on the MHT, of surviving members of the SMS1947 from the Edinburgh area began in 2004 (Wave One testing), with 1091 eventually comprising the final sample.9, 10 Starting in 2007 (hereinafter referred to as Wave Two), 866 of these subjects were invited to have a detailed structural brain MRI scan.11
From a total of 866 Wave Two participants, 732 consented to MRI scanning, with 666 undergoing structural brain imaging of high enough resolution for adequate cortical thickness estimates. Of these, five individuals were excluded because of a Mini-Mental State Examination15 score of less than 24 or a history of dementia. A further 39 subjects did not have an age-11 intelligence quotient (IQ) score or age-70 IQ score, and 2 more subjects were removed because their age-11 IQ was more than 3 standard deviations below their age-70 IQ (that is, their age-11 IQ was between 42 and 44 but their age-70 IQ was above 100), suggesting some form of error. From the remaining subjects, 32 failed visual quality control of the gray and white matter surfaces (see image processing section for further details), leaving a final sample size of 588 (280 females/308 males) with a mean age±s.d. of 72 years and 8 months±8.8 months at the time of imaging (referred to as age 73 years). For this sample, the mean age of testing for age-11 IQ was 10 years and 11.2 months±3.3 months, whereas the mean age of testing for age-70 IQ was 69 years and 6.2 months±10 months.
Cognitive testing
The MHT is a reliable test of general cognitive ability comprising items aimed at assessing language comprehension, verbal and non-verbal reasoning, spatial ability and simple calculations. Here, age-adjusted MHT raw scores were used to derive IQ-type scores (mean=100, s.d.=15). MHT-derived IQ scores have high concurrent validity with ‘gold-standard' tests of intelligence such as the Stanford-Binet in childhood and old age and Wechsler Adult Intelligence Scale-III in old age.16
Whereas the MHT is sensitive to age decline, it is likely to be less subject to it than some memory function tests well known for their age sensitivity. Given this and the 3-year gap between MRI scanning at age 73 years and IQ testing at age 70 years, all analyses were replicated using a measure of memory function at age 73 years derived from known age-sensitive subtests of the third edition of the Wechsler Memory Scale17 (for further details, see section 1 of Supplementary Information).
MRI acquisition protocol
All MRI data were acquired using a GE Signa Horizon HDxt 1.5 T clinical scanner (General Electric, Milwaukee, WI, USA) equipped with a self-shielding gradient set (33 mT m−1 maximum gradient strength) and manufacturer supplied eight-channel phased-array head coil. The examination comprised a high-resolution whole-brain T1-weighted (T1W) volume sequence acquired in the coronal plane; for full details of the complete LBC1936 MRI protocol, see Wardlaw et al.11 In brief, the T1W volume scan was acquired with a field of view of 256 × 256 mm2, an acquisition matrix of 192 × 192 (zero-filled to 256 × 256) and 160 contiguous 1.3-mm thick slices giving a final voxel dimension of 1 × 1 × 1.3 mm3. The repetition, echo and inversion times were 10, 4 and 500 ms, respectively. To allow accurate measurement of the intracranial volume, slices were carefully placed to cover the complete intracranial contents from above the skull vertex to the upper cervical spine below the foramen magnum.
Image processing
In order to obtain local cortical thickness measurements for each subject, all T1W volume scans were processed by the CIVET pipeline (version 1.1.12) developed at the Montreal Neurological Institute (http://www.bic.mni.mcgill.ca) for fully automated structural image analysis. The CIVET pipeline processing steps were implemented using the Canadian Brain Imaging Network18 (http://www.cbrain.mcgill.ca). Steps, detailed elsewhere,4 include: (1) registering T1W images to a standardized space using an age-specific template for the population under study; (2) correcting for intensity nonuniformity artifacts (bias field); (3) producing high-resolution hemispheric surfaces with 40 962 vertices each; (4) registering surfaces to a high-resolution template to establish inter-subject correspondence of vertices; (5) applying a reverse of step 1 to allow cortical thickness estimations in the native space of each subject; (6) calculating cortical thickness at each vertex using the t-link metric; and (7) smoothing using a 20-mm kernel.
