Abstract
Purpose
This study examines template-based squared-difference registration for motion correction in dynamic contrast-enhanced (DCE) MRI studies of the carotid artery wall and compares the results of fixed-frame template-based registration with a previously proposed consecutive-frame registration method.
Materials and Methods
Ten T1-weighted black-blood, turbo spin-echo DCE-MRI studies of the carotid artery wall were used to test template-based squared-difference registration. An intermediate image from each series was selected as the fixed-frame template for registration. Squared-difference minimization was used to align each image and template. Time-intensity curves generated from data aligned with template-based squared-difference registration were compared with gold standard curves created by drawing regions of interest on each image in the series. The results were also compared with unregistered data and data after consecutive-frame squared-difference registration.
Results
An analysis of variance test of root mean-square error values between gold standard curve and curves from unregistered data and data registered with consecutive-frame and fixed-frame template-based methods was significant (P < 0.005) with template-based squared-difference registration producing curves that most closely matched the gold standard.
Conclusion
A fixed-frame template-based squared-difference registration method was proposed and validated for alignment of DCE-MRI of carotid arteries.
Keywords: registration, template-matching, vascular DCE-MRI
Identifying vulnerable/high-risk atherosclerotic plaques is critical for planning treatment and intervention for atherosclerosis. Inflammation and neovascularization are markers of vulnerable plaques (1–3). Dynamic contrast-enhanced (DCE) MRI has recently been applied to noninvasively quantify plaque neovascularization (4–6), and in preclinical (7,8) and multicenter clinical trials (9,10) to evaluate anti-atherosclerotic intervention. Accurately tracking signal enhancement in the vessel wall requires that all images in the DCE-MRI series be properly aligned. However, the acquisition time for these sequences can be quite long (~8–13 min), resulting in images degraded by patient motion during the scan. A registration method needs to be applied to re-align the DCE-MRI series images before performing further analysis.
Many registration methods have been developed for the alignment of DCE-MRI of organs such as the heart and kidneys (11). However, these methods cannot be directly applied to current vascular DCE-MRI protocols, because of the small size of the carotid arteries (less than 1 cm in diameter) with respect to other organs, and the prominent use of two-dimensional (2D) multislice, instead of 3D volumetric, acquisitions (12). Therefore, 2D intensity-based rigid registration techniques are best suited to correct translational shifts due to interframe motion during the scan. Intensity-based registration metrics such as squared-difference can be used to align images by maximizing the similarity in the signal intensities among different frames. Previously, a sequential registration method has been described for carotid DCE-MRI (13), where each image in the series is aligned to the image acquired immediately preceding it. This type of “consecutive-frame” registration can result in the accumulation of subpixel errors during registration (14). A better approach may be to align all the frames to a single image or fixed-frame “template” selected from the DCE-MRI series. In this study, we propose combining the squared-difference metric with template matching for registration of carotid artery DCE-MRI.
The fixed-frame template-based squared-difference registration method was applied to DCE-MRI data to correct for inter-frame motion artifacts. The results were quantified and validated by comparing to “gold-standard” data obtained by manually drawing regions of interest (ROIs) on the carotid artery wall on every image. The results of template-based registration were also compared with unregistered data and the results of consecutive-frame registration.
MATERIALS AND METHODS
Image Acquisition
DCE-MRI studies of common carotid arteries of 10 human subjects with risk factors for cardiovascular disease were used to test the registration methods. The study protocol was approved by the institutional review board and informed consent was obtained from every subject. The subjects were instructed to lie still and refrain from swallowing during the scan. All images were acquired on a 3 Tesla (T) MRI whole body scanner (Philips Achieva) using a dedicated eight-channel carotid coil. DCE-MRI was performed using a previously validated multislice, T1-weighted black blood turbo spin-echo sequence (see Table 1 for imaging parameters)(6). After three precontrast frames, 0.1 mmol/kg of Gd-DTPA was injected using a power injector at a rate of 2 mL/s, followed by a 20-mL saline chase (15).
Table 1.
