Abstract
Background
Obesity is an independent risk factor for cardiovascular diseases. The effects of obesity on left ventricular structure and function have been reported, but relatively little is known regarding right ventricular (RV) function in obesity.
Objective
To evaluate subclinical RV alterations in obese, but otherwise healthy, young adults by conventional echocardiography and tissue Doppler imaging (TDI).
Methods
In this study, we included 35 normal weight healthy subjects with a body mass index (BMI) < 25 kg/m2 (group I), 27 subjects with a BMI of 30-34.99 kg/m2 (group II), and 42 subjects with a BMI ≥ 35 kg/m2 (group III). All subjects underwent transthoracic echocardiography. In addition to standard echocardiographic measurements, tricuspid annular peak systolic (Sm), peak early (Em), and late diastolic (Am) velocities, isovolumetric contraction (ICTm), relaxation (IRTm) time, and ejection time (ETm) were obtained by TDI, and RV myocardial performance index (MPIm) was calculated.
Results
In group II, RV Em/Am was significantly decreased and IRTm and MPIm were significantly increased compared to group I (p < 0.01). RV Sm, Em, and the Em/Am ratio were significantly lower and RV IRTm and MPIm were significantly higher in group III than in group II (p < 0.05 for RV Sm and IRTm and p < 0.01 for others). RV Am differed significantly between groups III and I (p < 0.05). BMI was significantly and negatively correlated with RV Sm, Em, and the Em/Am ratio, but positively correlated with RV MPI (p < 0.01).
Conclusion
Our study showed that isolated obesity in young normotensive adults was associated with subclinical abnormalities in RV structure and function.
Keywords: Obesity; Young Adult; Ventricular Function, Right; Echocardiography, Doppler
Introduction
Obesity is a chronic, progressive disease with increasing prevalence in both developed and developing countries, and represents an independent risk factor for hypertension, diabetes mellitus, dyslipidemia, and cardiovascular diseases, such as coronary artery disease, atrial fibrillation, and congestive heart failure1-3. Although the exact mechanisms leading to heart failure in obese patients have not been clarified, obesity has been linked to a spectrum of cardiovascular changes from hyperdynamic circulation through subclinical changes in cardiac structure that eventually manifest in heart failure3.
Obesity increases cardiac workload by increasing total blood volume and cardiac output. Incremental increases in left ventricular (LV) filling pressure and volume may ultimately produce chamber dilatation and LV hypertrophy2. Many studies have shown eccentric LV hypertrophy, LV diastolic dysfunction, and occasionally LV systolic dysfunction in long standing obesity3-10. Similar to that of the LV, obesity may affect right ventricular (RV) function through increased cardiac output and obesity-related obstructive sleep apnea, both of which increase pulmonary artery pressure and might lead to RV dysfunction. However, previous studies have revealed conflicting data regarding the effects of obesity on the RV and still less is known about RV structure and function in obese subjects11-15.
Clinical assessment of RV function by echocardiography is challenging due to the retrosternal position and complex shape of the RV and although three-dimensional echocardiography has provided promising results, it is time consuming and has limited practicability. Tissue Doppler imaging (TDI) is a novel echocardiographic technique that allows noninvasive assessment of regional myocardial velocities and offers advantages over conventional imaging of RV because it is clinically useful and potentially less load-dependent than other echocardiographic markers of RV function16.
In this study, we evaluated the impact of isolated obesity on RV structure and function in normotensive young adults (age < 40 years) by use of TDI measurements in addition to conventional echocardiographic parameters17.
Methods
Patient selection
In the present study, we enrolled 69 obese subjects (30 males and 39 females; age < 40 years; mean age 32.0 ± 5.3 years) who were admitted to our cardiology clinic for routine cardiovascular assessment and found to be free of cardiovascular and pulmonary diseases. Age- and sex-matched, healthy, normal weight volunteers among hospital staff or their relatives served as the control group (group I; n = 35; 15 men and 20 women; mean age 30.1 ± 4.9 years). Obesity was defined as a body mass index (BMI) of ≥ 30 kg/m2 and normal weight as a BMI of < 25 kg/m2 17. Obese patients were further divided into two subgroups: (1) patients with a BMI of 30-34.9 kg/m2 (group II, n = 27) and (2) patients with BMI > 35 kg/m2 (group III, n = 42). We excluded subjects with poor quality echocardiographic images, hypertension, diabetes mellitus, coronary artery disease, heart failure, cardiac valve disease, arrhythmia, and hepatic, renal, endocrine, or respiratory diseases. All participants provided informed consent and the study protocol was approved by the institutional ethics committees of the participating institutions.
