Abstract
Anemia is a prevalent comorbidity and marker of a poorer prognosis in patients with heart failure (HF). Its clinical relevance, as well as its pathophysiology and the clinical management of these patients are important subjects in the specialized literature. In the present review, we describe the current concepts on the pathophysiology of anemia in HF, its diagnostic criteria, and the recommendations for iron supplementation. Also, we make a critical analysis of the major studies showing evidences on the benefits of this supplementation. The four main components of anemia are addressed: chronic disease, dilutional, "renal" and malabsorption. In patients with HF, the diagnostic criteria are the same as those used in the general population: serum ferritin levels lower than 30 mcg/L in patients without kidney diseases and lower than 100 mcg/L or serum ferritin levels between 100-299 mcg/L with transferring saturation lower than 20% in patients with chronic kidney diseases. Finally, the therapeutic possibilities for anemia in this specific patient population are discussed.
Keywords: Iron deficiency, Inflamation, Iron supplementation, Chronic disease, Cardiovascular disease
Introduction
Heart failure (HF) is the major cause of hospital admissions in the Unified Health System (Sistema Único de Saúde - SUS) in individuals older than 65 years, and its prevalence tends to grow because of the increase in life expectancy of the population and of the higher effectiveness of medications that decrease morbidity and mortality1. Despite improvements in its pharmacological treatment, the prognosis is still poor, with a five-year survival rate lower than 50%2,3. According to the Registration and Access Permission System developed by the Ministry of Health in the SUS Department of Information Technology (DATASUS), there were 261,361 admissions for heart failure in Brazil in 2011, accounting for 22.6% of hospitalizations for cardiovascular diseases4.
Several studies have demonstrated that anemia is a prevalent comorbidity in patients with HF, and a marker of a worse outcome, with increased left ventricular mass, rehospitalizations and increased mortality5-10. Data on the prevalence of anemia in HF vary because of the use of different definitions for anemia11; however, it is estimated at approximately 35%11,12.
Anemia is more prevalent when HF and chronic kidney disease (CKD) coexist13, and the degree of anemia is directly proportional to the degree of CKD. This association is explained by the role of the kidneys in the pathophysiology of anemia. However, patients with HF and CKD develop a higher degree of anemia than those with CKD alone14.
The degree of anemia is also associated with the severity of HF, i.e., with a worse exercise tolerance with loss of the NYHA (New York Heart Association) functional capacity; with the elevation of atrial natriuretic peptide (BNP); left ventricular dilatation and/or hypertrophy; systolic and/or diastolic dysfunction; increased pulmonary artery pressure; reduction in oxygen consumption during exercise or at rest; with the degree of fluid retention; and reduction of the quality of life15. Iron deficiency is an independent risk factor for mortality or need for cardiac transplantation in HF16.
Based on these evidences, the Brazilian Guideline for Chronic Heart Failure, published in 2012, suggests intravenous iron supplementation in patients with iron deficiency for improvement of symptoms. The dosage is guided by ferritin levels and/or transferin saturation (ferritin < 100 mcg/L or ferritin 100-299 mcg/L with transferrin saturation < 20%), with indication IIA and level of evidence B17. In this guideline, the authors mention a study which demonstrated the beneficial effect of ferric carboxymaltose18.
Objectives
In view of the relevance of the topic, the objectives of this review are to make a summarized description of the pathophysiology of anemia in HF and the criteria for its diagnosis, and to critically analyze the major studies that support the recommendation of iron supplementation in this clinical situation.
