Abstract
Background
There is considerable controversy regarding the diagnosis of Acute Kidney Injury (AKI), and there are over 30 different definitions.
Objective
To evaluate the incidence and risk factors for the development of AKI following cardiac surgery according to the RIFLE, AKIN and KDIGO criteria, and compare the prognostic power of these criteria.
Methods
Cross-sectional study that included 321 consecutive patients (median age 62 [53-71] years; 140 men) undergoing cardiac surgery between June 2011 and January 2012. The patients were followed for up to 30 days, for a composite outcome (mortality, need for dialysis and extended hospitalization).
Results
The incidence of AKI ranged from 15% - 51%, accordingly to the diagnostic criterion adopted. While age was associated with risk of AKI in the three criteria, there were variations in the remaining risk factors. During follow-up, 89 patients developed the outcome and all criteria were associated with increased risk in the univariate Cox analysis and after adjustment for age, gender, diabetes, and type of surgery. However, after further adjustment for extracorporeal circulation and the presence of low cardiac output, only AKI diagnosed by the KDIGO criterion maintained this significant association (HR= 1.89 [95% CI: 1.18 - 3.06]).
Conclusion
The incidence and risk factors for AKI after cardiac surgery vary significantly according to the diagnostic criteria used. In our analysis, the KDIGO criterion was superior to AKIN and RIFLE with regard its prognostic power.
Keywords: Acute kidney failure, Cardiac Surgery, Hemodialysis, Coronary Artery Bypass
Introduction
Every year approximately one million heart surgeries are performed worldwide and these surgeries cause pathophysiological changes in various organs. This fact is further aggravated by the progressive aging of the general population, bringing with it an increased prevalence of chronic diseases that increase the surgical risk1.
The probability for development of acute kidney injury after cardiac surgery varies from 1% to 30%, according to the diagnostic criterion adopted and the type of cardiac surgery performed2,3. The development of AKI has a deep impact on the prognosis of patients undergone cardiac surgery, with mortality estimated at 15% - 30%. It is interesting to note that even small increases in plasma creatinine are significantly associated with mortality3-8. Furthermore, the prognostic impact is even greater in patients requiring dialysis, increasing in approximately eight times the risk of death, even after adjusting for other comorbidities and postoperative complications4.
Despite the recognized importance of acute kidney injure, one of the major problems in conducting studies on the subject is the lack of consensus regarding the diagnosis, as there are more than 30 different definitions9. Currently, three diagnostic criteria for AKI are highlighted: RIFLE ("Risk, Injury, Failure, Loss of kidney function and End-stage renal failure")9; AKIN ("Acute Kidney Injury Network")10, and KDIGO ("Kidney Disease: Improving Global Outcomes")11. The objective of the present study was to determine the incidence and risk factors for the development of AKI in the post cardiac-surgery accordingly to each criterion, as well as to compare the prognostic power of these criteria.
Methods
Study design
We conducted a cross-sectional study with a prospective follow-up, which included 321 patients (median age 62 [53-71] years, 140 men) undergoing cardiac surgery, including valve replacement, myocardial revascularization or both (combined surgery) at the Dante Pazzanese Institute of Cardiology (DPIC), between June 3rd, 2011 and January 23rd, 2012.
Patient Selection
We included all patients between 18 and 80 years of age, consecutively admitted for myocardial revascularization surgery, valve replacement or both procedures (combined surgery) at DPIC. Exclusion criteria were age below 18 or above 80, surgeries to repair congenital heart diseases or aortic aneurysms and patients with serum creatinine equal or greater than than 2.5 mg/dL in the preoperative period. Patients were followed from the immediate postoperative period for up to 30 days in order to identify cases of AKI, as well as need for hemodialysis, extended hospitalization and death. Serum creatinine was measured preoperatively and daily after surgery for a period of seven days, by the Central Laboratory of DPIC. Glomerular filtration rate was assessed by the Cockroft-Gault equation. The pre- and perioperative data were collected by chart / surgery description review and the researchers had no influence on the management of patients. The study was approved by the Research Ethics Committee of DPIC.