As a final step, and blinded to each subject's demographic and cognitive characteristics, visual quality control of the native cortical gray and white matter surfaces was implemented to make sure that there were no important aberrations in cortical thickness estimations for a given participant. Subjects in whom there were obvious problems with the cortical thickness maps because of ringing or other such artifacts (mainly caused by movement in the scanner) were eliminated from further analysis.
Statistical analyses
Statistical analyses were performed using SurfStat (http://www.math.mcgill.ca/keith/surfstat), a statistical tool for analyzing surface-based data, implemented in MATLAB 7 (http://www.mathworks.com). As a first step, we used multiple regression to examine each subject's absolute native-space cortical thickness at each vertex measured at the age of approximately 73 years against their age-70 IQ scores. This was done while accounting for the effects of gender, precise age at scanning in days (in order to further account for any residual age effect), and a measure of brain volume that does not overcorrect for the local effects of cortical thickness (for details, see appendix of Reference 3). In a second step, the same analysis was performed after replacing age-70 IQ by age-11 IQ. In a third step, the first analysis was repeated but age-11 IQ was added as a covariate in the model. The first and third steps were redone after replacing age-70 IQ by age-73 memory function.
Finally, in order to assess whether or not there were gender differences in the association between cortical thickness and age-70 IQ, a ‘Gender by age-70 IQ' interaction term was added to the original first step model (see above paragraph). A similar assessment was done for age-11 IQ where, instead, a ‘Gender by age-11 IQ' interaction term was added to the original second step model. Further, although the main analysis was conducted on the group as a whole, males and females were also analyzed separately.
Thresholds of significance for the resulting t-test values of the regressor coefficients were calculated by taking into account multiple comparisons through false discovery rate;19 a method that controls for the proportion of false positives among one's discoveries. For instance, under a false discovery rate threshold of 0.05, it is expected that one positive finding out of every 20 on a brain map will be a false positive. Resultant false discovery rate thresholded statistical maps were projected on an average surface template for the purpose of visualization.