Pathophysiology
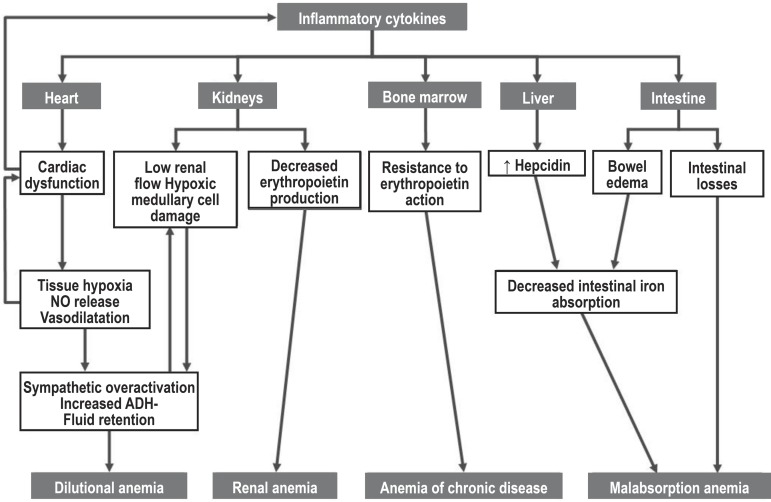
The pathophysiology of anemia in HF is multifactorial and has been didactically divided into four main mechanisms: anemia of chronic disease, dilutional anemia, "renal" anemia, and malabsorption anemia19. These mechanisms are summarized in Figure 1.
Figure 1.
Main mechanisms of the pathophysiology of anemia in HF.
The common link between these mechanisms are the increased levels of cytokines, such as IL6 and TNF-alpha, which are directly related to the degree of HF, acting in endothelial dysfunction, oxidative stress, induction of anemia, myocyte apoptosis, in the gradual loss of skeletal muscle mass, and BNP release in response to myocardial stress20. Progression of cardiac dysfunction causes tissue hypoxia, with proinflammatory activation and tissue nitric oxide release, which leads to peripheral vasodilatation and decreased blood pressure, and consequent reactive sympathetic overactivation with tachycardia, renal vasoconstriction, renin-angiotensin-aldosterone system activation15,21, and dilutional anemia. This neurohumoral activation deriving from hypoxia causes the progression of cardiac dysfunction and also worsens the anemia that results from the action of inflammatory cytokines (anemia of chronic disease), that lead to decreased renal production and resistance to the erythropoietin action in the bone marrow22,23. Worsening of the systolic function and/or renal vasoconstriction also cause a reduction in the renal erythropoietin production due to a decreased renal flow and hypoxic damage to erythropoietin-producing renal medullary cells (anemia of renal disease)15. Thus, a vicious cycle is closed that has been called cardio-renal anemia syndrome24,25.
Another mechanism of induction of anemia in HF is explained by the inflammatory cytokines action in the liver, stimulating the hepatic production of hepcidin and reducing both the intestinal iron absorption and the iron reserve release from the macrophages for hematopoiesis, leading to iron-deficiency anemia15. Thus, iron-deficiency/deprivation anemia in patients with HF may be secondary to a reduction in cytokine-hepcidin-mediated iron absorption20-25; to decreased food intake, since the proinflammatory state leads to loss of appetite; to malabsorption secondary to bowel edema or gastrointestinal losses in patients using antiplatelet and anticoagulant agents15; or to drug action. For instance, proton secretion inhibitors may impair iron absorption by reducing the acidic ph; ACE inhibitors and angiotensin-receptor blockers may inhibit erythropoietin production by blocking angiotensin production and action (erythropoiesis stimulator). Also, in the case of ACE inhibitors, levels of bone marrow depressors may increase, leading to a slight reduction in hemoglobin20,26,27.
Diagnosis
According to the World Health Organization, anemia is defined as a hemoglobin concentration < 13 g/dL in men and < 12 g/dL in women27.
Iron deficiency in the general population is defined as serum ferritin levels < 30 mcg/L. In patients with chronic kidney disease, it is defined as serum ferritin < 100 mcg/L (absolute iron deficiency) or serum ferritin between 100-299 mcg/L, with a transferrin saturation < 20% (functional iron deficiency)19,28,29. These values were extrapolated for patients with HF in studies evaluating anemia in this clinical situation16,19,30.