Definition of extended hospitalization, low cardiac output and acute kidney injury
The definition of extended hospitalization was based on the median and percentiles (8 [6-14] days) of elapsed time between cardiac surgery and hospital discharge of study participants; thus we defined "patients with extended hospitalization" as those with a post-operative hospitalization period equal to or greater than 15 days. The diagnosis of low cardiac output was based on the need to use at least three vasoactive drugs or hemodynamic support by means of an intra-aortic balloon during admission in the intensive care unit (ICU)12. AKI diagnosis was based on serum creatinine variations over a period of seven days, according to the three different criteria, as follows:
AKIN: Increase in serum creatinine equal to or greater than 0.3 mg/dL, or equal to or greater than 1.5 times the baseline creatinine level at "a 48-hour moving window10.
RIFLE: Increase in serum creatinine equal to or greater than 1.5 mg/dL above baseline creatinine for up to seven days9.
KDIGO: Increa se in serum creatinine equal to or greater than 0.3 mg/dL in 48 hours, or equal to or greater than 1.5 times the baseline creatinine for up to seven days11.
Statistical Analysis
Variables were represented by mean and standard deviation, median and percentiles (25-75) or absolute (n) and relative (%) value, as appropriate. Comparisons of variables between two different groups were performed by the Mann-Whitney test, for continuous variables, and Pearson chi-square test, for categorical variables. We conducted uni and multivariate logistic regression analyses to determine the factors associated with the development of AKI for each of the criteria studied. Survival analyses were performed by the Kaplan-Meier survival curves and the Cox proportional hazard model, using a composite outcome (death or need for dialysis in the first thirty days after surgery or prolonged hospitalization). Statistical significance was set at the level of P<0.05; and the statistical analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago IL, 2004).
Results
General characteristics of the study population and the incidence of AKI
The demographic and clinical characteristics of patients included in the study are summarized in Table 1. It is interesting to note that Extracorporeal Circulation (ECC) was used in all patients and AKI incidence varied from 15% to 51%, depending on the diagnostic criterion adopted.
Table 1.
Demographic and clinical characteristics of 321 patients undergoing cardiac surgery1
| Total Population | |||
| (n = 321) | |||
| Pre-operative | |||
| Age (years) | 62 (53 - 71) | ||
| Men (n, %) | 140 (44%) | ||
| BMI (Kg/m2) | 27.1 ± 5.2 | ||
| Diabetes (n, %) | 95 (30%) | ||
| Previous AMI (n, %) | 102 (32%) | ||
| Previous Cardiac Surgery (n, %) | 39 (12%) | ||
| Use of Statins (n, %) | 220 (69%) | ||
| Use of Insulin (n, %) | 27 (8%) | ||
| Hemoglobin (g/dL) | 13.5 ± 3.9 | ||
| GFR (ml/min) | 73.9 (58.8 - 98.1) | ||
| Perioperative | |||
| Type of Surgery (n, %) | |||
| MR | 158 (49%) | ||
| Valvular Surgery | 139 (43%) | ||
| Combined Surgery | 24 (8%) | ||
| Time of ECC (h) | 1.5 (1.2- 1.9) | ||
| Volume Balance (ml) | -240 (-855 - 339) | ||
| Post-operative | |||
| Low cardiac output (n, %) | 15 (5%) | ||
| Acute Kidney Injury | |||
| AKIN (n, %) | 163 (51%) | ||
| RIFLE (n, %) | 48 (15%) | ||
| KDIGO (n, %) | 62 (19%) | ||
1Data presented as average ± SD, median (percentiles 25-75) or absolute (n) and relative values (%).
BMI: Body Mass Index; AMI: Acute myocardial infarction, GFR: Glomerular filtration rate; MR: Myocardial revascularization; ECC: Extracorporeal Circulation.