Clinical assessment
Demographic information including age, gender, family history, personal habits (i.e., alcohol intake, tobacco consumption, drug ingestion, and known pathological conditions), functional status, and duration of obesity were obtained from all patients. Arterial blood pressure was measured after subjects had rested for > 5 min in a sitting position in a quiet room. A detailed physical examination was conducted to exclude endocrine and cardiovascular comorbidities and a 12-lead ECG was obtained. Routine hematological and biochemical variables were determined from fasting blood samples and included glucose, total cholesterol, triglycerides, high density lipoprotein cholesterol, urea, creatinine, liver function tests, free triiodothyronine, free thyroxine, thyroid-stimulating hormone, and complete blood count. Patients complaining of chest pain were administered exercise stress tests and additional myocardial perfusion scintigraphy when indicated to exclude coronary artery disease.
Standard echocardiographic measurements
All of the study participants were subjected to transthoracic echocardiographic examinations (Vivid 7 Pro; 2-4 MHz phased-array transducer; GE Vingmed Ultrasound AS, Horten, Norway) by a cardiologist unaware of the clinical data. M-mode and conventional Doppler measurements were made according to the American Society of Echocardiography guidelines and averaged from three cardiac cycles18,19. The RV diameter and wall thickness were measured from the M-Mode tracings at the end-diastole in the parasternal long axis view. The inner diameter of the right atrium was measured as the horizontal linear dimension from the apical four-chamber view. Pulsed wave Doppler measurements of tricuspid inflow velocities, namely peak E (early diastolic) and peak A (late diastolic), were obtained by placing the sample volume at the tip of the leaflets and averaged from 3 consecutive beats. The velocity of the pulmonary outflow tract was recorded by pulsed wave TDI at a level just distal to the pulmonary valve tips, and pulmonary acceleration time (PAT) was measured.
Tissue Doppler Imaging
TDI was performed in the apical four-chamber view using a 5-mm sample volume placed at the lateral tricuspid annulus. We used the minimal gain to assure clear and well-defined pulsed TDI wave borders. Settings were adjusted for a frame rate of 120-180 Hz and myocardial peak systolic (RV Sm), peak early diastolic velocity (RV Em), peak late diastolic velocity (RV Am), isovolumetric contraction (RV ICTm), relaxation time (RV IRTm), and ejection time (RV ETm) were measured. All measurements were averaged from three consecutive recordings. The RV myocardial performance index (RV MPIm) was calculated from the formula: RV MPIm = (IRTm + ICTm) / ETm. The patients with an RV Em / Am ration < 1 were considered to have RV diastolic dysfunction20.
Statistical Analysis
All analyses were conducted using SPSS statistical software for Windows 15.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were presented as percentages. The chi-square statistic was used to assess differences between categorical variables. Analysis of variance was used to compare parameters among the three groups. Pearson's correlation coefficient was used to assess the strength of the relationship between continuous variables. A p-value < 0.05 was considered statistically significant.
Results
Characteristics of the study groups
Clinical patient characteristics are presented in Table 1. Only weight and BMI were significantly different between groups. The average duration of obesity was 7.3 ± 2.9 and 7.7 ± 2.3 years (range, 2-13 years) in groups II and III, respectively. Although systolic, diastolic, and mean arterial blood pressures were slightly higher in group III, as compared to groups I and II, the differences did not reach statistical significance.
Table 1.
