Abstract
One of the current challenges in the field of advanced prosthetics is the development of artificial limbs that provide the user with detailed sensory feedback. Sensory feedback from our limbs is not only important for proprioceptive awareness and motor control, but also essential for providing us with a feeling of ownership or simply put, the sensation that our limbs actually belong to ourselves. The strong link between sensory feedback and ownership has been repeatedly demonstrated with the so-called rubber hand illusion (RHI), during which individuals are induced with the illusory sensation that an artificial hand is their own. In healthy participants, this occurs via integration of visual and tactile signals, which is primarily supported by multisensory regions in premotor and intraparietal cortices. Here, we describe a functional magnetic resonance imaging (fMRI) study with two upper limb amputees, showing for the first time that the same brain regions underlie ownership sensations of an artificial hand in this population. Albeit preliminary, these findings are interesting from both a theoretical as well as a clinical point of view. From a theoretical perspective, they imply that even years after the amputation, a few seconds of synchronous visuotactile stimulation are sufficient to activate hand-centered multisensory integration mechanisms. From a clinical perspective, they show that a very basic sensation of touch from an artificial hand can be obtained by simple but precisely targeted stimulation of the stump, and suggest that a similar mechanism implemented in prosthetic hands would greatly facilitate ownership sensations and in turn, acceptance of the prosthesis.
Keywords: Rubber hand illusion, Amputees, Multisensory integration, fMRI, Prosthetics
One of the main aims within the field of advanced prosthetics is the development of artificial limbs that can be fully incorporated into the user's body representation and consequently experienced as if they were real limbs. Two crucial criteria such prosthetic limbs would ideally have to fulfill are to enable the execution of fine voluntary motor commands and to provide the user with detailed sensory feedback. To date, the most significant advances have been made in terms of enabling motor control via direct cortical recordings (e.g., Nicolelis, 2003; Schwartz, 2004; Velliste, Perel, Spalding, Whitford, & Schwartz, 2008), and in clinical practice, the devices most used for this purpose are myoelectric prostheses (Jackson & Fetz, 2011; Sebelius, Rosén, & Lundborg, 2005). These involve the use of electromyography (EMG) recordings from the stump to control simple movements of the prosthesis, for example, flexion and extension of the elbow or opening and closing of the hand. However, even for these basic movements, the user needs extensive training to achieve a satisfactory level of control of the prosthesis. This could be rooted in the lack of somatosensory feedback, which is crucial for the optimization of motor control and learning (Wolpert, Diedrichsen, & Flanagan, 2011; Wolpert & Ghahramani, 2000). Increasing research over the past decade has therefore been aimed at developing methods to provide new somatosensation. These include targeted reinnervation (Kuiken, Marasco, Lock, Harden, & Dewald, 2007), sensory substitution (whereby signals from the prosthesis are used to activate a sensory substitution device that the user learns to interpret as a sensory signal related to the prosthesis) (Jones, 2011; Lundborg & Rosén, 2001), as well as direct simulation of afferent fibers in the arm, the spinal cord, or actual neurons in the somatosensory cortex (Hsiao, Fettiplace, & Darbandi, 2011). The main purpose of these technically highly advanced studies is to promote sensorimotor feedback loops that optimize the usability of prosthetic devices. In fact, in a normal hand, detailed sensory feedback is a prerequisite for the precise regulation of muscle force and the fine manipulation of finger position that enable precise grasping movements (Johansson & Flanagan, 2009).
The sensory feedback from our limbs is, however, not only relevant for optimal motor control and learning; but is also an essential aspect of what is referred to as a feeling of body ownership, or the sensation that our limbs are actually part of our body (Ehrsson, 2012; Gallagher, 2005; Tsakiris, Schutz-Bosbach, & Gallagher, 2007). A well-known experimental paradigm highlighting this fact is the so-called rubber hand illusion (RHI), first described by (Botvinick & Cohen, 1998). In short, it demonstrates how being stroked on ones unseen hand while viewing synchronous strokes applied to an aligned visible rubber hand can evoke the illusion that the sensation of touch originates from the rubber hand, and consequently that the rubber hand is a part of one's own body. Since its first description, numerous behavioral studies with healthy participants have explored the processes underlying the RHI in more detail. Crucial experimental criteria for the illusion to arise include that the tactile stimulation of the real hand and the visually perceived stimulation of the rubber hand occur in temporal and spatial synchrony, that the rubber hand is in a plausible anatomical orientation with respect to the real body, and that the rubber hand has a sufficient degree of shape resemblance with that of a real hand (Ehrsson, Spence, & Passingham, 2004; Pavani, Spence, & Driver, 2000; Tsakiris, Carpenter, James, & Fotopoulou, 2010; Tsakiris & Haggard, 2005). In addition to these behavioral studies, functional magnetic resonance imaging (fMRI) investigations have shed light on the neural correlates of the RHI (Bekrater-Bodmann, Foell, Diers, & Flor, 2012; Ehrsson et al., 2004). In healthy individuals, the RHI seems to be mainly driven by the activity of multisensory areas in premotor and intraparietal areas, with the former being particularly relevant for the self-attribution aspect of the illusion. In addition, cerebellar activity seems to play an important role in the recalibration process, possibly reflecting the shift of the perceived position of one's own hand toward the location of the rubber hand (Ehrsson et al., 2004).