Phenotype of patients diagnosed according to AKI diagnoses by the different criteria
Table 2 compares the demographic and clinical characteristics of patients according to the development of renal injury for each criterion evaluated. In summary, when using the AKIN criterion patients who developed AKI were older and had a lower glomerular filtration rate (GFR), in addition to a higher prevalence of insulin use, as well as, a higher prevalence of valve and/or combined surgery and low cardiac output. By RIFLE criterion, patients who had AKI differed from the others only by an older age and a higher prevalence of low cardiac output. Finally, when KDIGO criterion was applied, in addition to older age, patients who developed acute kidney injury also had a higher prevalence of insulin use and low cardiac output.
Table 2.
Demographic and clinical characteristics of 321 patients undergoing cardiac surgery according to the presence or absence of AKI by different criteria1
| AKIN | RIFLE | KDIGO | ||||||||
| p | p | p | ||||||||
| (n = 158) | (n = 163) | (n = 273) | (n =48) | (n = 259) | (n =62) | |||||
| Age (years) | 0.001 | 0.003 | 0.006 | |||||||
| (51 - 68) | (55 - 73) | (52 - 71) | (58 - 76) | (52 - 71) | (56 - 75) | |||||
| Men (n, %) | 0.806 | 0.768 | 0.991 | |||||||
| (44%) | (43%) | (44%) | (42%) | (44%) | (44%) | |||||
| BMI (Kg/m2) | 0.369 | 0.473 | 0.559 | |||||||
| (±5.3) | (±5.1) | (±5.2) | (±4.9) | (±5.2) | (±5.0) | |||||
| Diabetes (n, %) | 0.584 | 0.679 | 0.609 | |||||||
| (31%) | (28%) | (30%) | (27%) | (29%) | (32%) | |||||
| Previous AMI (n, %) | 0.250 | 0.932 | 0.831 | |||||||
| (35%) | (29%) | (32%) | (31%) | (32%) | (31%) | |||||
| 0.784 | 0.690 | 0.286 | ||||||||
| surgery (n,%) | (13%) | (12%) | (13%) | (10%) | (11%) | (16%) | ||||
| Use of statins (n, %) | 0.883 | 0.475 | 0.481 | |||||||
| (70%) | (70%) | (69%) | (75%) | (69%) | (74%) | |||||
| Use of insulin (n, %) | 0.011 | 0.097 | 0.013 | |||||||
| (4%) | (12%) | (7%) | (15%) | (7%) | (16%) | |||||
| Hemoglobin (g/dL) | 13.5 ± 5.2 | 13.4 ± 2.0 | 0.416 | 13.7 ± 4.2 | 13.1 ± 1.8 | 0.094 | 13.8 ± 4.3 | 13.1 ± 1.7 | 0.074 | |
| GFR (ml/min) | <0.001 | 0.674 | 0.959 | |||||||
| ( 67.1 - 102.7) | ( 53.0 - 94.2) | ( 58.9- 96.8) | ( 54.7 - 108.6) | ( 59.1 - 97.0) | ( 55.5 - 105.8) | |||||
| Combined surgery | 4 (2%) | 20 (12%) | 17 (6%) | 7 (15%) | 15 (6%) | 9 (14%) | ||||
| Time of ECC (h) | 0.071 | 1.4 (1.2- 1.8) | 0.206 | 0.247 | ||||||
| (1.2- 1.8) | (1.2 - 2.1) | (1.2 - 2.3) | (1.2- 1.8) | (1.1 - 2.3) | ||||||
| Volume Balance (ml) | 0.567 | 0.432 | 0.275 | |||||||
| (-895 - 300) | (-820 - 397) | (-895 - 300) | (-789 - 560) | (-903 - 295) | (-775 - 553) | |||||
| 0.020 | 0.005 | < 0.001 | ||||||||
| Output (n, %) | (2%) | (7%) | (3%) | (13%) | (2%) | (15%) | ||||
| 0.086 | 0.715 | 0.246 | ||||||||
| hospitalization (n, %) | (19%) | (28%) | (24%) | (21%) | (22%) | (29%) | ||||
| Hemodialysis (n, %) | 0.030 | 0.005 | 0.014 | |||||||
| (0) | (4%) | (1 %) | (8%) | (1%) | (7%) | |||||
| Death (n, %) | 0.011 | <0.001 | < 0.001 | |||||||
| (1%) | (6%) | (1%) | (17%) | (1%) | (13%) | |||||
| Combined events (n, %) | 0.002 | 0.013 | < 0.001 | |||||||
| (20%) | (36%) | (25%) | (44%) | (23%) | (47%) | |||||
BMI: Body mass index; AMI: Acute myocardial infarction; GFR: Glomerular filtration rate; MR: Myocardial revascularization; ECC: Extracorporeal circulation