DCE-MRI Acquisition Parameters in the 2D Double-Inversion Recovery (DIR) Turbo Spin-Echo (TSE) Sequence for DCE-MRI of Carotid Arteries
| Imaging parameter | 2D DIR TSE DCE |
|---|---|
| Acquisition plane | Axial |
| Number of slices | 4 |
| Slice thickness | 3 mm |
| Inter-slice gap | 0.3 mm |
| Field of view | 160 mm × 160 mm |
| Spatial resolution | 0.5 mm × 0.5 mm |
| Repetition time | 1000 ms |
| Echo time | 8.27 ms |
| Inversion time | 162 ms |
| Turbo factor | 15 |
| SENSE parallel imaging | 3 |
| Time resolution | 32s |
| Number of frames | 25 |
| Total acquisition time | ~13 min |
Image Analysis
In all subjects, either the right or left carotid artery from one slice were randomly chosen for analysis, thus yielding a dataset consisting of five left and five right carotid DCE-MRI series. DICOM images were imported into a custom-made MATLAB (MathWorks, Natick, MA) software package for analysis. Images were cropped to a 64 × 64-pixel area surrounding the artery as shown in Figure 1a, where registration was performed using either template-based or consecutive-frame registration. Results of both registrations were compared with the original data before registration and to gold standard data generated by manually tracing the vessel wall on every frame in the series. A detailed description of the registration methods follows.
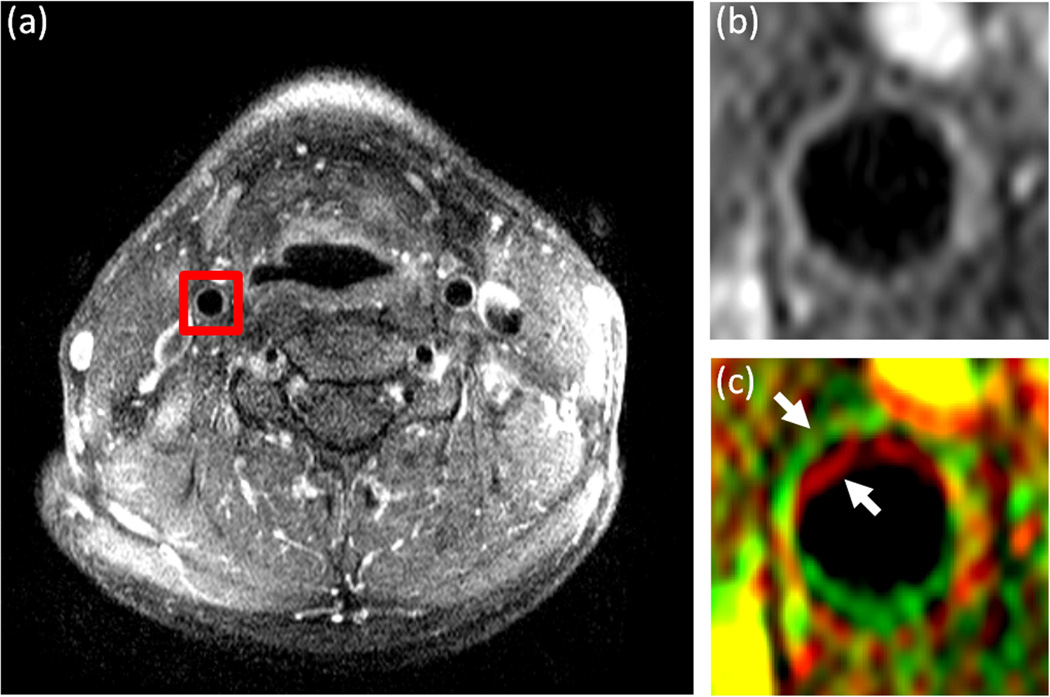
Figure 1.