The fact that healthy individuals can be tricked into experiencing an artificial hand as being their own, raises the intriguing question of whether the same could be achieved with amputees who have lost their real hand. In a behavioral study with 18 upper limb amputees, Ehrsson and colleagues (Ehrsson et al., 2008) demonstrated that this is in fact the case. Just as in the studies with healthy individuals, following simultaneous stroking of the amputees’ stump and the finger of a rubber hand, the participants reported sensations of touch from the artificial hand. Albeit the strengths of the illusion varied across individual amputees, on a group level, the effect was supported by subjective reports in the form of questionnaires, objective behavioral data in the form of proprioceptive drift, and physiological evidence in the form of skin conductance responses to threats applied to the artificial hand. In a subsequent study, Rosén and colleagues (Rosén et al., 2009) built on these findings by demonstrating that illusory referral of touch can also be elicited for an advanced hand prosthesis with a robotic-like appearance. And more recently, Marasco and colleagues (Marasco, Kim, Colgate, Peshkin, & Kuiken, 2011) have shown how a similar approach can also be extended to upper limb amputees with surgically redirected nerves. These findings are of particular clinical relevance, as they show the possibility of eliciting conscious tactile feedback from prosthetic limbs in a relatively simple and non-invasive way. What remains unknown however, are the neural correlates of these illusory sensations in amputees. Is the RHI in amputees supported by the same neural network as in healthy participants? In the current study, we addressed this question in an fMRI investigation with two upper limb amputees involving the RHI. We hypothesized that, as in healthy participants, the illusory ownership of an artificial hand would be reflected by activation in multisensory regions of premotor and intraparietal areas. In addition, we hypothesized there to be an involvement of the cerebellum which has been previously found to be activated during the rubber hand illusion (Ehrsson, Holmes, & Passingham, 2005; Ehrsson et al., 2004), and which is more generally known to be associated with the processing of proprioceptive signals from the limb as well as visually based recalibration of limb position (Hagura et al., 2009; Naito, Roland, & Ehrsson, 2005). Exploring the neural correlates of the RHI in amputees is of both theoretical and clinical relevance. From a theoretical point of view, it allows to explore the brain activity underlying limb ownership in the light of supposed previous cortical reorganization due to the amputation. From a clinical perspective, it allows to explore the neural validity of a non-invasive approach aimed at providing sensory feedback from a prosthetic hand.
MATERIALS AND METHODS
Participants
Two upper limb amputees (TA and LO), recruited through the Arm Prosthesis Unit of Red Cross Hospital, Stockholm, Sweden, participated in this study. They were selected from a group of eight amputees who had participated in a previous study conducted in our Lab (Schmalzl et al., 2011) on the basis of having a strong response to the RHI and MR compatibility (i.e., absence of metal in their body). Both participants had lost their limb due to a traumatic accident, and neither of them had any significant medical history apart from the amputation. More details of both participants are provided in Table 1. The study was approved by the Regional Ethics Review Board of Stockholm, and informed written consent was obtained from both the participants.
TABLE 1.
Participants. Details of participants TA and LO
| Participant | Gender | Age | Time since amputation | Side of amputation | Stump length from elbow | Phantom hand | Telescoping | Phantom pain |
|---|---|---|---|---|---|---|---|---|
| TA | F | 38 | 5 years | Right | 15 cm | Yes | Yes | Yes |
| LO | M | 53 | 36 years | Left | 24 cm | Yes | Yes | No |
fMRI data acquisition
fMRI data acquisition was performed at Karolinska University Hospital Huddinge, Stockholm, Sweden, with a Siemens TIM Trio 3T scanner equipped with a 12-channel head coil. Functional scans were obtained using a T2- weighted gradient-echo echo-planar (EPI) sequence and blood oxygenation level-dependent (BOLD) contrast as an index of brain activity (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). The scanning parameters were as follows: Slices: 47; Resolution: 3 × 3 × 3 mm; Interslice gap: 0.1 mm; Orientation: Near axial (parallel to the anterior–posterior commissure); TR: 3000 ms. These parameters ensured that the whole brain was within the field of view (FOV: 58×76 mm, In-plane resolution; 3×3 mm; TE: 40 ms). For each participant, a total of 705 volumes were acquired (235 volumes for each of the three experimental runs—see details below). To facilitate anatomical localization of statistically significant activations, following the functional fMRI experiment, a high-resolution structural image was acquired for each participant using a three-dimensional magnetization-prepared rapid gradient-echo (3D-MPRAGE) sequence with the following parameters: Slices: 176; Resolution: 1 × 1 × 1 mm; TR: 1900 ms; FOV: 250 ×250 mm; TE: 2.27 ms; Flip angle: 9°. The delivery of instructions to the participants and the experimenter during the fMRI experiment, as well as the recording of the participants’ responses, was monitored with presentation software (Neurobehavioral Systems, Albany, CA; http://www.neurobs.com/).
Data analysis
Both functional and anatomical scans were converted with MRIConvert (http://s.edu/≃jolinda/MRIConvert/). Processing and data analysis were performed with SPM 8 (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm/). Before performing the statistical analyses, the images were preprocessed following the standard steps (reorientation, slice timing correction, motion correction, coregistration, segmentation, normalization, and smoothing) and default parameters of SPM 8. Details of the statistical analyses are provided in the Results section below.
Experimental setup
For the current experiment, we used an adapted version of the RHI (Botvinick & Cohen, 1998). We performed the RHI with the participants on two occasions, once for a behavioral screening, and once within the fMRI setting for the conduction of the actual imaging experiment. The setup for the behavioral screening was based on that described in a previous RHI study with amputees (Ehrsson et al., 2008), and the setup for the fMRI experiment was adapted from that of a previous RHI study with healthy participants (Ehrsson et al., 2004). In both the behavioral and fMRI setup, the induction of the RHI illusion was obtained through simultaneous visuotactile stimulation applied to the participants’ stump which was out of view, and an artificial hand which was in full view (details of both setups and the experimental conditions are provided below).
Behavioral setup
For the behavioral screening, participants sat at a table facing the experimenter, and were asked to place both their intact and amputated arms in front of them at a distance of about 70 cm from each other. The intact arm was simply resting on the table, whereas the stump was covered by a plastic box with a front opening, so that it was out of view for the participant but accessible to the experimenter. A life-sized rubber hand was then placed on the table in full view, at a distance of about 20 cm from the stump toward the midline. The rubber hand always corresponded to the participants’ amputated hand, so we used a female right hand for TA and a male left hand for LO. In addition, a cloth was placed so as to cover the participants’ shoulder and part of the rubber forearm, to create the visual impression that the artificial hand was in direct continuation with the participants’ arm. Stroking of the stump and the rubber hand was performed with two identical small brushes.
fMRI setup
For the fMRI experiment, participants laid in a supine position on the MRI table. Their head was tilted forward by approximately 30°, which was obtained by slanting the head coil with a custom-made wooden wedge with an angle of 11°, and by tilting the participants’ heads by an additional 20° using towels and foam pads. The purpose of the head tilt was to enable the participants to have a direct view of a small MR-compatible table of the size 42 ×35 cm with an adjustable slope, which was attached to the sides of the MRI table and positioned above their waist. The participants’ stump was supported with towels so that it was at the same height as the table in a comfortable position and completely relaxed. In addition, a thin vertical foam pad was positioned on the inside of the arm in order to create a little “wall” which occluded the view of the stump on the part of the participant. The rubber hand was then placed on the table, again displaced by about 20 cm toward the midline with respect to the stump as in the behavioral experiment. Similar to the behavioral setup, a cloth was placed so as to cover the participants’ chest and part of the rubber forearm, to create the visual impression that the artificial hand was in direct continuation with the participants’ arm. Stroking of the stump and the rubber hand was performed with two MR-compatible stroking rods. Recording of illusion onset times was obtained via a foot pedal operated by the participant, which was mounted on the lower end of the MRI table (as in Ehrsson et al., 2004).