Clinical characteristics of the groups
| Group I (n = 35, BMI < 25 kg/m2) | Group II (n = 27, BMI 30–34.9 kg/m2) | Group III (n = 42, BMI ≥ 35 kg/m2) | |
| Age (years) | 30.1 ± 4.9 | 30.9 ± 6.0 | 32.7 ± 4.6 |
| Gender (M/F) | 15/20 | 14/13 | 16/26 |
| Weight (kg) | 58.8 ± 5.9 | 88.7 ± 8.2††† | 106.3 ± 14.5*** |
| Height (m) | 1.63 ± 0.06 | 1.64 ± 0.07 | 1.64 ± 0.11 |
| BMI (kg/m2) | 22.0 ± 2.1 | 33.1 ± 1.3††† | 39.6 ± 2.9*** |
| SBP (mmHg) | 114.3 ± 7.6 | 114.6 ± 5.7 | 117.4 ± 4.7 |
| DBP (mmHg) | 73.0 ± 5.2 | 73.5 ± 5.3 | 74.9 ± 4.7 |
| MAP (mmHg) | 86.8 ± 5.6 | 87.2 ± 5.0 | 89.0 ± 4.2 |
| Heart rate (beats/min) | 78.0 ± 10.6 | 81.3 ± 9.8 | 81.6 ± 10.7 |
| Duration of obesity (years) | _ | 7.3 ± 2.9 | 7.7 ± 2.3 |
| Smoking (%) | 25.7 | 29.6 | 21.4 |
BMI: body mass index; DBP: diastolic blood pressure; F: female; M: male; MAP: mean arterial pressure; SBP: systolic blood pressure. ***, p < 0.001 vs. group II; †††, p < 0.001 vs. group I
Conventional echocardiographic parameters
Structural and functional echocardiographic indices of the RV obtained by standard and tissue Doppler echocardiography are shown in Table 2. RV wall thickness, right atrial diameter, and RV diameter were significantly higher in the obese groups (groups II and III) as compared to the control group (p < 0.01, p < 0.01, and p < 0.001, respectively). The measurements of the right cardiac chambers were also significantly higher in group III than group II (p < 0.01 for RV wall thickness and p < 0.05 for other measurements). The tricuspid early (E) and late (atrial - A) (E/A) ventricular filling velocity ratio was significantly decreased in group II as compared to the control group (p < 0.001). Although the E/A ratio was further decreased in group III, as compared to group II, the difference was not statistically significant. PAT was found to be significantly shortened in groups II and III (p < 0.001), and also differed significantly between groups II and III (p < 0.05).
Table 2.
Echocardiographic measurements of right ventricular morphology and functions
| Group I (n = 35, BMI < 25 kg/m2) | Group II (n = 27, BMI 30–34.9 kg/m2) | Group III (n = 42, BMI ≥ 35 kg/m2) | |
| Standard parameters | |||
| RA diameter (mm) | 29.1 ± 2.8 | 31.9 ± 3.8†† | 34.6 ± 3.5* |
| RV diameter (mm) | 25.6 ± 3.5 | 29.4 ± 2.9††† | 31.3 ± 3.1* |
| RV FWT (mm) | 2.4 ± 0.3 | 3.9 ± 0.9†† | 4.9 ± 1.1*** |
| Tricuspid E/A | 1.57 ± 0.24 | 1.32 ± 0.25††† | 1.21 ± 0.31 |
| PAT (ms) | 139.0 ± 16.4 | 124.3 ± 12.3††† | 115.7 ± 16.8* |
| TDI parameters | |||
| RV Sm (cm/s) | 13.7 ± 1.6 | 13.3 ± 1.4 | 12.6 ± 1.6* |
| RV Em (cm/s) | 15.5 ± 2.6 | 14.1 ± 2.0† | 12.7 ± 2.0** |
| RV Am (cm/s) | 12.0 ± 3.2 | 13.2 ± 3.1 | 14.4 ± 3.2& |
| RV Em/Am | 1.37 ± 0.42 | 1.1 ± 0.21†† | 0.92 ± 0.20** |
| RV IRTm (ms) | 55.1 ± 11.5 | 69.3 ± 17.1†† | 78.6 ± 17.3* |
| RV ICTm (ms) | 64.4 ± 16.5 | 67.6 ± 12.9 | 71.6 ± 15.6 |
| RV ETm (ms) | 266.1 ± 18.3 | 256.7 ± 23.5 | 249.9 ± 20.1& |
| RV MPIm | 0.45 ± 0.07 | 0.53 ± 0.07††† | 0.60 ± 0.12** |
| Prevalence of RV DD (%) | 11.4 | 44.4†† | 69.0* |
A: late diastolic velocity of tricuspid inflow; Am: late diastolic myocardial velocity; DD: diastolic dysfunction; E: early diastolic velocity of tricuspid inflow; Em: early diastolic myocardial velocity; ETm: ejection time of myocardium; FWT: free wall thickness; ICTm: isovolumetric contraction time of myocardium; IRTm: isovolumetric relaxation time of myocardium; MPIm: myocardial performance index; PAT: pulmonary acceleration time; RA: right atrial; RV: right ventricular; Sm: systolic myocardial velocity; TDI: tissue Doppler imaging. *, p < 0.05 vs. group II; **, p < 0.01 vs. group II; ***, p < 0.001 vs. group II; †, p < 0.05 vs. group I; ††, p < 0.01 vs. group I; †††, p < 0.001 vs. group I; &, p < 0.01 vs. group I.