DCE-MR images of the carotid arteries in the neck of a representative subject. a: A single image from a DCE-MRI series with a red square surrounding the right carotid artery indicating the cropped region used for analysis. b: The 14th frame in the DCE-MRI series used as the template image for registration and (c) the 15th frame in the series (rendered in green) overlaid on the 14th frame (rendered in red). The two consecutive frames are overlaid to show the misalignment between images in the series before registration. The arrows indicate the position of the carotid artery wall in each of the frames.
Squared-Difference Metric
The squared-difference metric aligns two images by minimizing the sum of the squared intensity difference between the images. Squared-difference (SD) is defined as (13)
| (1) |
where I is the image to be aligned and x and d are the position and displacement in the x–y plane, respectively. Iref is the reference image to which the image I is registered. The reference image is either the template in template-based registration, or the preceding image in consecutive-frame registration. By varying d and displacing I, it is possible to find shifts in x- and y-directions that generate a minimum SD value.
Each image was displaced iteratively through ±10-pixel range of shifts in both the x- and y-directions and the SD between the two images was computed at each displacement. A quadratic patch was fit to the 3 × 3 SD values centered on the minimum SD value. The minimum of the quadratic patch was the final displacement calculated to subpixel accuracy. The image I was translated by this final displacement in the Fourier domain, using the Fourier shift theorem.
Template-Based Registration
For template-based registration, one image in each series was selected as a fixed-frame “template” image (16,17), and all images in the series were registered to this template. An image midway through the series was chosen as the template, as an intermediate image would have intensities and motion comparable to the images acquired before and after it. Therefore, before registration, the 13th image of each series was examined visually to determine if it was an appropriate template using the following criteria: (i) The contours of the carotid artery wall were clearly visible in the image; (ii) No flow artifacts in the lumen of the vessel in the image; (iii) Good contrast-to-noise ratio between the vessel wall and the lumen.
If the 13th frame met all the criteria, it was designated as the template for the series. If not, adjacent images (either the 12th or the 14th frame) were examined and selected as the template instead. Figure 1b shows the 14th image from a 25-frame DCE-MRI sequence used as the registration template for that series. Figure 1c displays the 15th frame overlaid on the template from Figure 1b to the show the misalignment between sequential frames and the need for registration. Once the template was selected, all the remaining frames in the series were registered to the fixed-frame template using the squared-difference measure.
Consecutive-Frame Registration
In consecutive-frame registration each image was registered to the preceding frame in the series using the squared-difference in intensities as the metric for minimization. Thus, each image served as the template for matching the following frame.
Validation of Registration
Various methods were used to validate the results of registration and quantify improvement after application of the registration techniques. The simplest validation method consisted in visual inspection of the average image of each DCE-MRI series before and after registration. For a more quantitative approach, enhancement curves were generated from the DCEMRI series. A gold standard time-intensity curve (18,19) was created by drawing a ROI on the carotid artery wall in each DCE-MRI frame from in the original data to get the mean signal intensity at that time point. While this gold standard curve shows the actual tissue uptake of the contrast agent, in practice this approach would increase time and effort needed to analyze a study. A more efficient method was to trace the artery wall on the average image of the registered series and to propagate this ROI throughout the whole series to generate enhancement curves. Curves produced by drawing ROIs on the average original and postregistration images were compared with the gold standard curves. All ROIs for this study were drawn by the first author (SR).
Root mean squared-error (RMSE) values were computed between the gold standard curve, the original unregistered data curve and curves generated after application of each registration method for each subject. A one-way, repeated-measures analysis of variance (ANOVA) test was used to compare RMSE values for all subjects before and after registration with consecutive- frame and template-based registration methods.
Average absolute registration errors were computed as the absolute difference between the manual shifts introduced in the gold standard method and the shifts generated by each registration method, respectively, and averaging across the subject population.
RESULTS
Figure 2 displays some frames from the DCE-MRI series of the right common carotid artery of a representative subject before and after registration. The absolute difference between the images and template before and after registration shows the “correction” made to align the images and template.