Experimental procedures and design
Interview
A detailed interview was conducted with both participants in order to document their medical history, the details of the accidents that led to their amputation, as well as their currently experienced phantom sensations (for a summary see Table 1).
Stump mapping
Prior to both the behavioral screening and the fMRI experiment, we performed a so-called “stump mapping” for each participant (Figure 1). During this procedure, systematic touches were applied to the distal portion of the stump in order to determine the points giving rise to referred sensations in specific parts of the phantom hand or phantom fingers. For both participants, the point triggering the strongest referred sensations was then marked on the stump (for both TA and LO, it corresponded to the phantom thumb). This point was then used as a reference for the tactile stimulation during the RHI experiment.
Figure 1.
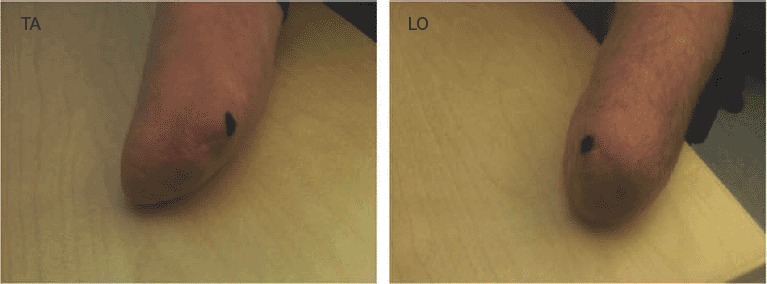
Stump mapping. For both participants, the point triggering referred sensations on the phantom thumb was marked on the stump. This point was then used as a reference for the tactile stimulation during the RHI experiment.
Behavioral screening
The aim of the behavioral screening was to determine whether the participants would experience the RHI, that is, whether they would experience ownership of and/or referral of touch from the rubber hand, and therefore, be suitable candidates for the fMRi experiment. There were three experimental conditions, one illusion condition (Synchronous), and two control conditions (Asynchronous and Incongruent). During all three conditions, the experimenter simultaneously stroked the point of the participants’ stump (which was out of view), evoking referred sensations on the phantom thumb, and the thumb of the rubber hand (which was in full view). During the Synchronous condition, the strokes were applied in temporal synchrony, that is, the participants visually perceived the strokes applied to the rubber hand at the exact same time as they perceived the referred sensations on their phantom hand. Such synchronous visuotactile stimulation has previously been shown to evoke ownership sensations of artificial hands in amputees (Ehrsson et al., 2008). During the Asynchronous condition, the strokes were applied in temporal asynchrony, that is, the participants visually perceived the strokes applied to the rubber before they perceived the referred sensations on their phantom hand. As with healthy participants, this manipulation has been shown to reduce ownership sensations of artificial hands also in some amputees (Ehrsson et al., 2008). During the Incongruent condition, the strokes were applied in temporal synchrony, but the rubber hand was rotated by 180° so that its fingertips were facing toward the participant. This type of manipulation is known to abolish the RHI in healthy participants (Ehrsson et al., 2004, Tsakiris & Haggard, 2005), and we hypothesized that the same would be the case for amputees. For all three conditions, strokes were applied for 60 seconds with a frequency of approximately one stroke per second, and the stroke length was kept between one and two cm. The overall temporal stroking pattern was kept irregular in order to avoid expectations about the timing of the visuotactile stimulation events in the participants. Following each condition, participants were asked to fill out a questionnaire consisting of six statements aimed at capturing the subjective experience of the experimental effects. Three of them were “Illusion statements” aimed at capturing potential sensations of ownership and referral of touch, whereas the remaining three were “Control statements” aimed at capturing the participants’ suggestibility and task compliance. The order of the questions was randomized for each condition, and participants were asked to affirm or deny each statement on a seven-point Likert scale (+3 = Strongly agree; − 3 = Strongly disagree) (Figure 2).
Figure 2.
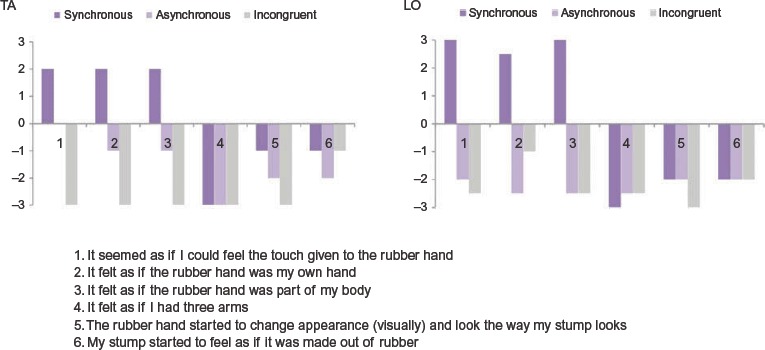
Questionnaire data. After each experimental condition, participants were administered a questionnaire consisting of six statements (Illusion statements 1–3; Control statements 4–6) aimed at capturing the subjective experience of the experimental effects. Participants were asked to affirm or deny each statement on a seven-point Likert scale (+3 =Strongly agree; −3 = Strongly disagree). Both participants confirmed experiencing the RHI in the Synchronous, but not in the Asynchronous and Incongruent condition.