Tissue Doppler imaging findings
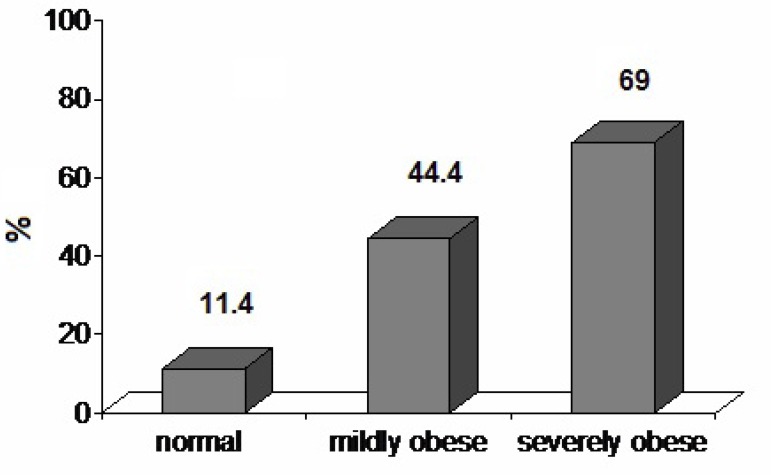
Considering RV diastolic function, RV Am did not significantly differ between groups I and II, although it was significantly increased in group III (p < 0.01). RV Em was significantly lower in group III (p < 0.01). RV Em/Am was significantly lower in group II compared to the control group (p < 0.01) and lower in group III than group II (p < 0.01). RV IRTm was significantly increased in group II compared to the control group (p < 0.01) and increased in group III compared to group II (p < 0.05). RV ICTm did not differ significantly between groups. The number of subjects with an Em/Am ratio < 1 was 4 (11.4%), 12 (44.4%), and 25 (69%) in groups I, II, and III, respectively, and the differences between groups were statistically significant (p < 0.01 for group I vs. II and p < 0.05 for group II vs. III) (Figure 1).
Figure 1.
Prevalence of subclinical diastolic dysfunction in the study groups.
RV Sm was significantly decreased in group III as compared to groups I and II (p < 0.01 and p < 0.05, respectively), but did not differ significantly between groups I and II. There were statistical differences between groups in regard to RV MPIm, which increased with BMI and was significantly increased in groups II and III as compared to the control group (p < 0.001), and also increased in group III as compared to group II (p < 0.01).