Treatment
Oral vs. Intravenous iron
Most of the studies on iron supplementation have been conducted in populations of patients with CKD, and have provided evidence of a better clinical response with intravenous iron in comparison to the oral route. Less side effects, better absorption, and better compliance to treatment have been described, without the need for the use of erythropoiesis stimulating agents for the hemoglobin target between 11.5 and 12 g/dL to be achieved31-33.
Some studies have reported an absence of response of increase in hemoglobin and/or improvement of cardiac parameters within one year of treatment34, whereas others have reported a hematological response between 3 and 6 months19.
There are several types of parenteral iron: iron dextran, iron gluconate, iron saccharate, and ferric carboxymaltose35. The latter two compounds were used in the two major studies on iron supplementation in HF.
Iron dextran may be administered intravenously or intramuscularly. It is a molecule with high antigenicity and requires a pre-treatment test to evaluate the development of symptoms of allergic or anaphylactic reaction, which occur in 0.5-1% of cases. Late hypersensitivity reactions, i.e., between 24 and 48 hours up to weeks after the administration, can also be observed; these reactions are characterized by fever, malaise, headache, lymphadenomegaly, arthralgia and myalgia, and may occur in more than 10% of the patients treated36.
Iron gluconate is a compound more stable than iron dextran, and its administration is associated with the occurrence of severe anaphylactic reaction and late reaction in 0.04% and 0.4% of cases, respectively. This medication has been validated for use in hemodialysis patients, in the treatment of anemia in patients with cancer, and in severely ill patients in intensive care units. However, it is not commercially available in Brazil36.
Iron saccharate is a medication with minimum immunogenicity (occurrence of allergic reaction < 1/100,000 infusions). In Brazil, ferric hydroxide saccharate is the only option for the treatment with parenteral iron. It is marketed in vials containing 2 mL and 100 mg of elemental iron for intramuscular use, and vials containing 5 mL and 100 mg of elemental iron for intravenous use. The Ganzoni formula is used for the calculation of the total dose required: [Hb (g/dL) desired - Hb (g/dL) found] x body weight (Kg) x 2.4 + 50036. For intravenous administration, it is necessary to observe the maximum dose limit of 200 mg per application (two vials) and the maximum weekly dose of 500 mg. Also, it is necessary to observe the minimum 24-hour interval between applications37.
Ferric carboxymaltose has the advantage of enabling the administration of 1,000 mg of iron in only 15 minutes, with a minimum risk of adverse effects. This dose facilitates treatment, and prevents waste of time and the need for several appointments during therapy38.
The most frequent adverse effects of these ferric compounds are gastrointestinal disturbances described as nausea, vomiting, abdominal pain, diarrhea or constipation and metallic taste. These side effects are significantly more frequent with oral replacement36,37.
The two major studies corroborating iron supplementation in patients with iron-deficiency anemia are FERRIC-HF, published in 2008, and FAIR-HF, published in 2009 and cited in the updating of the Guideline on Chronic Heart Failure of the Brazilian Society of Cardiology, published in 201217.
The FERRIC-HF study (Effect of Intravenous Iron Sucrose on Exercise Tolerance in Anemic and Nonanemic Patients with Symptomatic Chronic Heart Failure and Iron Deficiency) included 35 patients who were followed up for only 18 weeks, which limits data extrapolation for the clinical practice. However, it was a relevant study given the scarcity of information on this topic. In this study, anemic (Hb < 12.5 g/dL) and nonanemic patients (Hb between 12.5 and 14.5 g/dL) with ferritin < 100 ng/mL or between 100-300 ng/mL when associated with transferrin saturation < 20%, from two European centers, were randomized to receive placebo or intravenous iron sucrose at a 200 mg dose per week to achieve the correction of the calculated iron deficiency. Clinical and laboratorial reassessment of the patients was carried out at weeks 1, 4, 8, 12, 16, and 18. Treatment was discontinued for two weeks if serum ferritin was > 500 ng/mL and/or transferrin saturation > 45% or Hb > 16 g/dL. Treatment was resumed when: ferritin < 500 ng/mL, transferrin saturation < 45% and Hb < 16 g/dL. Among all patients studied, the fatigue score was significantly lower in the group treated in relation to the placebo group. This was the most important finding of that study. A statistically significant improvement in the peak oxygen consumption (VO2)/kg and functional capacity was also observed, however with little clinical relevance37.