1 Data presented as average ± SD, median (25-75 percentiles) or absolute (n) and relative (%) values.
AKI determinants after cardiac surgery
Aiming to determine the factors independently associated with the development of AKI using the different diagnostic criteria studied, we developed a series of logistic regression analyses, which are presented in Table 3. We noted that the factors independently associated with AKI development by AKIN criterion were age, use of insulin and valve surgery (pure and combined). Under the RIFLE criterion, the risk factors identified were age, duration of ECC and presence of low cardiac output. When the diagnostic of AKI was made by KDIGO criterion, the factors associated with increased risk were age, insulin use and low cardiac output.
Table 3.
Risk factors for AKI in 321 patients undergoing cardiac surgery, according to different criteria
| AKIN | RIFLE | KDIGO | |||||
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | ||
| Age (years) | 1.03 (1.01 - 1.05) | 1.03 (1.01 - 1.05) | 1.04 (1.01 - 1.07) | 1.04 (1.02 - 1.07) | 1.03 (1.01 - 1.06) | 1.03 (1.01 - 1.06) | |
| Men (n, %) | 1.06 (0.68 - 1.64) | ------ | 1.09 (0.59 - 2.04) | ------ | 1.00 (0.57 - 1.75) | ------ | |
| BMI (Kg/m2) | 0.98 (0.94 - 1.03) | ------ | 0.99 (0.93 - 1.05) | ------ | 0.99 (0.94 - 1.05) | ------ | |
| Diabetes (n, %) | 0.88 (0.54 - 1.41) | ------ | 0.87 (0.44 - 1.72) | ------ | 1.17 (0.64 -2.12) | ------ | |
| Previous AMI (n, %) | 0.76 (0.47 - 1.22) | ------ | 0.97 (0.50 - 1.88) | ------ | 0.94 (0.51 - 1.71) | ------ | |
| 0.91 (0.47 - 1.78) | ------ | 0.82 (0.30 - 2.21) | ------ | 1.53 (0.70 - 3.33) | ------ | ||
| Surgery (n, %) | |||||||
| Use of Statins (n, %) | 1.04 (0.64 - 1.68) | ------ | 1.29 (0.64 - 2.62) | ------ | 1.25 (0.67 - 2.35) | ------ | |
| Use of Insulin (n, %) | 3.04 (1.25 - 7.40) | 3.46 (1.36 - 8.83) | 2.15 (0.86 - 5.41) | ------ | 2.79 (1.21 - 6.45) | 3.10 (1.28 - 7.51) | |
| Hemoglobin (g/dL) | 0.96 (0.88 - 1.04) | ------ | 0.89 (0.77 - 1.04) | ------ | 0.90 (0.79 - 1.04) | ------ | |
| GFR (ml/min) | 0.98 (0.98 - 0.99) | 0.99 (0.98 - 1.01) | 1.01 (0.99 - 1.02) | ------ | 1.01 (0.99 - 1.02) | ------ | |
| Combined Surgery | 6.62 (2.16 - 20.26) | 4.09 (1.20 - 13.94) | 2.42 (0.90 - 6.47) | ------ | 2.67 (1.06 - 6.69) | 1.51 (0.48 - 4.70) | |
| Time of ECC (h) | 1.66 (1.16 - 2.39) | 1.39 (0.92 - 2.10) | 1.61 (1.04 - 2.47) | 1.76 (1.11 - 2.79) | 1.50 (1.01 - 2.24) | 1.55 (0.94 - 2.56) | |
| Volume Balance (ml) | 1.00 (1.00 - 1.00) | ------ | 1.00 (1.00 - 1.00) | ------ | 1.00 (1.00 - 1.00) | ------ | |
| 4.11 (1.14 - 14.84) | 3.79 (0.98 - 14.69) | 4.19 (1.42 - 12.38) | 3.67 (1.18- 11.38) | 7.16 (2.45 - 20.97) | 7.05 (2.28 - 21.78) | ||
| Output (n, %) | |||||||
1 Patients without AKI were used as reference. 2 Type of surgery: Patients underwent myocardial revascularization were used as reference. BMI: Body mass index; AMI: Acute myocardial infarction; GFR: Glomerular filtration rate; MR: Myocardial revascularization; ECC: Extracorporeal Circulation.