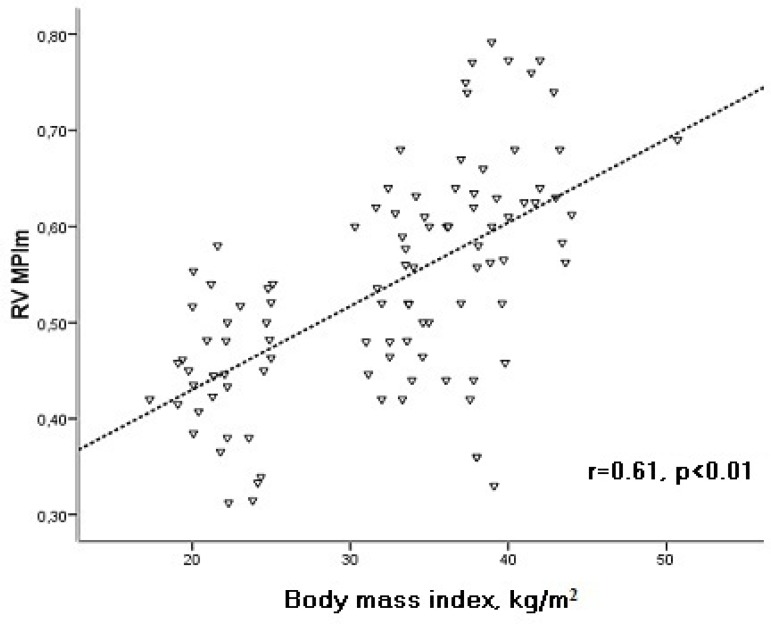
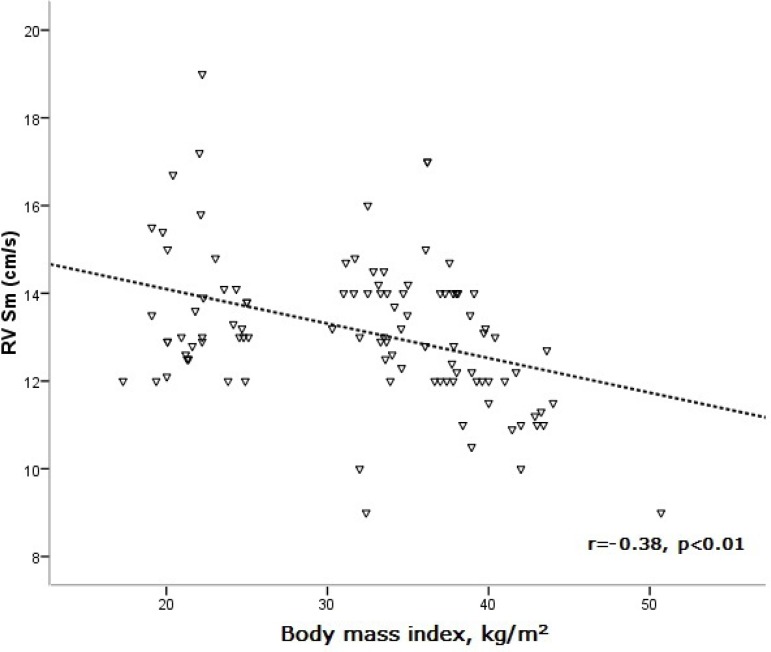
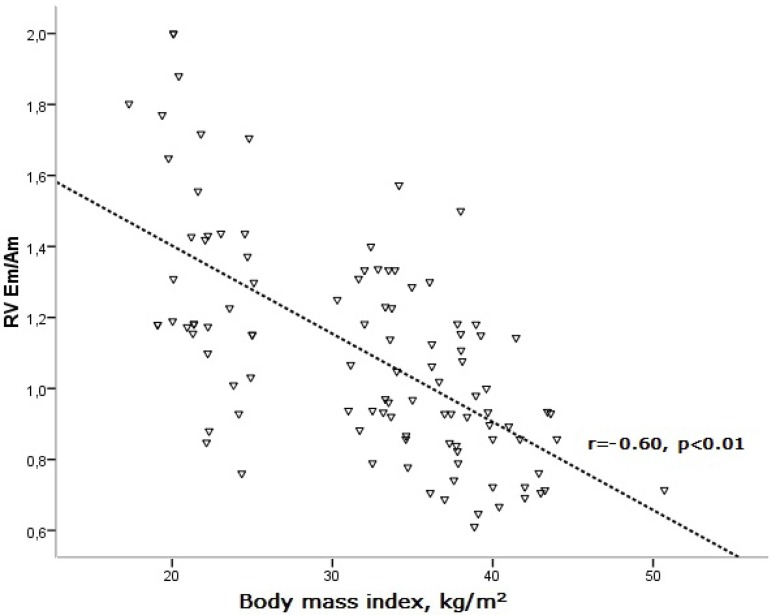
There was a significant correlation between BMI and echocardiographic parameters in the study patients (Table 3). BMI was found to be positively correlated with RV MPIm, while it was negatively correlated with RV Sm and Em/Am (Figures 2, 3, and 4).
