The hunt for predictive biomarkers in diseases of the cardio-, cerebro- and peripheral vascular systems is an ongoing effort and one whose importance cannot be overstated. Identification of subjects most at risk for death or major adverse cardiovascular events (MACE) may provide a means to stratify diagnostic and therapeutic interventions based on validated predictive algorithms. In this issue of Arteriosclerosis, Thrombosis and Vascular Biology, de Vos and colleagues report that in 252 eligible subjects with documented peripheral artery disease (PAD) studied at a single center, measurement of skin autofluorescence (SAF) was independently associated with all-cause mortality and fatal or nonfatal MACE after a follow-up period of five years.1 Importantly, the authors excluded subjects with hemodialysis, kidney transplantation, recent myocardial infarction or recent stroke and report that even after adjustment for cardiovascular risk factors and the use of lipid-lowering drugs, increased SAF remained associated with increased risk for death and MACE. A previous report from these authors compared subjects with PAD vs. control PAD-free subjects and showed that increased SAF was significantly associated with PAD in a manner independent of traditional cardiovascular risk factors, although such risk factors were associated with further increases in SAF.2
The AGE reader™ is used as a biological marker of advanced glycation endproducts (AGEs) in the skin and is usually tested in the forearm. AGEs are the products of nonenzymatic glycation and oxidation of proteins and lipids. AGEs are increased not only in diabetes, but also in natural aging, oxidative stress and inflammatory conditions, and in renal failure.3 AGEs form on lysine and arginine residues and are heterogeneous; some AGEs are associated with cross-linking (such as pentosidine) and some AGEs do not fluorescence, such as the carboxy methyl lysine (CML) AGEs. Based on defined fluorescent wavelengths of excitation and emission, SAF is reflective of skin levels of AGEs. Although few studies to date have compared directly the measures of SAF with the skin levels of AGEs, Meerwaldt and colleagues measured specific skin AGEs in diabetic and non-diabetic subjects in the same arm as that used to measure the SAF. They reported that SAF correlated with measured levels of collagen-linked fluorescence (CLF), pentosidine, CML and carboxy ethyl lysine (CEL) AGEs.4 In a second study, this same author group examined specific AGEs in subjects with end stage renal disease (ESRD) and reported that SAF correlated with measured levels of CLF, pentosidine, CEL and CML AGEs in the skin.5 Hence, in these two reports, the evidence suggested that SAF did indeed correlate with measured levels of a variety of cross-linked and non-cross-linked AGEs in the skin. The broader question is, of course, to what extent do SAF measures in the skin forearm correlate with AGE levels at the site of vascular pathology? Although the present study by de Vos and colleagues measured SAF and the authors proposed links to the pathogenesis of PAD, they did not directly measure vascular AGE content. Hofmann and colleagues sought to address this question experimentally. In their study, they related SAF to AGE intrinsic fluorescence measured in collagenase digestible collagen fraction retrieved from discarded excess vein tissue explanted for bypass graft material.6 The authors reported that the SAF and pulse wave velocity (a measure of vessel stiffness) both correlated with the AGE measurements in the collagenase digestible collagen fraction.
A major question is to what degree does SAF, as a surrogate measure for AGE content or, perhaps, yet-to-be-identified specific mediators of vascular damage, reflect underlying vascular perturbation? Although de Vos and colleagues do not assess measures beyond traditional risk factors, other studies have suggested that SAF correlates with such factors as hsCRP (in subjects with hemodialysis); impaired HDL antioxidative capacity, and endothelial dysfunction.7-9 Beyond their ability to cause vascular damage by virtue of basement membrane thickening, vascular “leakiness” and other pathologies, AGEs interact with specific cellular receptors, the best-characterized of these is receptor for AGE (RAGE).10 The extracellular domain of RAGE, composed of one V-type and two C-type immunoglobulin domains, acts as a ligand decoy in cultured cells exposed to RAGE ligands and in vivo, in diabetic or aged animals.10 In human subjects, accruing evidence suggests that circulating soluble RAGEs (sRAGE) correlate with the degree of diabetic or inflammatory pathologies,11 although studies differ, intriguingly, on whether “high” or “low” sRAGE levels reflect protection against or vulnerability to complications and tissue stress.12 Skrha and colleagues measured SAF in subjects with type 1 or type 2 diabetes vs. control subjects and reported that in the diabetic groups, SAF levels were higher vs. controls and correlated positively with levels of sRAGE.13 Significantly higher SAF also correlated with indices of endothelial dysfunction such as levels of von Willebrand factor, Intercellular Adhesion Molecule 1 (ICAM1), and Vascular Cell Adhesion Molecule 1 (VCAM1) in that study. Levels of sRAGEs were not measured in the present study by de Vos and colleagues but others measured sRAGE in human subjects with coronary artery disease and PAD and found lower levels of sRAGE in subjects with both sites of disease.14 Clearly, the relationships between ligand, sRAGE levels and affected vascular beds are complex and require further study to determine if SAF combined with measures such as sRAGEs or distinct markers of oxidative or endothelial stress may be a superior biomarker “panel” to add predictive value for long-term outcomes. Certainly, one solution to the quandary may be to measure multiple putative biomarkers in all study subjects, where feasible.