Association between diagnosis of AKI and events
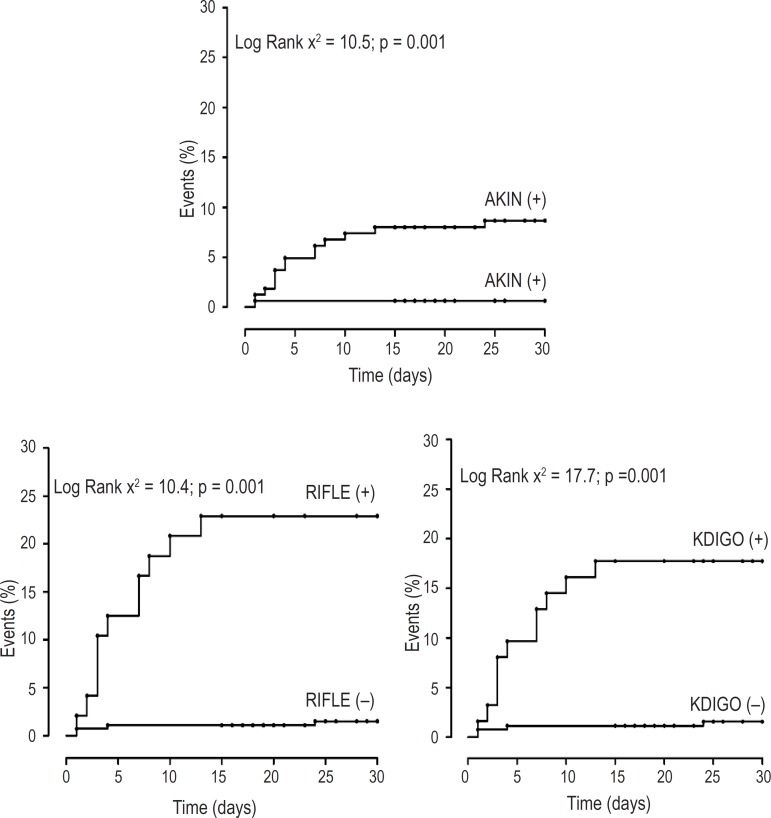
During follow-up there were eleven deaths (seven from septicemia and four from cardiogenic shock), six patients required hemodialysis and 75 had an extended hospitalization, comprising a total of 92 combined events in 89 patients (Table 2). Patients with AKI diagnosed by AKIN, RIFLE and KDIGO criteria presented an increased risk for development of events in the Kaplan-Meyer analysis (figure 1). In univariate Cox analysis, patients diagnosed with AKI by AKIN (HR: 1.99; [95% CI: 1.29 - 3.08]), RIFLE (HR: 2.15 [95% CI: 1.32 - 3.51]) and KDIGO (HR: 2.45 [95% CI: 1.57 - 3.82] presented an increased risk for the development of adverse events and this association persisted even after adjustment for age, gender, diabetes and type of surgery. However, after further adjustment for extracorporeal circulation and the presence of low cardiac output, only the diagnosis of AKI by KDIGO criterion maintained this significant association (HR = 1.89 [95% CI: 1.18 to 3.06]), as shown in Table 4.
Figure 1.
Kaplan-Meyer curves for combined events in 321 patients undergoing cardiac surgery, according to different diagnostic criteria of acute renal injury.