fMRI experiment
The fMRI experiment was performed on a separate day with respect to the behavioral screening. After being given a detailed explanation about the procedure of the fMRI experiment, the participants were set up on the MRI table as described above. We then performed a test stroking session to ensure that they would still experience the RHI in the context of the fMRI setup, and had them practice pressing the foot pedal. Specifically, the participants were instructed that in the Synchronous trials, they had to press the foot pedal as soon as they began to experience the illusion, so that we could take their response as an indicator for the illusion onset time. In addition, for each of the Asynchronous and Incongruent trials, they were told to press the foot pedal as soon as they heard a beep tone, the timing of which was always matched to that of the response signaling the illusion onset during the previous Synchronous trial. This was done in order to keep the task demands equivalent across the experimental conditions. Participants were then moved inside the scanner to begin the actual experiment. Both participants completed three runs, with a duration of 12 minutes each. During each run, there were three trials of each experimental condition with a duration of 60 seconds each, for a total of nine Synchronous, nine Asynchronous, and nine Incongruent trials. Each of the three experimental runs had to begin with a Synchronous trial, so that the illusion onset time of that trial could then be used as reference for the timing of the beep tone during the first Asynchronous and Incongruent trials. The order of the remaining trials of each run was then randomized. In addition, after every third trial, there was a 20-second rest period which was used as baseline. Throughout the experiment, both the participants and the experimenter received instructions via separate sets of MR-compatible headphones. The outline of the trials was as follows: Each trial began with an instruction for the experimenter announcing the type of trial to be performed (i.e., “Synchronous”, “Asynchronous”, or “Incongruent”). This was followed by a 10-second preparation period, during which the experimenter had time to make the necessary adjustments for the specific trial, e.g., rotate the hand for Incongruent trials, etc. At the end of this period, the participant received the foot pedal instructions (i.e., “Press the foot pedal when you feel the illusion” for the Synchronous trials; “Press the foot pedal when you hear the beep tone” for the Asynchronous and Incongruent trials). Then the stroking session began, and the experimenter applied the strokes by following the exact timing of a metronome which she listened to via the headphones. This ensured that the number of strokes (45 strokes for each 60-second period) was kept consistent across the experimental conditions and across the two participants. Stroking was always continued for the entirety of the 60-second period, irrespective of the timing of the foot pedal press.
Preprocessing and statistical analysis of fMRI data
We analyzed our data using a general linear model (GLM), and defined seven independent conditions of interest. For each of the three experimental conditions, we separately modeled the time period before the foot pedal press (i.e., Synchronouspre, Asynchronouspre, and Incongruentpre), and the time period after the foot pedal press (i.e. Synchronouspost, Asynchronouspost, and Incongruentpost). The average illusion onset time was 9 seconds for TA (Range: 9–21 seconds) and 11 seconds for LO (Range: 5–22 seconds), which is in agreement with previously published onset times for healthy individuals (Ehrsson et al., 2004; Lloyd, 2007). In addition, we defined a baseline condition (Rest), consisting of all 20-second rest periods following each third experimental trial. All foot pedal responses, as well as the motion parameter estimates generated during the preprocessing, were modeled as regressors of no interest.
The main focus of our analyses was to test the hypothesis that the illusory ownership of an artificial hand in amputees would be reflected by activity in premotor and intraparietal areas, as has been previously documented in healthy individuals (Bekrater-Bodmann et al., 2012; Ehrsson et al., 2004, 2005; Ehrsson, Weich, Weiskopf, Dolan, & Passingham, 2007). In addition, we were interested in documenting any illusion-related activity in the cerebellum as well as inferior parietal areas, which are known to be involved in the processing of proprioceptive signals (Naito et al., 2005) and multisensory processing (Gentile, Petkova, & Ehrsson, 2011). In order to test these hypotheses, we designed a contrast that allowed us to examine which brain areas would show a significantly higher BOLD response during the Synchronous condition as compared to the Control conditions. That is, we performed a direct comparison of the activation elicited in the Synchronous condition following the illusion onset (i.e., post foot pedal press), with the average activation elicited in the Control conditions during the same time frame [(Synchronouspost) – (Asynchronouspost + Incongruentpost)]. Hence, we looked for activity associated with the illusion condition that cannot be simply accounted for by the effects of viewing a hand in a congruent position and feeling synchronous brushstrokes. Given the hypothesis-based individual case approach of our study, we report all activations that were significant at p < .001 uncorrected. For brain regions that we had strong anatomical hypotheses for, (i.e., premotor cortex, intraparietal cortex, and cerebellum—Ehrsson et al., 2004), we also report additional clusters that were significant at p .01 uncorrected. For all activations reported in the figures and table, we confirmed that there was stronger activity in the Synchronouspost condition compared to the baseline rest period, thereby excluding the possibility that the activations were simply produced by a deactivation in one or more of the control conditions. Our decision to report uncorrected statistics, which as stated above was based on the fact that we had only two participants as well as restricted scanning time, makes our approach descriptive in nature. Nevertheless, we reasoned that observing activity in the key set of hypothesized areas in both participants would constitute encouraging pilot evidence for the fact that amputees can experience the rubber and illusion and that they do so by engaging similar multisensory mechanisms as healthy individuals.
RESULTS
Behavioral screening
As mentioned above, the aim of the behavioral screening was to determine whether the participants would experience the RHI. Participants were subjected to each of the experimental conditions once, and at the end of each stroking session, they were asked to rate the six questionnaire statements. The ratings for both participants are depicted in Figure 2. As can be clearly seen from the graphs, both TA and LO affirmed experiencing the RHI in the Synchronous but not in the Asynchronous and Incongruent conditions. That is, both participants affirmed the illusion statements (statements 1–3) in the Synchronous condition (by giving them a rating of +1 or higher), but not in the two control conditions (by giving a rating of −1 or lower). The reliability of the results is further strengthened by the fact the both participants did not affirm any of the three control statements (statements 4–6) in either of the conditions. On the basis of this result, we then proceeded with the fMRI experiment, adopting the Synchronous condition as illusion condition and the Asynchronous and Incongruent conditions as control conditions.
fMRI experiment
As hypothesized, in both participants, we found significant activation in the premotor cortex (Figure 3a) as well as intraparietal cortex (Figure 3b). Specifically, for TA, we found activation in the contralateral dorsal premotor cortex (PMd), and the depth of the contralateral intraparietal sulcus. For LO, we found activation in the contralateral PMd as well as ventral premotor cortex (PMv), and again in the contralateral intraparietal sulcus, where three adjacent clusters could be identified (see Figure 3b). Furthermore, for both participants, we found bilateral cerebellar activation, as well as significant clusters in inferior parietal cortex with peaks in the supramarginal gyrus (TA) and angular gyrus (LO). An additional activation of interest for LO included the contralateral occipitotemporal cortex, possibly corresponding to what has been previously defined as the extrastriate body area (EBA) (Downing, Jiang, Shuman, & Kanwisher, 2001). For details of these activations see Table 2.