Table 3.
Association of echocardiographic parameters with BMI in study patients
| Pearson's r | p | |
| RA diameter | 0.68 | < 0.01 |
| RV diameter | 0.70 | < 0.01 |
| RV free wall thickness | 0.80 | < 0.01 |
| Tricuspid E/A | -0.51 | < 0.01 |
| Pulmonary acceleration time | -0.59 | < 0.01 |
| RV myocardial systolic velocity | -0.38 | < 0.01 |
| RV myocardial early diastolic velocity | -0.50 | < 0.01 |
| RV Em/Am | -0.60 | < 0.01 |
| RV isovolumic relaxation time | 0.54 | < 0.01 |
| RV ejection time | -0.35 | < 0.01 |
| RV myocardial performance index | 0.61 | < 0.01 |
E/A: early/late diastolic velocity of tricuspid inflow; Em/Am: myocardial early diastolic velocity / late diastolic velocity; RA: right atrial; RV: right ventricular.
Figure 2.
Correlation of body mass index with RV MPIm. RV: right ventricular; MPIm: myocardial performance index.
Figure 3.
Correlation of body mass index with RV Sm. RV: right ventricular; Sm: peak systolic wave.
Figure 4.
Correlation of body mass index with RV Em/Am. Am: peak late diastolic wave; Em: peak early diastolic wave; RV: right ventricular.
Discussion
The most important findings of the present study were that (1) isolated obesity was associated with subclinical impairment in RV diastolic and systolic functions and dilatation of right cardiac chambers in young healthy subjects, and (2) these unfavorable changes were more pronounced in severely obese subjects, and appeared to be related to the degree of obesity.
Obesity has been shown to have many effects on cardiovascular structure and function. Excess adiposity imposes an increased metabolic demand on the body and both cardiac output and total blood volume are elevated in obesity leading to hyperdynamic circulation, which causes LV and RV structural changes in obesity and subsequently leads to increased ventricular mass and cavity dilatation2,3. Previous studies have suggested that obesity is associated with LV hypertrophy, LV dilatation, LV diastolic dysfunction, and occasionally LV systolic dysfunction3-10,21. RV structure and function have also been evaluated in several studies, but the results are conflicting11-15,22.
The present study showed that uncomplicated obesity was associated with RV and right atrial dilatation, and increased thickness of the RV free wall. Also, these structural indices were found to be positively correlated with BMI. Similarly, in a previous study evaluating RV structure and function by echocardiography, RV mass and volume was found to be increased in obese subjects11. Recently, Chahal et al13 reported that RV end-diastolic volume and mass were increased in overweight and obese subjects, which was in accordance with our results. However, the mean patient age in that study was greater and RV morphology was assessed by cardiac magnetic resonance imaging13. On the other hand, some studies revealed no relation between obesity and RV diameter14,15. We also found that the tricuspid E/A ratio was decreased, indicating impaired RV relaxation, and PAT was significantly shortened, indicating increased pulmonary vascular resistance in obese subjects.
Likely causes of the increased RV mass, RV dilatation, shortened PAT, and subsequent impairment of RV function in obese persons include increased total blood volume and preload, hyperinsulinemia and insulin resistance, changes in respiratory workload, presence of obstructive sleep apnea, which has been frequently associated with severe obesity, among other mechanisms. Each of these changes may occur in obesity to different degrees and play a role in alterations of pulmonary vascular resistance and RV structure and function23-25.