Table 4.
Association between AKI diagnosis by different criteria and the risk of development of combined events (dialysis, death and/or prolonged hospitalization) in 321 patients undergoing to cardiac surgery
| Model | Variables | |||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | ||
| 1 | Univariate analysis | 1.99 (1.29- 3.08) | 2.15 (1.32 - 3.51) | 2.45 (1.57 - 3.82) |
| 2 | 1.62 (1.02 - 2.55) | 1.89 (1.15 - 3.14) | 2.19 (1.39 - 3.44) | |
| Type of surgery 1 | ||||
| 3 | 1.41 (0.89 - 2.25) | 1.58 (0.93 - 2.67) | 1.89 (1.18 - 3.06) | |
| Output |
1 Patients who underwent myocardial revascularization were used as reference. ECC: Extracorporeal circulation.
Discussion
The incidence of acute kidney injury after cardiac surgery in our population was 15% by RIFLE criterion, 19% by KDIGO criterion and 51% by AKIN criterion. To the best of our knowledge, this was the first study to compare these three criteria in this kind of population. Patients with AKI diagnosis, regardless of the criterion used, had higher mortality and a higher risk of combined events (death, need for dialysis and/or extended hospitalization); our results are in agreement and support previous evidences regarding the poor prognosis associated with the development of AKI4,13-16. It is interesting to highlight, however, that two patients with AKI diagnosed by AKIN criterion, but without renal injury by RIFLE and KDIGO criteria, required dialytic therapy.
Despite all advances in knowledge regarding the pathogenesis and treatment of AKI, it is still a relatively common complication in critically ill patients and is associated with mortality independently of other risk factors4,9,17. In addition, there are still several controversies regarding the matter, particularly about the definition of AKI18. The lack of consensus leads to inaccuracy in data regarding the actual incidence of this complication, as can be confirmed by the large variation (1% - 30%) in the reported incidence of AKI post cardiac-surgery3. In fact, the incidence in our study showed a wide variation (15% - 51%) according to the criterion adopted. In order to compare the incidence of AKI described here with that of other populations undergoing similar interventions, we looked for studies that have used the criteria that we assessed. In this regard, when the AKIN criterion was evaluated, AKI incidence ranged between 26 % and 49 %16,19,20, while with RIFLE criterion the incidence varied between 19% and 30%19-21. We found only one study that had evaluated AKI incidence through KDIGO criterion and this was 15%22. Taken together, these data show that the incidence of AKI described here is comparable to those reported in the literature; however, it is also clear that even with the use of similar criteria, AKI incidence after cardiac surgery is still quite variable.
Among the clinical variables assessed, age was associated with AKI risk regardless of the criterion used, possibly reflecting the lower tolerance of older patients to hemodynamic and electrolyte fluctuations commonly observed with this type of surgical intervention13,15. Interestingly, the presence of diabetes mellitus was not associated with a higher risk of AKI in our patients, but the use of insulin increased this risk by more than three times by AKIN and KDIGO criteria, which probably indicates the existence of different degrees of risk related to the time of exposure and seriousness of diabetes2,23. Compared with myocardial revascularization, valvular and combined surgery increased the risk of AKI in the AKIN criteria, respectively, two to four times, reinforcing previous evidence pointing out the increased risk of renal injury in this type of surgery2, 12,24,25 The augmentation on ECC time increased by nearly two-fold the risk of renal function decline according to the RIFLE criterion; in fact, an ECC time above 90 minutes is related to an increased urinary excretion of renal tubular damage markers26 and evidences show that surgeries without ECC are associated with a smaller risk of acute renal failure27,28. The presence of low cardiac output was the factor most strongly associated with AKI risk, according to RIFLE (approximately four-fold) and KDIGO (approximately seven-fold); in fact, low cardiac output is a recognized AKI predictor12,29-31. Considered all together, our results show that there is a large variation between the factors that determine AKI, according to the diagnosis criteria used. It is important to highlight this finding, as it may indicate the inaccuracy of predictive scores for AKI developed based on a single diagnosis criterion.