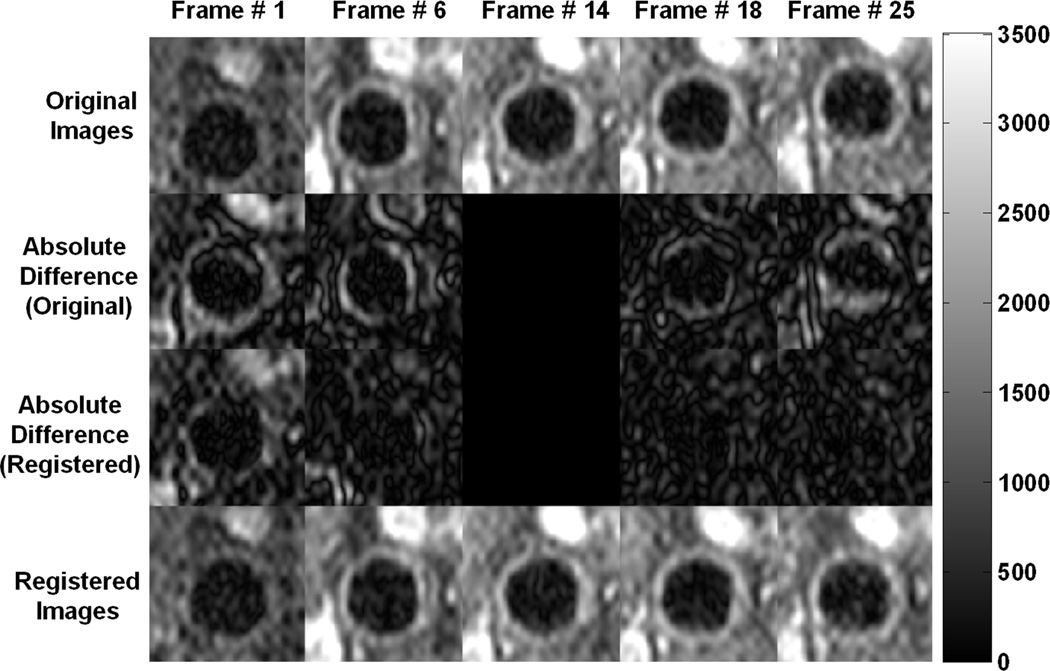
Figure 2.
Several frames from a DCE-MRI series of a typical subject are presented to illustrate the motion of the carotid artery over the course of the sequence acquisition and the need for registration. In the original images (top row), the vessel changes position from near the base of the image in frame # 1 to closer to the top in frame # 25. In the registered images (bottom row), the position of the vessel remains relatively stable in all the frames. Frame # 14 was the template image for this subject (middle column). The second row shows the absolute difference between the original images and the template image, and the third row shows the corresponding difference between the registered images and the template. After registration, there is much less difference between the various frames and the template, indicating that the images were aligned appropriately. All images are displayed with the same intensity levels for comparison.
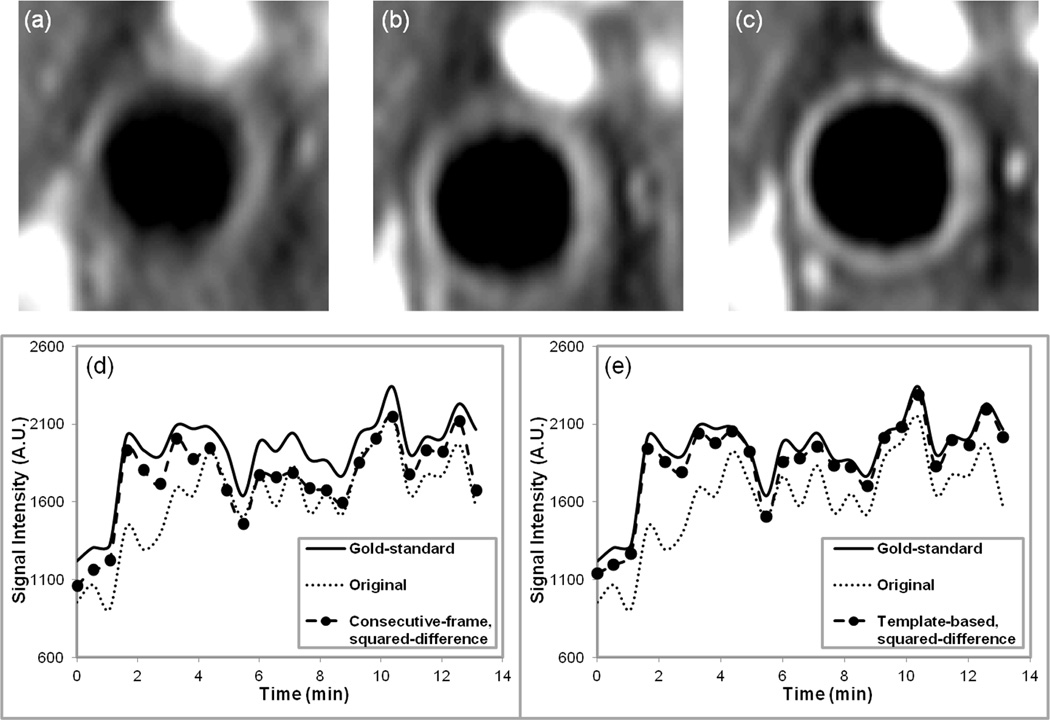
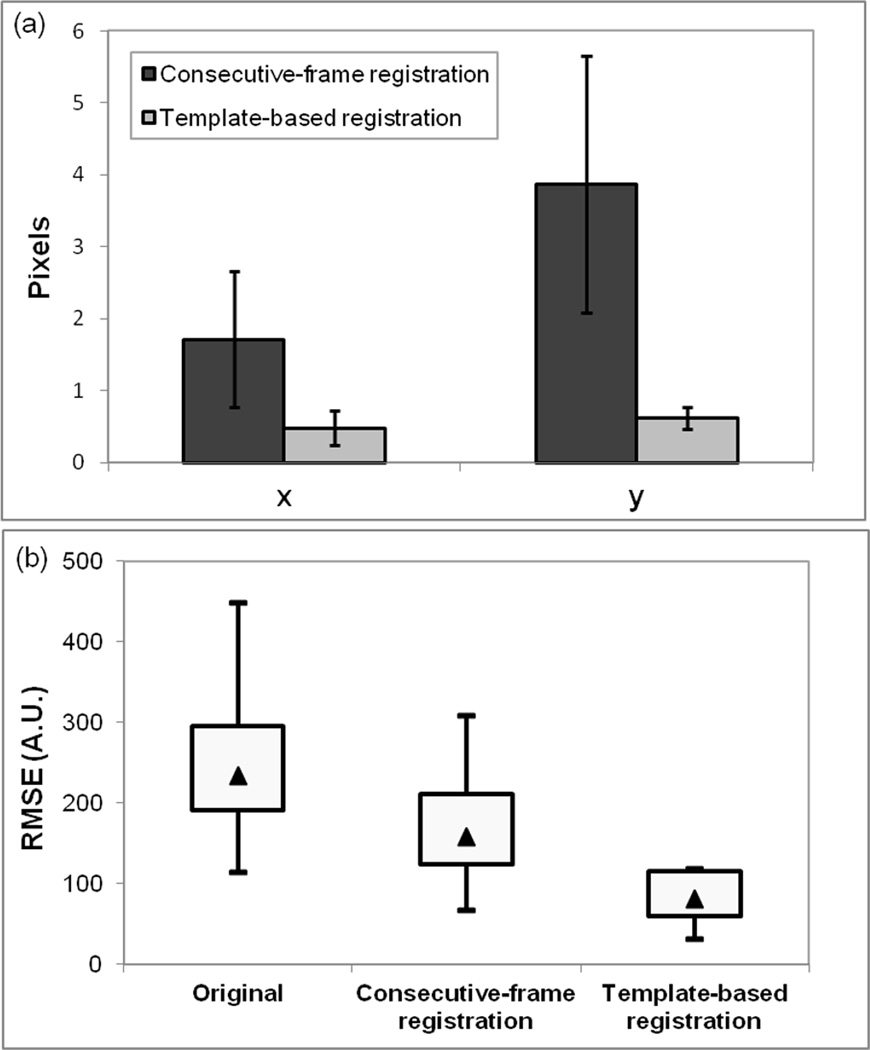
The improvement due to registration may be demonstrated by taking the mean of all the images in a series before and after registration. Figure 3a–c shows the average images for the original DCE-MRI data from the same representative subject shown in Figure 2, and after consecutive-frame and template-based registration. In the unregistered data the position of the artery changed from image to image in the series, which was reflected in the average (Fig. 3a) by the blurring of the artery wall. The average image after template-based registration (Fig. 3c) is much sharper and shows better contrast-to-noise ratio between vessel lumen and wall, therefore, making it easier to trace the vessel wall contours. The average image after consecutive-frame registration (Fig. 3b) showed some improvement over the original average image, but the edges of the carotid artery wall were still indistinct in