Figure 3.

Activation associated with the Synchronous condition compared to the Control conditions. The figure depicts activation in (a) premotor and (b) intraparietal areas reflecting a significantly higher BOLD activation in the Synchronous condition compared to the Control conditions. The activation maps correspond to a direct comparison of the activation elicited in the Synchronous condition following the illusion onset (i.e., post foot pedal press), with the average activation elicited in the Control conditions during the same time frame [(Synchronouspost) – (Asynchronouspost + Incongruentpost)]. The activation maps are displayed on either sagittal or axial slices of the mean high-resolution structural scan of each participant. The plots on the right of each activation map show the corresponding parameter estimates for each condition compared to rest, i.e., (Synchronouspost – Rest) etc. Parameter estimates were calculated on the peak voxel of each respective cluster (xyz coordinates in MNI space are shown in the heading of each plot). The error bars denote SEs. The threshold for the activation maps was set at p < .001 or p < .01 uncorrected— see Table 2 for details.
TABLE 2.
Key areas displaying significant activation related to the experience of the RHI. For each contrast, the table displays the details of significant activation clusters found in each participant. Specifically, it shows the anatomical region, the peak xyz coordinates in MNI space, and the corresponding peak t values. The basic threshold for the calculation of significant clusters was set at p < .001 uncorrected. Additionally, reported clusters in regions that we had strong anatomical hypotheses for and that were significant at a threshold of <.01 are marked with °
| Synchronous (post) – Control (post) | Peak xyz | Peak t∗ | Cluster size | ||
|---|---|---|---|---|---|
| TA | |||||
| L Precentral Sulcus (PMd) | -62 | -8 | 46 | 4.93 | 17 |
| L Intraparietal Sulcus | -46 | -44 | 50 | 3.21 | 30 |
| L Inferior Parietal Cortex (Supramarginal Gyrus) | -58 | -42 | 28 | 5.51 | 57 |
| Medial Cerebellum | 0 | -76 | -30 | 3.62 | 71 |
| L Cerebellum | -52 | -68 | -30 | 4.56 | 23 |
| R Cerebellum | 46 | -60 | -34 | 4.50 | 145 |
| LO | |||||
| R Precentral Gyrus (PMd) | 46 | -2 | 60 | 3.65 | 4 |
| R Precentral Sulcus (PMv) | 62 | -8 | 14 | 2.95° | 41 |
| R Intraparietal Sulcus | 46 | -54 | 52 | 3.59 | 23 |
| 50 | -40 | 50 | 2.95° | 33 | |
| 28 | -58 | 48 | 2.58° | 27 | |
| L Inferior Parietal Cortex (Angular Gyrus) | -60 | -58 | 28 | 3.29° | 58 |
| R Lateral Occipitotemporal Cortex | 38 | -62 | -18 | 4.77 | 75 |
| L Cerebellum | -26 | -42 | -22 | 3.37° | 122 |
| R Cerebellum | 24 | -58 | -38 | 3.37 | 14 |
Note: p <.001 uncorrected.
p < .01 uncorrected.
DISCUSSION
In the current study, we used fMRI to explore the neural correlates of the RHI in two upper limb amputees. In line with our hypothesis, in both particpants, the experience of the RHI was associated with premotor and intraparietal activity. In addition, we documented cerebellar as well as inferior parietal activation. These observations are interesting as they provide the first preliminary BOLD evidence for the fact that the rubber hand illusion can be induced in amputees. In addition, our study outlines an experimental procedure that can be used in future large-group imaging studies with this patient population. We will now discuss our findings in more detail.
The observed activation of premotor and intraparietal areas specific to the illusion condition is consistent with previous studies investigating the neural mechanisms underlying the RHI (Bekrater-Bodmann et al., 2012; Brozzoli, Gentile, & Ehrsson, 2012; Ehrsson et al., 2004, 2005) as well as the illusory ownership of an entire artificial body (Petkova et al., 2011) in healthy populations. Since self-attribution of a body part or an entire body is heavily reliant on a match between the visual and tactile signals originating from it (Botvinick & Cohen, 1998; Petkova & Ehrsson, 2008; Ehrsson, 2012), it has been proposed that the contribution of these areas is primarily of multisensory nature. That is, neuronal populations in these areas seem to be responsible for the binding of synchronous visuotactile events relating to a body part or body that is seen from a first-person perspective, with a consequent arising of ownership sensations (Ehrsson et al., 2004). This interpretation is supported by findings of studies with non-human primates revealing that both premotor and intraparietal areas contain neurons that integrate visual, tactile, and proprioceptive information in body part-centered reference frames (Avillac, Deneve, Olivier, Pouget, & Duhamel, 2005; Fogassi et al., 1996; Graziano, 1999).
We would like to note that the most significant premotor activation peaks of our two participants were in the more dorsal portions of the premotor cortex with respect to the peaks reported in previous RHI studies with healthy participants (Bekrater-Bodmann et al., 2012; Ehrsson et al., 2004). Whether this observation was merely specific to our two participants, or whether it might be found in other amputees as well, remains an open question. In any case, we did note activation of the ventral premotor cortex in one of the participants, and the observed the observed dorsal premotor activity is also very likely to reflect multisensory processing as there is evidence from both primate (Fogassi et al., 1999; Graziano & Gandhi, 2000) as well as human (Brozzoli, Gentile, Petkova, & Ehrsson, 2011; Gentile et al., 2011) studies that this portion also contains multisensory neurons and is involved in hand-centered visuotactile integration.