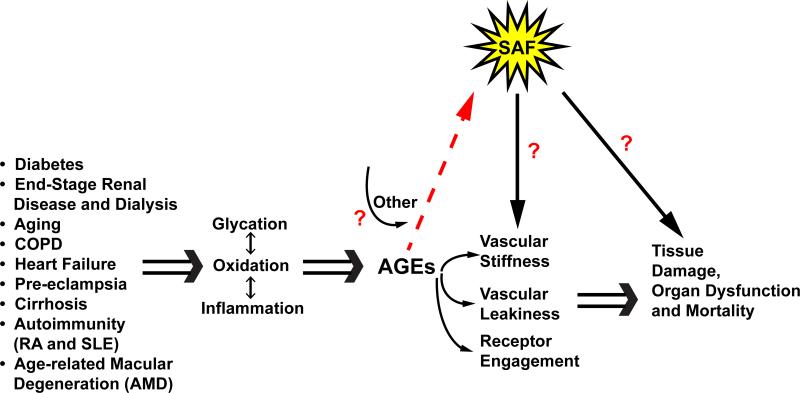
Studies reporting SAF may be complicated by the reports suggesting that, not surprisingly, SAF, and by inference, AGEs, accumulate in diverse disorders and, at least in some cases, correlate with the extent of disease or its complications. Extensive evidence links elevated SAF to not only ESRD and mortality and the complications of types 1 and 2 diabetes,15-17 but also to such disorders as pre-eclamptic pregnancies, chronic obstructive pulmonary disease, rheumatoid arthritis, systemic lupus erythematosus, cirrhosis and age-related macular degeneration, as examples (Figure 1).18-23 These considerations reflect two key points: first, that SAF increases and AGEs accumulate in metabolic, inflammatory and oxidative stresses; and second, that studies reporting SAF must take care to exclude subjects displaying advanced stages of these disorders. In this context, de Vos and colleagues did exclude patients with hemodialysis or kidney transplantation.
Figure 1. SAF mirrors AGE burden and the state of vascular dysfunction.
Beyond hyperglycemia, oxidative and inflammatory stresses are independently linked to the generation of AGEs. AGE production occurs in diverse disorders such as end stage renal disease (ESRD) (complicated by certain dialysis fluids), aging, chronic obstructive pulmonary disease, congestive heart failure, pre-eclampsia in pregnancy, cirrhosis of the liver, arthritis, systemic lupus erythematosus and age-related macular degeneration (AMD), as examples. AGE production and accumulation is systemic and may be measured by inference using skin autofluorescence (SAF) as certain of the AGEs, such as pentosidine, bear this property. AGEs cause damage by multiple mechanisms such as vascular stiffness, vascular leakiness and via receptor engagement. At least certain AGE formation is irreversible and therefore AGEs are linked to chronic tissue damage, organ dysfunction and as suggested by de Vos and colleagues, mortality in PAD. Others linked SAF to mortality in ESRD. The study by de Vos and colleagues suggests that simple and painless measures of the AGE burden, through the SAF surrogate, may predict long-term (5 year) mortality in subjects with PAD, even after correcting for usual cardiovascular risk factors. Studies are essential to correlate SAF with vascular AGE burden, receptor (RAGE) engagement and the mechanisms of chronic vascular stress to bring full circle the AGE hypothesis to disease mechanism and to discover novel treatments for which measures of SAF may be a bona fide target engagement biomarker.
Despite the promise of SAF as a biomarker for AGE deposition, there are caveats, some of which were noted by de Vos and colleagues. It is known that in subjects with dark skin pigmentation, SAF may not be reliable.24 It is possible that intense vasodilation, vasoconstriction certain skin creams used to induce skin browning, or high AGE meals might affect SAF, even if just temporarily.25 On the other hand, SAF is painless and is said not to be affected by short-term glycemic variations.26 Although it must be acknowledged that AGEs may be surrogate markers for yet-to-be-identified pathogenic mediators being measured by the SAF, this is not highly likely. Hence, although there are some caveats to measurements of SAF, careful study design and patient inclusion and exclusion criteria should aid in improving the predictive value of this marker.