The FAIR-HF study (Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency) was a randomized double-blind clinical study which included 459 patients with NYHA functional class (FC) II-III patients with left ventricular ejection fraction ≤ 40% for FC II or ≤ 45% for FC III, iron deficiency (serum ferritin < 100mcg/L or between 100-299 mcg/L if transferring saturation < 20%) and hemoglobin level between 9.5 and 13.5 g/dL. The patients were randomized to receive ferric carboxymaltose either 200 mg or saline solution. The total iron dose was calculated using the Ganzoni formula, and the weekly dose administered was 200 mg. Then, a monthly maintenance iron dose of 200 mg was administered. Clinical and laboratorial reassessment of patients was made at weeks 4, 12, 24 and 26. If ferritin was higher than 800 mcg/L or between 500-800 mcg with transferrin saturation > 50% or Hb > 16 g/L, iron supplementation was discontinued. When ferritin levels dropped to 400 mcg/L, transferrin saturation < 45% and Hb < 16 g/dL, treatment was resumed 45% for FC III, iron deficiency (serum ferritin< 100mcg/L or between 100-299 mcg/L if transferring saturation < 20%) and hemoglobin level between 9.5 and 13.5 g/dL. The patients were randomized to receive ferric carboxymaltose either 200 mg or saline solution. The total iron dose was calculated using the Ganzoni formula, and the weekly dose administered was 200 mg. Then, a monthly maintenance iron dose of 200 mg was administered. Clinical and laboratorial reassessment of patients was made at weeks 4, 12, 24 and 26. If ferritin was higher than 800 mcg/L or between 500-800 mcg with transferrin saturation > 50% or Hb > 16 g/L, iron supplementation was discontinued. When ferritin levels dropped to 400 mcg/L, transferrin saturation < 45% and Hb < 16 g/dL, treatment was resumed 18.
Assessment of the quality of life and functional capacity at week 24 comprised the primary endpoint and at weeks 4 and 12, the secondary endpoint. With respect to the primary endpoint, among patients who received the ferric compound, 50% reported improvement of their functional capacity in comparison to 28% of those receiving saline solution (OR = 2.51; 95% CI: 1.75-3.6). At week 24, among patients of the ferric carboxymaltose group, 47% reported FC I-II in comparison to 30% of patients of the placebo group (OR = 2.4; 95% CI: 1.55-3.71). In relation to the secondary endpoint, there was a significant improvement in the quality of life and functional capacity at weeks 4 and 12, with stabilization of the distance walked in the 6-minute walking test at week 12. Mortality and adverse events rates were similar between the groups. In this study, the fact that the improvement in the functional capacity was limited to the subgroup with ischemic heart disease is noteworthy. Maybe this finding results from the small number of nonischemic patients included (20%). However, this is a topic that remains to be elucidated in the literature.
Erythropoiesis-stimulating agents (erythropoietin, darbepoetin)
Recent studies on chronic kidney disease have demonstrated that these agents are associated with an increased incidence of cardiovascular events including hypertension, stroke, and thromboembolic disorders38,39, as well as with a worse prognosis in oncologic patients, with a significant increase in the incidence of thromboembolic phenomena40,41.
Erythropoiesis-stimulating agents may also promote an increase in blood pressure and vascular injury by means of several mechanisms, including the increase in cytosolic calcium concentration, renin-angiotensin system activation, increased endothelin production, coagulation system activation, and lower nitric oxide production42.