some areas. Tracking the boundaries of the carotid artery in the original average image and after consecutive-frame registration was difficult and could cause errors in the analysis. The signal-intensity versus time graphs generated from gold standard data, original data, and data registered using the consecutive-frame and template-based methods for the representative subject are also plotted in Figure 3d,e. The original data significantly underestimated the signal from the influx of contrast agent into the artery wall. The results of consecutive-frame registration (Fig. 3d) captured the upslope of the gold-standard curve, however, at all other time-points, the signal intensity values were consistently below those of the gold-standard curve. Template-based registration (Fig. 3e) produced the best curve that closely followed contours of the gold standard curve. Because the template-based registration curve was generated by drawing a single ROI on the average image after registration and propagating the ROI, nearly identical results to the gold standard were achieved in a fraction of the time. The average absolute registration errors, presented in Figure 4a, for template-based registration (compared with the gold standard ROIs) were 0.48 ± 0.24 pixels and 0.62 ± 0.15 pixels in the x- and y-direction, respectively. The average absolute registration errors for consecutive-frame registration were 1.71 ± 0.95 pixels and 3.87 ± 1.79 pixels, respectively.
Figure 3.
Average images and time-intensity curves. Images obtained after averaging together all 25 frames in a DCE-MRI series (a) original data, before registration, (b) after registration with the consecutive-frame squared-difference method, (c) after registration with the template-based squared-difference method. All images are displayed with the same intensity levels for comparison. Both plots in (d) and (e) display the gold-standard and original data time-intensity curves along with the consecutive- frame curve in (d) and the template-based time-intensity curve in (e).