Pulsed TDI is a unique method to measure systolic and diastolic myocardial velocities. The determination of velocities of tricuspid annular motion has been confirmed as a new tool to assess RV systolic and diastolic function in various diseases16,26. This study detected impaired RV diastolic function, as indicated by a decreased RV Em/Am ratio and increased RV IRT in obese subjects. Moreover, RV diastolic function was further impaired in severely obese subjects and closely related with BMI. To avoid the contributory effect of aging to impaired RV relaxation, our study population consisted of young adults (age < 40 years). We also found subclinical impairment in RV systolic function demonstrated by decreased RV Sm in obese subjects with a BMI ≥ 35 kg/m2. The preservation of RV Sm in mildly obese subjects, while significantly decreasing RV Sm in those severely obese, suggested that RV systolic function was affected late in the course of obesity. Since obese subjects with co-morbidities were not included in the present study and blood pressures were similar in obese and normal weight groups, impaired RV function can be attributed to the isolated incidence of obesity in this study. In the literature, studies have revealed normal RV systolic and diastolic function14,27 or impaired RV diastolic function together with preserved systolic function12,22. However, consistent with the present study, most of previous studies reported impaired RV functions in obese subjects11,12,15,22 with some of them reporting improvement in RV function after continuous weight loss28,29.
The MPIm introduced by Tei is a Doppler-derived index to reflect both systolic and diastolic function of the ventricles30. MPIm obtained via TDI has the advantage of measuring systolic and diastolic time intervals within the same cardiac cycle. The MPIm is a useful tool to noninvasively measure RV function31. In the literature, there are few studies that have investigated RV MPI in obese subjects and they revealed conflicting results14,32,33. In our study, TDI-derived RV MPIm was found to be increased in otherwise healthy obese subjects and was significantly correlated with BMI. Similarly, Maniscalco et al32 found that RV MPI was significantly increased in uncomplicated severe obesity and improved after continuous weight loss. On the other hand, Yildirimturk et al14 reported no change in RV MPIm in mildly obese subjects; however, this may be explained by the demographic features of the study population as well as the duration and severity of obesity. Although we found significant increases in RV MPIm in both obese groups, the increase was more pronounced in patients with a BMI ≥ 35 kg/m2, which was most likely due to subclinical impairment of RV systolic function in addition to RV diastolic dysfunction in severe obesity.
Study limitations
Obesity was measured using only BMI and no measurements of body fat distribution were made. A better correlation might have been found between abdominal obesity and echocardiographic alterations. We did not perform a sleep study to determine the contribution of obstructive sleep apnea to RV changes. However, patients with obstructive sleep apnea symptoms, such as habitual snoring and witnessed apnea, were excluded. Another limitation to the present study was the lack of a longitudinal follow-up of the obese subjects to document the development of heart failure. Finally, the number of patients was relatively limited in this study; therefore, the results may not be identical when applied to larger populations.
Conclusion
This study demonstrated that isolated obesity in young adults was associated with subclinical abnormalities in RV structure and function. The early detection of impaired RV function may be important in obesity management and TDI may provide a useful tool to monitor subclinical cardiac involvement in obese subjects.
Author contributions
Conception and design of the research: Sokmen A; Acquisition of data: Sokmen A, Sokmen G, Acar G, Akcay A, Koroglu S, Koleoglu M, Yalcintas S, Aydin MN; Analysis and interpretation of the data: Sokmen A, Sokmen G; Statistical analysis and Writing of the manuscript: Sokmen A, Acar G; Critical revision of the manuscript for intellectual content: Sokmen A, Sokmen G, Akcay A.
Footnotes
Potential Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Sources of Funding: There were no external funding sources for this study.
Study Association: This study is not associated with any post-graduation program.