One of the possible causes for the increased cardiovascular mortality is iron depletion secondary to increased erythropoiesis, which would lead to reactive thrombocytosis and eventually to the development of cardiovascular and embolic events43.
The major study on the use of darbepoetin in anemic patients with heart failure (STAMINA study - Study of Anemia in a Heart Failure Population) was a prospective, randomized, double-blind clinical study including 319 patients with hemoglobin < 12.5 g/dL and FC II-IV HF with ejection fraction < 40%. The individuals were randomized to receive placebo or darbepoetin alpha, with the objective of keeping hemoglobin at 14 g/dL. The results did not show improvement of the functional capacity among the groups44.
When administered in combination with oral or intravenous iron, the erythropoiesis-stimulating agents were associated with an improvement of the severity of heart failure, characterized by decreased hospitalization; improvement of the functional class; 6-minute walking test showing a decrease in oxygen consumption during the test; reduction in BNP levels; increase in ejection fraction; and improvement of the quality of life45,46. There were also evidences of improvement of the renal function; reduction of diuretic dose, heart rate, and depression signs; improvement of sleep apnea; reduction of plasma volume; and improvement of the ventricular function and oxidative stress12,16,47,51.
Treatment prescription
Intravenous iron supplementation(52)
In determined conditions, when intravenous iron supplementation may improve symptoms, the medication is prescribed at a dose of one iron vial per week for ten weeks, or two vials every two weeks for five weeks. In these patients, Hb/Ht levels are monitored monthly and iron reserve, every three months. Barretto et al described a practical formula for the calculation of the total iron amount (in mL):
N(mL)= [(Weight in Kg x Dhb x 2.4) + 500 mg]/20, where
N = amount of iron, in mL, to be administered intravenously
Dhb = difference between hemoglobin desired and found
500 mg = iron reserve required
Erythropoietin supplementation53
Human recombinant erythropoietin supplementation should be started using the subcutaneous route at a weekly dose of 4,000 U to 5,000 U (up to 10,000 U per week) until hemoglobin levels higher than 12.5 g/dL are achieved.
In these patients, hemoglobin levels should be determined monthly and iron reserve, every three months.
Conclusion
Scientific evidences corroborate intravenous iron supplementation in symptomatic patients with HF due to systolic dysfunction and anemia or iron deficiency in the presence of optimized clinical treatment. Nonetheless, the long-term effects of iron supplementation have not been studied in this group of patients. Iron is known to be pro-oxidant; it may inhibit the nitric oxide signaling and lead to irreversible cell injury. Iron overload is associated with endothelial dysfunction and increased risk of coronary events54. The duration of follow-up of these patients in the studies was short and the maintenance dose and administration of iron supplementation were variable. There is no clear definition in the literature of how to proceed after correction of anemia or iron deficiency. Thus, the indication of iron supplementation should be customized and judicious. It seems reasonable to suggest a customization of the therapeutic approach by monitoring the laboratory profile in every case, while following the recommendations of the Brazilian Guidelines on chronic HF17:
• Intravenous iron in patients with iron deficiency (ferritin < 100 or ferritin between 100 and 299 with transferrin saturation < 20%) for improvement of symptoms, as class IIa recommendation and level of evidence B;
• Blood transfusion in anemic patients (Hb < 7 g/dL) and dilated cardiomyopathy of ischemic and nonischemic etiology, as class IIa recommendation and level of evidence C;
• Intravenous erythropoietin/iron for the correction of anemia (Hb < 12 g/dL) in patients with dialytic or pre-dialytic chronic kidney failure and HF, as class IIb recommendation and level of evidence B.