In addition to premotor and intraparietal activity, in both participants, we also observed bilateral cerebellar activation. This is also consistent with previous observations on the rubber hand illusion in healthy participants (Ehrsson et al., 2004, 2005), and it is possible to reflect the processing of proprioceptive signals from the limb and the visually driven recalibration of limb position sense (Hagura et al., 2009; Naito et al., 2005). In fact, in both healthy participants and amputees, the RHI has been shown to cause a shift in the felt position of one's own limb (Ehrsson et al., 2008; Tsakiris & Haggard, 2005). Furthermore, in healthy participants, it has been found to influence reaching movements (Zopf, Truong, Finkbeiner, Friedman, & Williams, 2011). The observed activations in the lateral cerebellar hemispheres were located posterior to the classical “motor” sections of the anterior cerebellar hemispheres. While the anterior regions of the cerebellum are connected to the primary motor cortices, these more posterior regions are known to be connected to premotor and posterior parietal cortices (Clower, Dum, & Strick, 2005; Clower, West, Lynch, & Strick, 2001; Dum, Li, & Strick, 2002; Orioli & Strick, 1989). The co-activation of posterior regions of the cerebellum in combination with the premotor and intraparietal cortices in both participants provides thus a rather compelling pilot fMRI evidence for the fact that amputees can experience the rubber hand illusion.
We also noted activations in some further regions that we would like to comment on. First, in both participants, we observed activity in the inferior posterior parietal cortex, with significant peaks of activation located in the supramarginal gyrus (TA) and angular gyrus (LO), respectively. These regions represent important nodes in a network encompassing premotor, parietal, insular, and extrastriate visual areas involved in the perception of the own body (Berlucchi & Aglioti, 2010). In fact, lesions to the inferior parietal cortex can result in a variety of body representation deficits, for example, in the inability to localize one's own body parts (i.e., autotopagnosia) (Corradi Dell'Acqua & Rumiati, 2007). Moreover, the supramarginal gyrus is a known site of multisensory convergence and visuotactile integration in particular in relation to the hand (Gentile et al., 2011), and has been shown to be active during more complex somatosensory illusions that involve a perceived movement of the hand and hand–object interaction (Naito & Ehrsson 2006). Similarly, the angular gyrus has been shown to be involved in complex somatosensory illusions (Blanke, Ortigue, Landis, & Seeck, 2002). The current pilot data from the two participants do not allow us to make any specific inferences about the potential involvement of the inferior parietal cortex in mechanisms producing the rubber hand illusion in amputees. However, they support the formulation of the interesting hypothesis that in this population, the illusion may represent a more dramatic change to the body representation (due to the nature of telescoped phantoms and the more drastic change in the visual appearance of the limb), which in turn might speculatively require additional processing in inferior parietal areas with respect to healthy individuals.
Lastly, for LO, we observed additional activation in a region of the occipitotemporal cortex, consistent with the EBA. Known for its general response selectivity for visually presented bodies and body parts (Downing et al., 2001; but see Weiner & Grill-Spector, 2013 for a critical review), the right EBA in particular has been found to differentially respond to pictures of body parts that are presented on a computer screen from a first-vs. a third-person perspective, and hence proposed to be involved in the discrimination of own body parts (Saxe, Jamal, & Powell, 2006). Previous studies on the rubber hand illusion (Ehrsson et al., 2004) and the full-body version of this illusion (Petkova et al., 2011), have observed activations in similar locations of the extrastriate cortex. Specifically, during these illusions, the activation was stronger for hands or entire bodies viewed from a first-person perspective. It can therefore be speculated that the occipitotemporal activation observed in our study might correspond to the EBA, in which case its activation might have been driven by viewing the rubber hand in a congruent position, and additionally strengthened during the illusion condition when the hand was actually perceived as belonging to oneself.
Albeit preliminary, the findings of our study are interesting from both a theoretical and clinical point of view. From a theoretical perspective, they provide the first demonstration that the illusory ownership of an artificial hand in amputees is driven by activity in the same multisensory brain regions as in healthy individuals. This implies that even years after the amputation, a few seconds of synchronous visuotactile stimulation are sufficient to activate hand-centered multisensory integration mechanisms. On a more general level, it also underlines the remarkable robustness of the RHI in the context of a perceived substantial change to the body configuration. In fact, as opposed to healthy participants, amputees are missing their real hand. Hence, in order for the RHI to be experienced, the amputees’ brain has to allow for a greater change to the body representation than in healthy individuals. First of all, it has to override the prior visual experience of the hand no longer being there. Second, in amputees with telescoped phantoms as the ones in our study, it has to accommodate a larger shift in perceived hand position as compared to limbed individuals. That is, the RHI does not merely represent a recalibration of the felt position of the phantom toward the midline, but also toward a more distal point with respect to the stump. We have previously demonstrated this malleability of the perceived position of telescoped phantom limbs following visuotactile stimulation in the context of a full-body illusion (Schmalzl et al., 2011). In sum, in amputees, the recalibration of the central body representation has to result in the perceptual fusion of the phantom hand and the rubber hand into a single physical hand that is a part of their own body. In order for this to occur, it is critical to stimulate according to an exact match between the point on the stump that evokes referred sensations in the phantom hand, and the corresponding point on the artificial hand.
From a clinical perspective, our findings underline the importance of sensory feedback from prosthetic limbs, by highlighting how it can facilitate ownership sensations. There is no doubt that technically more advanced techniques such as targeted reinnervation or direct stimulation of nerve fibers are necessary to provide the user with a more fine-tuned spectrum of somatic sensations. However, our observations suggest that in the context of congruent visual feedback, a very basic sensation of touch from an artificial hand can be obtained by simple but precisely targeted stimulation to even just a single point of the stump that triggers referred sensations. Such a mechanism could be easily implemented in prosthetic hands with an array of devices that connect sensors attached to a specific part of the artificial hand to stimulators attached to the corresponding part of the user's stump. So in a nutshell, the idea would be that each time the specific part of the prosthetic hand gets in contact with a surface or touches an object, this would trigger an immediate tactile stimulation on the stump, which in turn would trigger ownership sensations of the hand provided that the user would look at the hand at the same time (see Antfolk, Balkenius, Lundborg, Rosén, & Sebelius, 2010 for an example of a prototype tactile stump stimulation display). Of course, for clinical use, it would be ideal to be able to maintain a sense of ownership even while the user is not directly looking at the hand, as well as during temporary absence of tactile stimulation. Whether ownership sensations could temporarily be maintained even during the absence of either one type of stimulation through prolonged and repeated training, remains an open question. In any case however, the fact that ownership sensations can arise after only a few seconds of stimulations indicates that even if they vanished, they could be easily resurrected at any stage.