Known IQ range restriction in the LBC1936 sample12 could lead to overly conservative findings. For this reason, corrected results are also provided using the method described by Wiberg and Sundström20 (for further details, see section 2 of Supplementary Information). Note that for the sake of clarity and simplicity of presentation, whereas figures presented in the main manuscript are uncorrected for IQ range restriction, corrected figures are provided as Supplementary Information.
Results
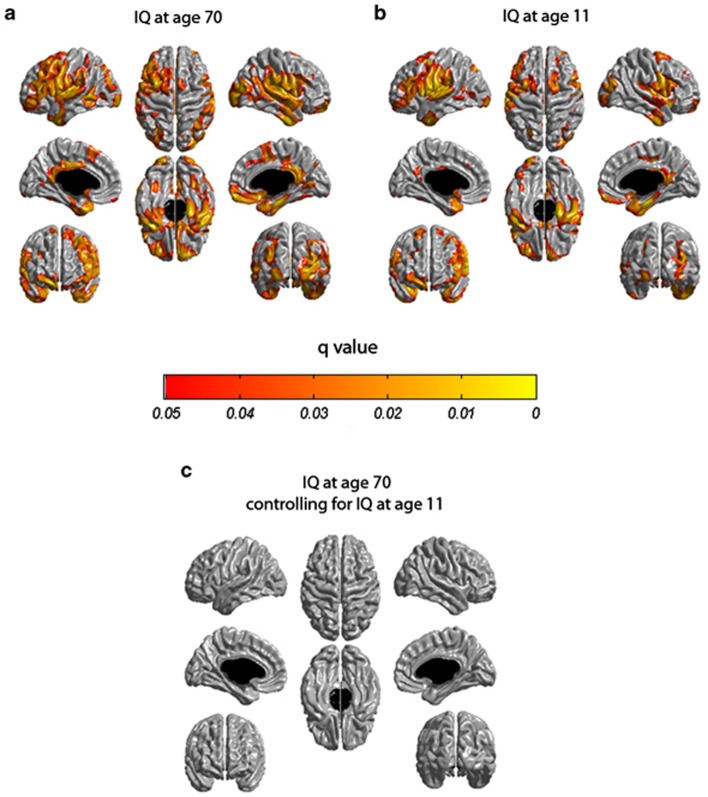
An extensive pattern of distributed, significant associations was observed between age-73 cortical thickness and age-70 IQ. There were significant associations in bilateral ventrolateral prefrontal areas, insular, cingulate and lateral occipital cortices as well as in regions of the temporal lobe including the planum temporale and parahippocampal gyri (Figure 1a; see Supplementary Figure 1A for results corrected for IQ range restriction). Regressing age-11 IQ against age-73 cortical thickness revealed a very similarly distributed pattern of significant associations (Figure 1b; see Supplementary Figure 1B for results corrected for IQ range restriction). In keeping with previous reports,4, 5 significant associations between cortical thickness and IQ were in the small to medium range of effect sizes, depending on the exact cortical location. The uncorrected Pearson correlation ranges were: 0.11–0.30 for age-11 IQ and 0.10–0.30 for age-70 IQ. However, the corrected (for range restriction) Pearson correlation ranges were: 0.15–0.40 for age-70 IQ and 0.15–0.41 for age-11 IQ. See Supplementary Table 1 for a list of significant correlations and their associated regions.
Figure 1.
Results of cortical thickness at age 73 years regressed against intelligence quotient (IQ) scores from the Moray House Test at 11 and 70 years of age. A false discovery rate threshold of 0.05 is used to control for multiple comparisons. Colors, representing q values, are superimposed on an average surface template. Results are corrected for gender, age in days at brain scanning and a measure of brain volume. (a) Cortical thickness at age 73 years is regressed against age-70 IQ. (b) Cortical thickness at age 73 years is regressed against age-11 IQ. (c) The same cortical thickness but regressed against age-70 IQ after adjusting for age-11 IQ.
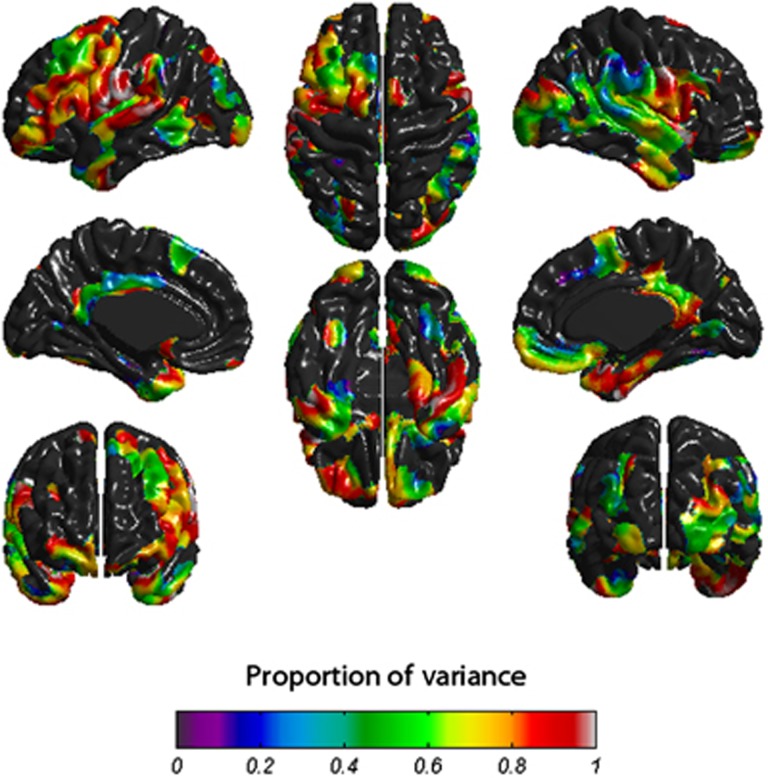
Repeating the analysis between age-73 cortical thickness and age-70 IQ with age-11 IQ as a covariate left no significant associations (Figure 1c). Within regions where there was an observed association between age-73 cortical thickness and age-70 IQ, a mean of 67.8% (mean value across the cortex) of this association was accounted for by age-11 IQ (Figure 2).