Figure 4.
a: Column graph showing the average absolute registration errors over the subject population in the x- and y-directions for consecutive-frame and template-based registration. b: Box plot of the average RMSE values over the test population before registration, and after registration with consecutive-frame and template-based methods. The bottom and top of each box represents the 25th and 75th percentile of RMSE values respectively and the ends of the whiskers in the box plot represent the maximum and minimum RMSE values for each method. The triangle in each box represents the median RMSE value for the method.
Comparison of the average RMSE between the various curves and the gold standard curve over the test population is shown in the boxplot in Figure 4b. The average RMSE values for template-based registration were much lower than those for original data and consecutive-frame registration for all subjects. The results of the ANOVA test showed there was a statistically significant difference in the RMSE values before and after registration with consecutive-frame and template-based methods (P < 0.005), with F = 13.673 and degrees of freedom 1.161 and 10.452 between groups and within groups, respectively. The average RMSE error value for original data with respect to the gold standard was 249.89 ± 93.42 A.U. Post hoc tests using the Bonferroni correction indicated that consecutive-frame registration resulted in slightly lower RMSE values (176.36 ± 81.61 A.U.) compared with unregistered data, but there was no significant difference from the original data (P = 0.126). After template-based registration, RMSE values were reduced to 82.58 ± 32.81 A.U., which was strongly significant when compared with the RMSE values of unregistered data (P < 0.0005). The template-based method also significantly differed from consecutive-frame registration (P < 0.005).