Lastly, consistently perceived ownership sensations can be expected to facilitate the general acceptance of the prosthesis and consequently positively influence the overall well-being of the amputee (Gallagher & MacLachlan, 1999). In fact, a prostheses providing the user not only with a cosmetic reconstitution of the lost limb but also with a more realistic experience of it, might support the restoring of a coherent feeling of the own body and in turn, the psychological and emotional adjustment to the amputation. Hence, our study highlights how principles stemming from research on body representation in healthy individuals can contribute to the understanding of the cognitive and neural mechanisms underlying body representation in amputees, the development of new prosthetic devices, as well as the implementation of clinical management strategies.
REFERENCES
- Antfolk C., Balkenius C., Lundborg G., Rosén B., Sebelius F. Design and technical construction of a tactile display for sensory feedback in a hand prosthesis system. BioMedical Engineering OnLine. 2010;9:50. doi: 10.1186/1475-925X-9-50. doi:10.1186/1475-925X-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avillac M., Deneve S., Olivier E., Pouget A., Duhamel J. R. Reference frames for representing visual and tactile locations in parietal cortex. Nature Neuroscience. 2005;8:941–949. doi: 10.1038/nn1480. [DOI] [PubMed] [Google Scholar]
- Bekrater-Bodmann R., Foell J., Diers M., Flor H. The perceptual and neuronal stability of the rubber hand illusion across contexts and over time. Brain Research. 2012;1452:130–139. doi: 10.1016/j.brainres.2012.03.001. doi:10.1016/j.brainres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Berlucchi G., Aglioti S. M. The body in the brain revisited. Experimental Brain Research. 2010;200:25–35. doi: 10.1007/s00221-009-1970-7. [DOI] [PubMed] [Google Scholar]
- Blanke O., Ortigue S., Landis T., Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Botvinick M., Cohen J. Rubber hands “feel” touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Brozzoli C., Gentile G., Ehrsson H. H. That's near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. Journal of Neuroscience. 2012;32:14573–14582. doi: 10.1523/JNEUROSCI.2660-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozzoli C., Gentile G., Petkova V. I., Ehrsson H. H. fMRI-adaptation reveals a cortical mechanism for the coding of space near the hand. Journal of Neuroscience. 2011;31:9023–9031. doi: 10.1523/JNEUROSCI.1172-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower D. M., Dum R. P., Strick P. L. Basal ganglia and cerebellar inputs to “AIP. Cerebral Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Clower D. M., West R. A., Lynch J. C., Strick P. L. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. Journal of Neuroscience. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi Dell'Acqua C., Rumiati R. I. What the brain knows about the body: Evidence for dissociable representations. In: Santoianni F., Sabatano C., editors. Brain development in learning environments: Embodied cognition and perceptual learning. Newcastle: Cambridge Scholars Publishing; 2007. pp. 50–64. [Google Scholar]
- Downing P. E., Jiang Y., Shuman M., Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Dum R. P., Li C., Strick P. L. Motor and non-motor domains in the monkey dentate. Annals of the New York Academy of Sciences. 2002;978:289–301. doi: 10.1111/j.1749-6632.2002.tb07575.x. [DOI] [PubMed] [Google Scholar]
- Ehrsson H. H. The concept of body ownership and its relation to Multisensory integration. In: Stein B. E., editor. The New Handbook of Multisensory Processes. Cambridge, MA: MIT Press; 2012. pp. 775–792. [Google Scholar]
- Ehrsson H. H., Holmes N. P., Passingham R. E. Touching a rubber hand: Feeling of body ownership is associated with activity in multisensory brain areas. Journal of Neuroscience. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson H. H., Rosén B., Stockselius A., Ragnö C., Köhler P., Lundborg G. Upper limb amputees can be induced to experience a rubber hand as their own. Brain. 2008;131:3443–3452. doi: 10.1093/brain/awn297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson H. H., Spence C., Passingham R. E. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Ehrsson H. H., Weich K., Weiskopf N., Dolan R. J., Passingham R. E. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. PNAS. 2007;104:9828–9833. doi: 10.1073/pnas.0610011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L., Gallese V., Fadiga L., Luppino G., Matelli M., Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4) Journal of Neurophysiology. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Raos V., Franchi G., Gallese V., Luppino G., Matelli M. Visual responses in the dorsal premotor area F2 of the macaque monkey. Experimental Brain Research. 1999;128:194–199. doi: 10.1007/s002210050835. [DOI] [PubMed] [Google Scholar]
- Gallagher S. How the body shapes the mind. Oxford: Oxford University Press; 2005. [Google Scholar]
- Gallagher P., MacLachlan M. Psychological adjustment and coping in adults with prosthetic limbs. Behavioral Medicine. 1999;25:117–124. doi: 10.1080/08964289909596741. [DOI] [PubMed] [Google Scholar]
- Gentile G., Petkova V., Ehrsson H. H. Integration of visual and tactile signals from the hand in the human brain: An fMRI study. Journal of Neurophysiology. 2011;105:910–922. doi: 10.1152/jn.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M. S. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. PNAS. 1999;96:10418–10421. doi: 10.1073/pnas.96.18.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M. S. A., Gandhi S. Location of the polysensory zone in the precentral gyrus of anesthetized monkeys. Experimental Brain Research. 2000;135:259–266. doi: 10.1007/s002210000518. [DOI] [PubMed] [Google Scholar]
- Hagura N., Oouchida Y., Aramaki Y., Okada T., Matsumura M., Sadato N., Naito E. Visuokinesthetic perception of hand movement is mediated by cerebro-cerebellar interaction between the left cerebellum and right parietal cortex. Cerebral Cortex. 2009;19:176–186. doi: 10.1093/cercor/bhn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao S. S., Fettiplace M., Darbandi B. Sensory feedback for upper limb prostheses. In: Green A., Venkatasamy G., editors. Progress in brain research: Enhancing performance for action and perception – Multisensory integration, neuroplasticity and neuroprosthetics. Oxford: Elsevier; 2011. pp. 69–81. [Google Scholar]