Perspectives
Further multicenter randomized controlled studies should be carried out to evaluate the impact of iron supplementation combined with erythropoiesis-stimulating agents on endpoints of morbidity and mortality of HF. Also, it is necessary to elucidate whether there are different responses on endpoints of patients with ischemic HF in comparison to those with nonischemic HF. Finally, it is necessary to evaluate whether the effect of iron sucrose is comparable to that of iron carboxymaltose.
Author contributions
Conception and design of the research: Pereira CA, Zanati SG; Acquisition of data: Pereira CA; Analysis and interpretation of the data and Writing of the manuscript: Pereira CA, Roscani MG, Zanati SG, Matsubara BB.
Footnotes
Potential Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Sources of Funding: There were no external funding sources for this study.
Study Association: This study is not associated with any post-graduation program.
References
- 1.Araujo DV, Tavares LR, Veríssimo R, Ferraz MB, Mesquita ET. Cost of heart failure in the Unified Health System. Arq Bras Cardiol. 2005;84(5):422–427. [PubMed] [Google Scholar]
- 2.Massie VM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133(6):703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 3.Tecce MA, Pennington JA, Segal BL, Jessup ML. Heart failure: clinical implications of systolic and diastolic dysfunction. Geriatrics. 1999;54(8):24-8, 31-3. [PubMed] [Google Scholar]
- 4.Ministério da Saúde. Datasus [Acesso em 2012 dez 10];2011 Internet. Disponível em http://www.datasus.gov.br.
- 5.Cardoso J, Brito MI, Ochiai ME, Novaes M, Berganin F, Thicon T, et al. Anemia in patients with advanced heart failure. Arq Bras Cardiol. 2010;95(4):524–528. doi: 10.1590/s0066-782x2010005000118. [DOI] [PubMed] [Google Scholar]
- 6.Okonko DO, Anker SD. Anemia in chronic heart failure: pathogenetic mechanisms. J Card Fail. 2004;10(1) Suppl:S5–S9. doi: 10.1016/j.cardfail.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Felker GM, Gattis WA, Leimberger JD, Adams KF, Cuffe MS, Gheorghiade M, et al. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003;92(5):625–628. doi: 10.1016/s0002-9149(03)00740-9. [DOI] [PubMed] [Google Scholar]
- 8.O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, et al. CHARM Committees and Investigators Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113(7):986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 9.Szachniewicz J, Petruk-Kowalczyk J, Majda J, Kaczmarek A, Reczuch K, Kalra PR, et al. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol. 2003;90(2-3):303–308. doi: 10.1016/s0167-5273(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 10.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39(11):1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 11.Adams KF, Jr, Pina IL, Ghali JK, Wagoner LE, Dunlap SH, Schwartz TA, et al. Prospective evaluation of the association between hemoglobin concentration and quality of life in patients with heart failure. Am Heart J. 2009;158(6):965–971. doi: 10.1016/j.ahj.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients: a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52(10):818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure-the anemia in chronic heart failure: Outcomes and Resource Utilization (ANCHOR) study. Circulation. 2006;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 14.Luthi JC, Flanders WD, Burnier M, Burnand B, McClellan WM. Anemia and chronic kidney disease are associated with poor outcomes in heart failure patients. BMC Nephrol. 2006;7:3–3. doi: 10.1186/1471-2369-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg DS. The role of erythropoiesis stimulating agents and intravenous (IV) iron in the cardio renal anemia syndrome. Heart Fail Rev. 2011;16(6):609–614. doi: 10.1007/s10741-010-9194-2. [DOI] [PubMed] [Google Scholar]