Figure 2.
Proportion of the association between age-70 intelligence quotient (IQ) and age-73 cortical thickness that is accounted for by age-11 IQ.
Findings for age-73 memory function mirrored those observed for age-70 IQ. Here too, repeating the analysis between age-73 cortical thickness and age-73 memory function after controlling for age-11 IQ left no significant associations. Further, within regions where there was an observed association between age-73 cortical thickness and age-73 memory function, a mean of 60.9% (mean value across the cortex) of this association was accounted for by age-11 IQ (see Supplementary Figures 2 and 3).
The patterns of age-70 and age-11 IQ associations with cortical thickness were similar, for both males and females, to those observed for the group as a whole. After accounting for multiple comparisons, associations were found to be significant only in males. Importantly, there were no significant ‘Gender by age-70 IQ' or ‘Gender by age-11 IQ' interactions anywhere on the cortex.
The Pearson correlation between MHT-derived IQ scores at age-11 and age-70 was 0.68 (P<0.001; the correlation between MHT-derived IQ scores at age-11 and memory function at age-73 was 0.51 (P<0.001)). In light of such a high correlation, it may appear unavoidable that these variables will produce similar cortical thickness maps. However, this is far from the case as such correlations indicate that there is substantial unshared variance between the variables. See section 3 of Supplementary Information for results of a simulation where normally distributed noise was added to age-70 IQ in order to generate a variable that correlates 0.68 with age-70 IQ. In contrast to age-11 IQ, the simulated variable showed no association with cortical thickness. Further, controlling for the simulated variable did not remove age-70 IQ associations with cortical thickness (see Supplementary Figure 4).
Discussion
The key novel finding here is that, in addition to having predictive value for cortical thickness over 60 years later, intelligence at 11 years of age accounts for more than two-third of the cross-sectional association between intelligence and cortical thickness in old age in many brain regions frequently reported to be involved in intelligence differences.21 Without early-life measures of cognitive ability, it would have been tempting to conclude that preservation of cortical thickness in old age is the main mechanism of successful cognitive aging when, instead, it is a lifelong association. This finding constitutes compelling evidence for a generally disregarded association between early cognitive development and the aging brain.
These results should not be construed, of course, as meaning that studies on the effects of aging on the brain and cognition absolutely require childhood measures of cognitive ability but rather as suggesting that proxy measures of premorbid function can be useful to take into consideration. One such example is the National Adult Reading Test (NART)/(NART-R).22, 23 Indeed, scores on the NART administered in old age have been shown to provide a good estimate of childhood IQ as well as of premorbid IQ.24, 25
Cognitive ability has been shown in previous work to be positively associated with cortical thickness in many of the same regions as found here in a completely independent sample of children.4 Therefore, our findings further suggest that each person's rank order in terms of cortical thickness at age 11 years may be mirrored at age 73 years despite the fact that the cortex is known generally to thin slowly throughout adulthood.26
Finding a positive association between IQ in childhood and cortical thickness in old age is also compatible with reports that those with a higher childhood IQ have a delayed onset or decreased chance of developing dementia in old age.27 Indeed, as dementia is associated with widespread and progressive cortical atrophy,28 those with a thicker cortex may have more time until cortical thinning has progressed to such an extent that efficient cognitive processing becomes significantly attenuated.