DISCUSSION
In this study, we evaluated a fixed-frame template-based squared-difference registration method for motion correction in DCE-MR images of the carotid artery wall. One of the challenges of vascular DCEMRI is the small size of the vessel wall, which requires imaging with at high in-plane spatial resolution (0.5–0.7 mm2) to capture plaque heterogeneity (12). The need for high spatial and temporal resolution for accurate perfusion quantification has limited vascular DCE-MRI to 2D multislice rather than true 3D volume acquisitions. Because the registration is an entirely intraseries process, intensity-based registration may be used. Therefore, a simple 2D intensity-based registration technique that corrects for in-plane rigid translational motion was developed for DCE-MRI of the common carotid artery. This type of registration aims to correct motion that happens between temporal frames acquired sequentially (interframe motion). Correction of any intraframe motion, i.e., movement that occurs during the acquisition of a frame of data which can significantly lower image quality of single frames, still remains a challenge, and is beyond the scope of this study.
Registration was achieved by matching all the images to a fixed-frame template chosen from within the series, using a squared-difference metric. Template selection required some user interaction as the template needed to be carefully chosen for each DCEMRI series using the criteria detailed in the Materials and Methods section. In practice, we found that selection of a good template image substantially increased registration efficacy. More development is needed to automate the template selection step, and thus the entire registration process.
The results of fixed-frame template-based squared-difference registration were compared with the original unregistered data and after registration with a consecutive-frame squared-difference method. The template-based registration technique performed consistently better than the consecutive-frame method. In the consecutive-frame method, any registration error between two consecutive images is propagated to the rest of the series when the inaccurately aligned image is used to register the next sequential frame (14). The fixed-frame template-based method avoids this error as each image is registered to the same template, restricting the alignment error to only that specific image, with no accumulation of errors over repeated sequential registrations. Group-wise registration methods try to avoid any bias to the template by aligning frames to the average image (20); however, we find that the average image before registration is too blurry to serve as a suitable template (Fig. 3a). Once all the images in a series are correctly registered, the average image can be used to obtain the enhancement curves as described in the Methods section. By providing a sharp average image after registration, the template-based method produced the most accurate time-intensity curves for all 10 test datasets compared with consecutive-frame registration. As it can be seen in Figure 3e, the template-based method produced time-intensity curves closest to the gold standard, which was obtained by drawing ROIs on the carotid artery wall on each frame to obtain the mean signal intensity at that time point. The ability to generate accurate time-intensity curves after drawing only one ROI substantially reduces the processing time for a study. After registration, Kalman filtering can be applied to the enhancement curves for smoothing, as described by Kerwin et al (13). For our purposes, the effects of temporal smoothing introduced by the Kalman filter can obscure the extent of motion correction achieved by any registration method, and may conceal subpixel errors in the consecutive-frame method. Therefore, filtering was not included in this study, although the template-based registration technique is flexible enough to incorporate the Kalman filter for temporal smoothing. Future work includes incorporation of temporal filtering to smooth the curves, development of an automated template-selection procedure, and application of template-based registration to a larger patient database of DCE-MRI studies.
In conclusion, fixed-frame template-based squared-difference registration was shown to be a robust, semiautomated method for interframe, rigid-body motion correction in DCE-MRI of the human carotid artery. The time-intensity curves generated from data registered with the template-based method were closest to the curves that would be generated by manually aligning all the images in the series.
ACKNOWLEDGMENTS
Funding for this research was provided in part by NIH NHLBI R01 HL071021 and Siemens Medical Solutions (Malvern, PA).