- 16.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31(15):1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 17.Bocchi EA, Marcondes-Braga FG, Bacal F, Ferraz AS, Albuquerque D, Rodrigues D, et al. Sociedade Brasileira de Cardiologia Atualização da Diretriz Brasileira de insuficiência cardíaca crônica - 2012. Arq Bras Cardiol. 2012;98(1) supl. 1:1–33. doi: 10.1590/s0066-782x2012001000001. [DOI] [PubMed] [Google Scholar]
- 18.Anker SD, Comin Colet J, Fillipatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New Engl J Med. 2009;361(25):2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 19.Von Haehling S, Anker MS, Jankowska EA, Ponikowski P, Anker SD. Anemia in chronic heart failure: can we treat? What to treat? Heart Fail Rev. 2012;17(2):203–210. doi: 10.1007/s10741-011-9283-x. [DOI] [PubMed] [Google Scholar]
- 20.Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50(17):1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Anand IS. Anemia chronic heart failure: implications and treatment options. J Am Coll Cardiol. 2008;52(7):501–511. doi: 10.1016/j.jacc.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 22.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107(2):223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 23.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg DS, Wexler D, Iaina A, Schwartz D. The role of anaemia in patients with congestive heart failure: a short review. Eur J Heart Fail. 2008;10(9):819–823. doi: 10.1016/j.ejheart.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg DS, Wexler D, Palazzuoli A, Iaina A, Schwartz D. The anemia of heart failure. Acta Haematol. 2009;122(2-3):109–119. doi: 10.1159/000243795. [DOI] [PubMed] [Google Scholar]
- 26.Le Jemtel TH, Arain S. Mediators of anemia in chronic heart failure. Heart Fail Clin. 2010;6(3):289–293. doi: 10.1016/j.hfc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein KE, Xiao HD, Frenzel K, Li P, Shen XZ, Adams JW, et al. Six truisms concerning ACE and the renin-angiotensin system educed from the genetic analysis of mice. Circ Res. 2005;96(11):1135–1144. doi: 10.1161/01.RES.0000169536.73576.66. [DOI] [PubMed] [Google Scholar]
- 28.Blanc B, Finch CA, Hallberg L, World Health Organization Nutritional anaemias (WHO) World Health Organ Tech Rep Ser. 1968;405:1–40. [Google Scholar]
- 29.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. FAIR-HF committees and investigators Rationale and design of Ferinject assessment in patients with iron deficiency and chronic heart failure (FAIRHF) study: a randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anemia. Eur J Heart Fail. 2009;11(11):1084–1091. doi: 10.1093/eurjhf/hfp140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S, United States Iron Sucrose (Venofer) Clinical Trials Group A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with non-dialysis dependent CKD. Kidney Int. 2005;68(6):2846–2856. doi: 10.1111/j.1523-1755.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 31.Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant. 2006;21(1):120–124. doi: 10.1093/ndt/gfi087. [DOI] [PubMed] [Google Scholar]
- 32.Kovesdy CP, Kalantar-Zadeh K. Iron therapy in chronic kidney disease: current controversies. J Ren Care. 2009;35(Suppl 2):14–24. doi: 10.1111/j.1755-6686.2009.00125.x. [DOI] [PubMed] [Google Scholar]
- 33.Palazzuoli A, Silverberg D, Iovine F, Capobianco S, Giannotti G, Calabrò A, et al. Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006;152(6):1096.e9–1096.e15. doi: 10.1016/j.ahj.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Beris P, Maniatis A. Role of intravenous iron therapy in anemia management: state of the art. Semin Hematol. 2006;43(6):S1–S2. [Google Scholar]
- 35.Cançado RD, Lobo C, Friedrich JR. Iron deficiency anemia treatment with parenteral iron. Rev Bras Hematol Hemoter. 2010;32(supl 2):121–128. [Google Scholar]
- 36.Cançado RD, Chiattone CS. Iron deficiency anemia in the adult - causes, diagnosis and treatment. Rev Bras Hematol Hemoter. 2010;32(3):240–246. [Google Scholar]