There are plausible reasons for having age-11 IQ substantially accounting for the association between IQ and brain cortical thickness in old age:
First, there is the possibility of a lifetime association between the traits, partly via genetic factors. Given the heritability of cortical thickness,29 this would be compatible with results from recent genome-wide association studies, which show that genetic factors affecting intelligence in childhood also affect intelligence in old age.30 Thus, it is possible that genes influence initial cortical growth and its maintenance across life in areas relevant for cognitive ability differences. This would be in keeping with a previous report showing that children and adolescents with average IQ have a different cortical thickness developmental trajectory than those with high IQ.5 Less directly, it is also possible that genetic factors may be responsible for a lifetime confounding effect if the same genes affect both cortical thickness and cognitive ability via a third factor or set of factors.
Second, it could be speculated that the association between childhood IQ and cortical thickness in old age is present because individuals with high IQ tend to keep more intellectually and physically active throughout life than those with lower IQ. This form of reverse causation is compatible with findings of increases in local cortical gray matter volume and thickness secondary to the practice of given tasks31, 32, 33, 34 and with the fact that people with a higher IQ tend to stay in school longer.35 Here too, however, a confounding effect is possible, potentially brought about by, for instance, environmental, including intrauterine, factors that influence both cortical thickness and IQ.
Finally, it is possible, of course, that different effects coexist and that there is a set of reciprocal, dynamic associations between cortical thickness and IQ. For example, greater cortical thickness may lead to greater IQ, which, in turn, may foster an increased propensity for stimulating activities and corollary cortical growth. Similarly, a higher IQ may be associated with healthier lifestyle choices and better understanding of and regard for health messages and the consequent avoidance of risk factors resulting in better maintenance of brain structure and, hence, cognition in old age. The end results of these types of dynamic feedback loops could be a developmental trajectory leading to a thicker cortex in old age for those having a higher IQ in childhood.
Our results do not apply to subjects with incipient dementia, in whom cortical loss is known to be accelerated. The MRI scans and measures of old age cognitive ability were acquired 3 years apart. Although this could result in changes in cognitive ability that are not accounted for, the availability of data from the same cognitive test across a gap of almost 60 years as well as the similarity of findings for age-73 memory function, estimated at the same age as scanning, greatly outweigh this concern; these data therefore provide a rare opportunity to examine changes in cognitive ability across most of the human lifespan. Further strengths of the study, all of which contributed to high statistical power and robust estimates of effects, include: a large sample of people; blinded quality-controlled imaging and cognitive assessment methods; and participants who were all scanned at the same age, thereby minimizing the confounding effect of chronological age differences.
Understanding associations between brain structure and cognitive function are crucial for addressing life course changes in cognitive ability. The present study demonstrates a long-standing association, accounting for what might otherwise have seemed like a compelling association within old age.