- Jackson A., Fetz E. Interfacing with the computational brain. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2011;19:534–541. doi: 10.1109/TNSRE.2011.2158586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R. S., Flanagan J. R. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nature Reviews Neuroscience. 2009;10:345–359. doi: 10.1038/nrn2621. [DOI] [PubMed] [Google Scholar]
- Jones L. A. Tactile communication systems: Optimizing the display of information. In: Green A., Venkatasamy G., editors. Progress in brain research: Enhancing performance for action and perception – Multisensory integration, neuroplasticity and neuroprosthetics. Oxford: Elsevier; 2011. pp. 113–128. [Google Scholar]
- Kuiken T. A., Marasco P. D., Lock B. A., Harden R. N., Dewald P. A. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. PNAS. 2007;104:20061–20066. doi: 10.1073/pnas.0706525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M. D. Spatial limits on referred touch to an alien limb may reflect boundaries of visuo-tactile peripersonal space surrounding the hand. Brain and Cognition. 2007;64:104–109. doi: 10.1016/j.bandc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Logothetis N. K., Pauls J., Augath M., Trinath T., Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lundborg G., Rosén B. Sensory substitution in prosthetics. Hand Clinics. 2001;17:481–488. [PubMed] [Google Scholar]
- Marasco P. D., Kim K., Colgate J. E., Peshkin M., Kuiken T. A. Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain. 2011;134:747–758. doi: 10.1093/brain/awq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E., Ehrsson H. H. Somatic sensation of hand-object interactive movement is associated with activity in the left inferior parietal cortex. Journal of Neuroscience. 2006;26:3783–3790. doi: 10.1523/JNEUROSCI.4835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E., Roland P. E., Ehrsson H. H. Role of area 2 and dominance of the right hemisphere in human kinaesthesia. Journal of Neurophysiology. 2005;93:1020–1034. doi: 10.1152/jn.00637.2004. [DOI] [PubMed] [Google Scholar]
- Nicolelis M. A. L. Brain–machine interfaces to restore motor function and probe neural circuits. Nature Reviews Neuroscience. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- Orioli P. J., Strick P. L. Cerebellar connections with the motor cortex and the arcuate premotor area: An analysis employing retrograde transneuronal transport of WGA-HRP. Journal of Comparative Neurology. 1989;288:612–626. doi: 10.1002/cne.902880408. [DOI] [PubMed] [Google Scholar]
- Pavani F., Spence C., Driver J. Visual capture of touch: Out of the body experiences with rubber gloves. Psychological Science. 2000;11:353–359. doi: 10.1111/1467-9280.00270. [DOI] [PubMed] [Google Scholar]
- Petkova V. I., Björnsdotter M., Gentile G., Jonsson T., Li T. Q., Ehrsson H. H. From part to whole-body ownership in the multisensory brain. Current Biology. 2011;21:1–5. doi: 10.1016/j.cub.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Petkova V. I., Ehrsson H. H. If I were you: Perceptual illusion of body swapping. PLoS One. 2008;3:e3832. doi: 10.1371/journal.pone.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén B., Ehrsson H. H., Antfolk C., Cipriani C., Sebelius F., Lundborg G. Referral of sensation to an advanced humanoid robotic hand prosthesis. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 2009;43:260–266. doi: 10.3109/02844310903113107. [DOI] [PubMed] [Google Scholar]
- Saxe R., Jamal N., Powell L. My body or yours? The effect of visual perspective on cortical body representations. Cerebral Cortex. 2006;16:178–182. doi: 10.1093/cercor/bhi095. [DOI] [PubMed] [Google Scholar]
- Schmalzl L., Thomke E., Ragnö C., Nilseryd M., Stockselius A., Ehrsson H. H. “Pulling telescoped phantoms out of the stump”: Manipulating the perceived position of phantom limbs using a full-body illusion. Frontiers in Human Neuroscience. 2011;5:121. doi: 10.3389/fnhum.2011.00121. doi:10.3389/fnhum.2011.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. B. Cortical neural prosthetics. Annual Review of Neuroscience. 2004;27:487–507. doi: 10.1146/annurev.neuro.27.070203.144233. [DOI] [PubMed] [Google Scholar]
- Sebelius F. C., Rosén B., Lundborg G. N. Refined myoelectric control in below-elbow amputees using artificial neural networks and a data glove. Journal of Hand Surgery. 2005;30:780–789. doi: 10.1016/j.jhsa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Carpenter L., James D., Fotopoulou A. Hands only illusion: Multisensory integration elicits sense of ownership for body parts but not for non-corporeal objects. Experimental Brain Research. 2010;204:343–352. doi: 10.1007/s00221-009-2039-3. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Haggard P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. Journal of Experimental Psychology: Human Perception & Performance. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Schutz-Bosbach S., Gallagher S. On agency and body-ownership: Phenomenological and neurocognitive reflections. Consciousness and Cognition. 2007;16:645–660. doi: 10.1016/j.concog.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Velliste M., Perel S., Spalding M. C., Whitford A. S., Schwartz A. B. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- Weiner K. S., Grill-Spector K. Neural representations of faces and limbs neighbor in human high-level visual cortex: Evidence for a new organization principle. Psychological Research. 2013;77:74–97. doi: 10.1007/s00426-011-0392-x. doi:10.1007/s00426-011-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D. M., Diedrichsen J., Flanagan J. R. Principles of sensorimotor learning. Nature Reviews Neuroscience. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- Wolpert D. M., Ghahramani Z. Computational principles of movement neuroscience. Nature Neuroscience. 2000;3:1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Zopf R., Truong S., Finkbeiner M., Friedman J., Williams M. A. Viewing and feeling touch modulates hand position for reaching. Neuropsychologia. 2011;49:1287–1293. doi: 10.1016/j.neuropsychologia.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