Finally, it is intriguing to consider the fate of SAF after putative therapeutic interventions. In one study in subjects with heart failure, the AGE cross link “breaker” alagebrium was administered for 36 weeks twice daily. In parallel with no changes in cardiac function or American Heart Association heart failure score in treated subjects, no changes in SAF were noted post-therapy.27 Issues such as dose, schedule and indeed in the context of SAF, the baseline AGE burden and character might have confounded the results. These findings underscore that future studies should focus on identifying the specific AGEs that mediate tissue damage in order to develop targeted therapies. In that context, it would then be critical to determine if/to what extent SAF might track the accumulation of the pathogenic specific AGEs and if and how they parallel vascular AGE burden.
In conclusion, in the study by de Vos and colleagues, meticulous attention to patient entry/exclusion criteria and the long-term follow up of nearly all of the initial study entrants provides a formidable window into the association between SAF, AGE burden, and mortality and MACE in PAD and suggests that further research is needed to establish SAF and AGE content as a mechanism and validated biomarker in this disorder. Indeed, the work of de Vos and co-workers supports that SAF highlights the pathogenic importance of glycation, oxidative and inflammatory stresses as these processes come of AGE as mechanisms of a diverse array of chronic diseases.
Acknowledgements
The author gratefully acknowledges the outstanding assistance of Ms. Latoya Woods in the preparation of this manuscript.
Sources of funding
Research in the Schmidt laboratory is funded by grants from the United States Public Health Service (HL60901, HL118565 and AG026467) and the JDRF.
Footnotes
Disclosures: None
References
- 1.de Vos LC, Mulder DJ, Smit AJ, Dullaart RPF, Kleefstra N, Lijfering WM, Kamphuisen PW, Zeebregts CJ, Lefrandt JD. Skin auto fluorescence is associated with 5-year mortality and cardiovascular events in patients with peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.113.302731. [DOI] [PubMed] [Google Scholar]
- 2.de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RP, Kamphuisen PW, Zeebregts CJ, Lefrandt JD. Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2013;33:131–138. doi: 10.1161/ATVBAHA.112.300016. [DOI] [PubMed] [Google Scholar]
- 3.Schalkwijk CG, Miyata T. Early- and advanced non-enzymatic glycation in diabetic vascular complications: the search for therapeutics. Amino Acids. 2012;42:1193–1204. doi: 10.1007/s00726-010-0779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans RO, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 5.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3687–3693. doi: 10.1681/ASN.2005020144. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann B, Adam AC, Jacobs K, Riemer M, Erbs C, Bushnaq H, Simm A, Silber RE, Santos AN. Advanced glycation end product associated skin autofluorescence: a mirror of vascular function? Exp Gerontol. 2013;48:38–44. doi: 10.1016/j.exger.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Nagano M, Fukami K, Yamagishi S, Sakai K, Kaida Y, Matsumoto T, Hazama T, Tanaka M, Ueda S, Okuda S. Tissue level of advanced glycation end products is an independent determinant of high-sensitivity C-reactive protein levels in haemodialysis patients. Nephrology (Carlton) 2011;16:299–303. doi: 10.1111/j.1440-1797.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- 8.Mulder DJ, de Boer JF, Graaff R, de Vries R, Annema W, Lefrandt JD, Smit AJ, Tietge UJ, Dullaart RP. Skin autofluorescence is inversely related to HDL anti-oxidative capacity in type 2 diabetes mellitus. Atherosclerosis. 2011;218:102–106. doi: 10.1016/j.atherosclerosis.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 9.de Groot L, Hinkema H, Westra J, Smit AJ, Kallenberg CG, Bijl M, Posthumus MD. Advanced glycation endproducts are increased in rheumatoid arthritis patients with controlled disease. Arthritis Res Ther. 2011;13:R205. doi: 10.1186/ar3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends in endocrinology and metabolism: TEM. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamagishi S, Matsui T. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Frontiers in bioscience (Elite edition) 2010;2:1184–1195. doi: 10.2741/e178. [DOI] [PubMed] [Google Scholar]