Acknowledgments
This research has been made possible by the use of computing resources provided by Compute/Calcul Canada. We thank the LBC1936 participants; Caroline Brett, Michelle Taylor and Caroline Cameron for data collection; the LBC1936 Study Secretary, Paula Davies; and the nurses, radiographers and other staff at the Wellcome Trust Clinical Research Facility, Edinburgh (http://www.wtcrf.ed.ac.uk). This work was supported by a Research into Ageing programme grant (to I.J.D. and J.M.S.) and the Age UK-funded Disconnected Mind project (http://www.disconnectedmind.ed.ac.uk; to I.J.D., J.M.S. and J.M.W.), with additional funding from the UK Medical Research Council (to I.J.D., J.M.S., J.M.W. and M.E.B.). J.M.W. is supported by the Scottish Funding Council through the SINAPSE Collaboration (http://www.sinapse.ac.uk). S.K. is supported by the Fonds de la Recherche en Santé du Québec. Imaging was performed at the Brain Research Imaging Centre, University of Edinburgh (http://www.bric.ed.ac.uk), a centre in the SINAPSE Collaboration. The work was undertaken within The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology (http://www.ccace.ed.ac.uk), part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the UK Biotechnology and Biological Sciences Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, and the Medical Research Council is gratefully acknowledged. We thank Paul Redmond for his technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cerebral Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, et al. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage. 2011;55:1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ad-Dab'bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, et al. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot RK, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, DeYoung CG, Lee MJ, Lee JM, et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 2008;28:10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. J NeuropsychiatryClin Neurosci. 2011;23:6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Gow AJ, Taylor MD, Corley J, Brett C, Wilson V, et al. The Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:28. doi: 10.1186/1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort profile: The Lothian Birth Cohorts of 1921 and 1936. Int J Epidemiol. 2012;41:1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Bastin ME, Valdes Hernandez MC, Maniega SM, Royle NA, Morris Z, et al. Brain aging, cognition in youth and old age and vascular disease in the Lothian Birth Cohort 1936: rationale, design and methodology of the imaging protocol. Int J Stroke. 2011;6:547–559. doi: 10.1111/j.1747-4949.2011.00683.x. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Johnson W, Pattie A, Brett CE, Roberts B, Starr JM, et al. Stability and change in intelligence from age 11 to ages 70, 79, and 87: the Lothian Birth Cohorts of 1921 and 1936. Psychol Aging. 2011;26:232–240. doi: 10.1037/a0021072. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Starr JM. A Lifetime of Intelligence: Follow-up Studies of the Scottish Mental Surveys of 1932 and 1947. American Psychological Association: Washington; 2009. [Google Scholar]
- Scottish Council for Research in Education . The Trend of Scottish Intelligence: A Comparison of the 1947 and 1932 Surveys of the Intelligence of Eleven-Year-Old Pupils. University of London Press Ltd: London; 1949. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging. Psychol Aging. 2010;25:219–228. doi: 10.1037/a0017750. [DOI] [PubMed] [Google Scholar]
- Wechsler D.WMS-III (UK) Administration and Scoring Manual3rd ednThe Psychological Corporation: London, UK; 1998 [Google Scholar]
- Frisoni GB, Redolfi A, Manset D, Rousseau ME, Toga A, Evans AC. Virtual imaging laboratories for marker discovery in neurodegenerative diseases. Nat Rev Neurol. 2011;7:429–438. doi: 10.1038/nrneurol.2011.99. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Wiberg M, Sundström A. A comparison of two approaches to correction of restriction of range in correlation analysis. Pract Assess Res Eval. 2009;14:1–9. [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Readind Test. NFER-Nelson: Windsor, UK; 1982. [Google Scholar]
- Nelson HE, O'Connell A. The Revised National Adult Reading Test-Test Manual. NFER-Nelson: Windsor, UK; 1991. [Google Scholar]
- McGurn B, Starr JM, Topfer JA, Pattie A, Whiteman MC, Lemmon HA, et al. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62:1184–1186. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8:847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology. 2008;71:1051–1056. doi: 10.1212/01.wnl.0000319692.20283.10. [DOI] [PubMed] [Google Scholar]
- Archer HA, Kennedy J, Barnes J, Pepple T, Boyes R, Randlesome K, et al. Memory complaints and increased rates of brain atrophy: risk factors for mild cognitive impairment and Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25:1119–1126. doi: 10.1002/gps.2440. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Yang J, Davies G, Harris SE, Tenesa A, Liewald D, et al. Genetic contributions to stability and change in intelligence from childhood to old age. Nature. 2012;482:212–215. doi: 10.1038/nature10781. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Johnson W, Deary IJ, Iacono WG. Genetic and Environmental Transactions Underlying Educational Attainment. Intelligence. 2009;37:466–478. doi: 10.1016/j.intell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.