References
- 1.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26 year-follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 4.Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89(10):1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumuklu MM, Etikan I, Kisacik B, Kayikcioglu M. Effect of obesity on left ventricular structure and myocardial systolic function: assessment by tissue Doppler imaging and strain/strain rate imaging. Echocardiography. 2007;24(8):802–809. doi: 10.1111/j.1540-8175.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- 6.Dorbala S, Crugnale S, Yang D, Di Carli MF. Effect of body mass index on left ventricular cavity size and ejection fraction. Am J Cardiol. 2006;97(5):725–729. doi: 10.1016/j.amjcard.2005.09.122. [DOI] [PubMed] [Google Scholar]
- 7.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43(8):1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 8.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110(19):3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 9.Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol. 2006;98(1):116–120. doi: 10.1016/j.amjcard.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 10.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracey RP, et al. The impact of obesity on the left ventricle. JACC Cardiovasc Imaging. 2010;3(3):266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CY, O'Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol. 2006;47(3):611–616. doi: 10.1016/j.jacc.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Otto ME, Belohlavek M, Khandheria B, Gilman G, Svatikova A, Somers V. Comparison of right and left ventricular function in obese and nonobese men. Am J Cardiol. 2004;93(12):1569–1572. doi: 10.1016/j.amjcard.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 13.Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, et al. Obesity and right ventricular structure and functions: the MESA-right ventricle study. Chest. 2012;141(2):388–395. doi: 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yildirimturk O, Tayyareci Y, Aytekin S. The impact of body mass index on right ventricular systolic functions in normal and mildly obese healthy: a velocity vector imaging study. Echocardiography. 2011;28(7):746–752. doi: 10.1111/j.1540-8175.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- 15.Orhan AL, Uslu N, Dayi SU, Nurkalem Z, Uzun F, Erer HB, et al. Effects of isolated obesity on left and right ventricular function: a tissue Doppler and strain rate imaging study. Echocardiography. 2010;27(3):236–243. doi: 10.1111/j.1540-8175.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 16.Kjaergaard J. Assessment of right ventricular systolic function by tissue Doppler echocardiography. Dan Med J. 2012;59(3):B4409–B4409. [PubMed] [Google Scholar]
- 17. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report: National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 18.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Cicala S, Galderisi M, Caso P, Petrocelli A, D'Errico A, de Divitiis O, et al. Right ventricular diastolic dysfunction in arterial systemic hypertension: analysis by pulsed tissue Doppler. Eur J Echocardiogr. 2002;3(2):135–142. doi: 10.1053/euje.2001.0124. [DOI] [PubMed] [Google Scholar]
- 21.Rider OJ, Petersen SE, Francis JM, Ali MK, Hudsmith LE, Robinson MR, et al. Ventricular hypertrophy and cavity dilatation in relation to body mass index in women with uncomplicated obesity. Heart. 2011;97(3):203–208. doi: 10.1136/hrt.2009.185009. [DOI] [PubMed] [Google Scholar]
- 22.Willens HJ, Chakko SC, Lowery MH, Byers P, Labrador E, Gallgher A, et al. Tissue Doppler imaging of the right and left ventricle in severe obesity (body mass index >35 kg/m2) Am J Cardiol. 2004;94(8):1087–1090. doi: 10.1016/j.amjcard.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 23.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321(4):225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109(18):2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 25.Di Bello V, Santini F, Di Cori A, Pucci A, Palagi C, Delle Donne MG, et al. Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization. J Am Soc Echocardiogr. 2006;19(8):1063–1071. doi: 10.1016/j.echo.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Gondi S, Dokainish H. Right ventricular tissue Doppler and strain imaging: ready for clinical use? . Echocardiography. 2007;24(5):522–532. doi: 10.1111/j.1540-8175.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 27.Her C, Cerabona T, Bairamian M, McGoldrick KE. Right ventricular systolic function is not depressed in morbid obesity. Obes Surg. 2006;16(10):1287–1293. doi: 10.1381/096089206778663887. [DOI] [PubMed] [Google Scholar]
- 28.Willens HJ, Chakko SC, Byers P, Chirinos JA, Labrador E, Castrillion JC, et al. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95(12):1521–1524. doi: 10.1016/j.amjcard.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, et al. Favorable changes in cardiac geometry and function following gastric bypass surgery. J Am Coll Cardiol. 2011;57(6):732–739. doi: 10.1016/j.jacc.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function- a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26(6):357–366. [PubMed] [Google Scholar]
- 31.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9(6):838–847. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 32.Maniscalco M, Arciello A, Zedda A, Faraona S, Verde R, Giardiello C, et al. Right ventricular performance in severe obesity: effect of weight loss. Eur J Clin Invest. 2007;37(4):270–275. doi: 10.1111/j.1365-2362.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 33.Garza CA, Pellikka PA, Somers VK, Sarr MG, Collazo-Clavell ML, Korenfeld Y, et al. Structural and functional changes in left and right ventricles after major weight loss following bariatric surgery for morbid obesity. Am J Cardiol. 2010;105(4):550–556. doi: 10.1016/j.amjcard.2009.09.057. [DOI] [PubMed] [Google Scholar]