- 12.Piarulli F, Sartore G, Lapolla A. Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update. Acta diabetologica. 2013;50:101–110. doi: 10.1007/s00592-012-0412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skrha J, Jr., Soupal J, Loni Ekali G, Prazny M, Kalousova M, Kvasnicka J, Landova L, Zima T, Skrha J. Skin autofluorescence relates to soluble receptor for advanced glycation end-products and albuminuria in diabetes mellitus. Journal of diabetes research. 2013;2013:650694. doi: 10.1155/2013/650694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falcone C, Bozzini S, Guasti L, D'Angelo A, Capettini AC, Paganini EM, Falcone R, Moia R, Gazzaruso C, Pelissero G. Soluble RAGE plasma levels in patients with coronary artery disease and peripheral artery disease. The Scientific World Journal. 2013;2013:584504. doi: 10.1155/2013/584504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerrits EG, Lutgers HL, Smeets GH, Groenier KH, Smit AJ, Gans RO, Bilo HJ. Skin autofluorescence: a pronounced marker of mortality in hemodialysis patients. Nephron extra. 2012;2:184–191. doi: 10.1159/000339282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noordzij MJ, Mulder DJ, Oomen PH, Brouwer T, Jager J, Castro Cabezas M, Lefrandt JD, Smit AJ. Skin autofluorescence and risk of micro- and macrovascular complications in patients with Type 2 diabetes mellitus-a multi-centre study. Diabet Med. 2012;29:1556–1561. doi: 10.1111/dme.12005. [DOI] [PubMed] [Google Scholar]
- 17.Genevieve M, Vivot A, Gonzalez C, Raffaitin C, Barberger-Gateau P, Gin H, Rigalleau V. Skin autofluorescence is associated with past glycaemic control and complications in type 1 diabetes mellitus. Diabetes Metab. 2013;39:349–354. doi: 10.1016/j.diabet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Blaauw J, Smit AJ, van Pampus MG, van Doormaal JJ, Aarnoudse JG, Rakhorst G, Graaff R. Skin autofluorescence, a marker of advanced glycation end products and oxidative stress, is increased in recently preeclamptic women. American journal of obstetrics and gynecology. 2006;195:717–722. doi: 10.1016/j.ajog.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 19.Gopal P, Reynaert NL, Scheijen JL, Engelen L, Schalkwijk CG, Franssen FM, Wouters EF, Rutten EP. Plasma advanced glycation end-products and skin autofluorescence are increased in COPD. The European respiratory journal. 2014;43:430–438. doi: 10.1183/09031936.00135312. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto T, Tsurumoto T, Baba H, Osaki M, Enomoto H, Yonekura A, Shindo H, Miyata T. Measurement of advanced glycation endproducts in skin of patients with rheumatoid arthritis, osteoarthritis, and dialysis-related spondyloarthropathy using noninvasive methods. Rheumatol Int. 2007;28:157–160. doi: 10.1007/s00296-007-0408-4. [DOI] [PubMed] [Google Scholar]
- 21.Nienhuis HL, de Leeuw K, Bijzet J, Smit A, Schalkwijk CG, Graaff R, Kallenberg CG, Bijl M. Skin autofluorescence is increased in systemic lupus erythematosus but is not reflected by elevated plasma levels of advanced glycation endproducts. Rheumatology (Oxford, England) 2008;47:1554–1558. doi: 10.1093/rheumatology/ken302. [DOI] [PubMed] [Google Scholar]
- 22.Maury E, Vergniol J, Ledinghen V, Rigalleau V. Skin autofluorescence is high in patients with cirrhosis - further arguing for the implication of Advanced Glycation End products. J Hepatol. 2011;54:1079–1080. doi: 10.1016/j.jhep.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Mulder DJ, Bieze M, Graaff R, Smit AJ, Hooymans JM. Skin autofluorescence is elevated in neovascular age-related macular degeneration. The British journal of ophthalmology. 2010;94:622–625. doi: 10.1136/bjo.2009.162990. [DOI] [PubMed] [Google Scholar]
- 24.Mulder DJ, Water TV, Lutgers HL, Graaff R, Gans RO, Zijlstra F, Smit AJ. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: an overview of current clinical studies, evidence, and limitations. Diabetes technology & therapeutics. 2006;8:523–535. doi: 10.1089/dia.2006.8.523. [DOI] [PubMed] [Google Scholar]
- 25.Noordzij MJ, Lefrandt JD, Graaff R, Smit AJ. Dermal factors influencing measurement of skin autofluorescence. Diabetes technology & therapeutics. 2011;13:165–170. doi: 10.1089/dia.2010.0123. [DOI] [PubMed] [Google Scholar]
- 26.Noordzij MJ, Lefrandt JD, Graaff R, Smit AJ. Skin autofluorescence and glycemic variability. Diabetes technology & therapeutics. 2010;12:581–585. doi: 10.1089/dia.2010.0014. [DOI] [PubMed] [Google Scholar]
- 27.Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, Hillege HL, Voors AA. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur J Heart Fail. 2011;13:899–908. doi: 10.1093/eurjhf/hfr067. [DOI] [PubMed] [Google Scholar]