REFERENCES
- 1.Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, Finn AV, Kolodgie FD, Gold HK, Virmani R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nat Clin Pract Cardiovasc Med. 2007;4:491–502. doi: 10.1038/ncpcardio0979. [DOI] [PubMed] [Google Scholar]
- 3.Langheinrich AC, Kampschulte M, Buch T, Bohle RM. Vasa vasorum and atherosclerosis - Quid novi? Thromb Haemost. 2007;97:873–879. [PubMed] [Google Scholar]
- 4.Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241:459–468. doi: 10.1148/radiol.2412051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaens ME, Backes WH, Rozel S, et al. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: model selection, reproducibility, and validation. Radiology. 2013;266:271–279. doi: 10.1148/radiol.12120499. [DOI] [PubMed] [Google Scholar]
- 6.Calcagno C, Cornily JC, Hyafil F, et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–1317. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobatto ME, Calcagno C, Metselaar JM, et al. Imaging the efficacy of anti-inflammatory liposomes in a rabbit model of atherosclerosis by non-invasive imaging. Methods Enzymol. 2012;508:211–228. doi: 10.1016/B978-0-12-391860-4.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vucic E, Dickson SD, Calcagno C, et al. Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. JACC Cardiovasc Imaging. 2011;4:1100–1109. doi: 10.1016/j.jcmg.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong L, Kerwin WS, Chen H, et al. Carotid artery atherosclerosis: effect of intensive lipid therapy on the vasa vasorum–evaluation by using dynamic contrast-enhanced MR imaging. Radiology. 2011;260:224–231. doi: 10.1148/radiol.11101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayad ZA, Mani V, Woodward M, et al. Rationale and design of dal-PLAQUE: a study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Am Heart J. 2011;162:214.e2–221.e2. doi: 10.1016/j.ahj.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Ghafourian P, Sharma P, Salman K, Martin D, Fei B. Nonrigid registration and classification of the kidneys in 3D dynamic contrast enhanced (DCE) MR Images. Proc SPIE. 2012;8314:83140B. doi: 10.1117/12.912190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balu N, Chu B, Hatsukami TS, Yuan C, Yarnykh VL. Comparison between 2D and 3D high-resolution black-blood techniques for carotid artery wall imaging in clinically significant atherosclerosis. J Magn Reson Imaging. 2008;27:918–924. doi: 10.1002/jmri.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerwin WS, Cai J, Yuan C. Noise and motion correction in dynamic contrast-enhanced MRI for analysis of atherosclerotic lesions. Magn Reson Med. 2002;47:1211–1217. doi: 10.1002/mrm.10161. [DOI] [PubMed] [Google Scholar]
- 14.Andersson JL. How to obtain high-accuracy image registration: application to movement correction of dynamic positron emission tomography data. Eur J Nucl Med. 1998;25:575–586. doi: 10.1007/s002590050258. [DOI] [PubMed] [Google Scholar]
- 15.Mani V, Wong SK, Sawit ST, et al. Relationship between particulate matter exposure and atherogenic profile in “Ground Zero” workers as shown by dynamic contrast enhanced MR imaging. Int J Cardiovasc Imaging. 2012 doi: 10.1007/s10554-012-0154-x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallippi C, Trahey G, Insana M, Leahy R. Information Processing in Medical Imaging. Vol. 2082. Springer Berlin/Heidelberg: Lecture Notes in Computer Science; 2001. Automatic image registration for MR and ultrasound cardiac images; pp. 148–154. [Google Scholar]
- 17.Gallippi CM, Kramer CM, Hu YL, Vido DA, Reichek N, Rogers WJ. Fully automated registration and warping of contrast-enhanced first-pass perfusion images. J Cardiovasc Magn Reson. 2002;4:459–469. doi: 10.1081/jcmr-120016384. [DOI] [PubMed] [Google Scholar]
- 18.Bidaut LM, Vallee JP. Automated registration of dynamic MR images for the quantification of myocardial perfusion. J Magn Reson Imaging. 2001;13:648–655. doi: 10.1002/jmri.1092. [DOI] [PubMed] [Google Scholar]
- 19.Dornier C, Ivancevic MK, Thevenaz P, Vallee JP. Improvement in the quantification of myocardial perfusion using an automatic spline-based registration algorithm. J Magn Reson Imaging. 2003;18:160–168. doi: 10.1002/jmri.10351. [DOI] [PubMed] [Google Scholar]
- 20.Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23(Suppl 1):S151–S160. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]