- 37.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51(2):103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith D. 2009: a requiem for rHuEPOs-but should we nail down the coffin in 2010? Clin J Am Soc Nephrol. 2010;5(5):929–935. doi: 10.2215/CJN.09131209. [DOI] [PubMed] [Google Scholar]
- 39.Singh AK. Does TREAT give the boot to ESAs in the treatment of CKD anemia? J Am Soc Nephrol. 2010;21(1):2–6. doi: 10.1681/ASN.2009111127. [DOI] [PubMed] [Google Scholar]
- 40.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomized trials. Lancet. 2009;373(9674):1532–1543. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 41.Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer associated anemia. JAMA. 2008;299(8):914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 42.Vaziri ND, Zhou XJ. Potential mechanisms of adverse outcomes in trials of anemia correction with erythropoietin in chronic kidney disease. Nephrol Dial Transplant. 2009;24(4):1082–1088. doi: 10.1093/ndt/gfn601. [DOI] [PubMed] [Google Scholar]
- 43.Streja E, Kovesdy CP, Greenland S, Kopple JD, McAllister CJ, Nissenson AR, et al. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52(4):727–736. doi: 10.1053/j.ajkd.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghali JK, Anand IS, Abraham WT, Fonarow GC, Greenberg B, Krum H, et al. Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation. 2008;117(4):526–535. doi: 10.1161/CIRCULATIONAHA.107.698514. [DOI] [PubMed] [Google Scholar]
- 45.van der Meer P, Groenveld H, Januzzi JL Jr, van Veldhuisen DJ. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009;95(16):1309–1314. doi: 10.1136/hrt.2008.161091. [DOI] [PubMed] [Google Scholar]
- 46.Ngo K, Kotecha D, Walters JA, Manzano L, Palazzuoli A, van Veldhuisen DJ, et al. Erythropoiesis-stimulating agents for anaemia in chronic heart failure patients. Cochrane Database Syst Rev. 2010 Jan;20(1): doi: 10.1002/14651858.CD007613.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandra SR, Finkelstein FO, Bennett AV, Lewis EF, Brazg T, Martin ML. Impact of erythropoiesis-stimulating agents on energy and physical function in non-dialysis CKD patients with anemia: a systematic review. Am J Kidney Dis. 2010;55(3):519–534. doi: 10.1053/j.ajkd.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Johansen KL, Finkelstein FO, Revicki DA, Gitlin M, Evans C, Mayne TJ. Systematic review and meta-analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis stimulating agents. Am J Kidney Dis. 2010;55(3):535–548. doi: 10.1053/j.ajkd.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 49.He SW, Wang LX. The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review. Congest Heart Fail. 2009;15(3):123–130. doi: 10.1111/j.1751-7133.2008.00030.x. [DOI] [PubMed] [Google Scholar]
- 50.Murphy CL, Fitzimmons RJ, Jardine AJ, Satar N, McMurray JJ. Routine assessment of iron status in all patients with heart failure may identify those at risk of developing anemia. Eur J Heart Fail. 2007;6(Suppl):24. [Google Scholar]
- 51.de Silva R, Rigby AS, Witte KK, Nikitin NP, Tin L, Goode K, et al. Anemia, renal dysfunction, and their interaction in patients with chronic heart failure. Am J Cardiol. 2006;98(3):391–398. doi: 10.1016/j.amjcard.2006.01.107. [DOI] [PubMed] [Google Scholar]
- 52.Barretto AC, Cardoso MN, Cardoso JN. Deficiência de ferro na insuficiência cardíaca. Rev Bras Hematol Hemoter. 2010;32(supl. 2):89–94. [Google Scholar]
- 53.Tello BS, Sales AL, Barcellos E, Lima LC, Steffen R, Wiefels C, et al. Anemia e disfunção renal na insuficiência cardíaca. Rev SOCERJ. 2007;20(6):434–442. [Google Scholar]
- 54.Jelani Q, Katz SD. Treatment of anemia in heart failure: potential risks and benefits of intravenous iron therapy in cardiovascular disease. Cardiol Rev. 2010;18(5):240–250. doi: 10.1097/CRD.0b013e3181e71150. [DOI] [PMC free article] [PubMed] [Google Scholar]