One of the most important contributions of our study was to compare the prognostic power of different AKI criteria. Accordingly, we demonstrated that despite the association of the three evaluated criteria with an increased risk of adverse events in univariate analysis, and ever after adjustment for comorbidities and type of surgery, only the KDIGO criterion maintained its prognostic power when hemodynamic variables were taken into consideration (i.e. time of ECC and low cardiac output); these results suggest the superiority of the KDIGO criterion in comparison to RIFLE and AKIN. However, several limitations should be taken into account when analyzing our data, including: 1) the relatively reduced number of patients, 2) the diagnosis of AKI, which was based only on variations in plasma creatinine, regardless of changes in glomerular filtration rate and urinary output, and 3) we did not take into consideration the stages of kidney injury, which could increase the specificity of certain criteria. Our goal, however, was to compare simple diagnostic instruments based on easily accessible information, even for non-nephrologists; and thus, we believe that the present study, despite the limitations described, provides an important contribution to the medical community.
Conclusion
The incidence of acute kidney injury after cardiac surgery and the risk factors for its development vary significantly according to the diagnostic criterion used, and this information should be taken into consideration when developing predictive scores for AKI. In our analysis, the KDIGO criterion was superior to AKIN and RIFLE with regard its prognostic power.
Author contributions
Conception and design of the research, Acquisition of data, Analysis and interpretation of the data and Writing of the manuscript: Sampaio MC, Máximo CAG, Montenegro CM, Mota DM, Fernandes TR, Cordeiro AC; Statistical analysis: Cordeiro AC; Critical revision of the manuscript for intellectual content: Bianco ACM, Amodeo C, Cordeiro AC.
Footnotes
Potential Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Sources of Funding: There were no external funding sources for this study.
Study Association: This article is the result of the scientific project required for conclusion of the Medical Residency Program in Cardiology at Dante Pazzanese Institute of Cardiology from the follow medical residents: Carlos Alberto Gonçalves Máximo, Carolina Moreira Montenegro, Diandro Marinho Mota, Márcio Campos Sampaio and Tatiana Rocha Fernandes
References
- 1.Rosner MH, Portilla D, Okusa MD. Cardiac surgery as a cause of acute kidney injury: pathogenesis and potential therapies. J Intensive Care Med. 2008;23(1):3–18. doi: 10.1177/0885066607309998. [DOI] [PubMed] [Google Scholar]
- 2.Grayson AD, Khater M, Jackson M, Fox MA. Valvular heart operation is an independent risk factor for acute renal failure. Ann Thorac Surg. 2003;75(6):1829–1835. doi: 10.1016/s0003-4975(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 5.Macedo E, Castro I, Yu L, Abdulkader RR, Vieira JM., Jr Impact of mild acute kidney injury (AKI) on outcome after open repair of aortic aneurysms. Ren Fail. 2008;30(3):287–296. doi: 10.1080/08860220701857522. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa RR, Cestari PF, Capeletti JT, Peres GM, Ibanez TL, da Silva PV, et al. Impact of renal failure on in-hospital outcomes after coronary artery bypass surgery. Arq Bras Cardiol. 2011;97(3):249–253. doi: 10.1590/s0066-782x2011005000075. [DOI] [PubMed] [Google Scholar]
- 7.Brito DJ, Nina VJ, Nina RV, Figueiredo JA, Neto, Oliveira MI, Salgado JV, et al. Prevalence and risk factors for acute renal failure in the postoperative of coronary artery bypass grafting. Rev Bras Cir Cardiovasc. 2009;24(3):297–304. doi: 10.1590/s0102-76382009000400007. [DOI] [PubMed] [Google Scholar]
- 8.Santos FO, Silveira MA, Maia RB, Monteiro MD, Martinelli R. Acute renal failure after coronary artery bypass surgery with extracorporeal circulation - incidence, risk factors, and mortality. Arq Bras Cardiol. 2004;83(2):150-4; 145-9. doi: 10.1590/s0066-782x2004001400006. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31–R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury . Kidney inter. 2012;2(Suppl):1–138. [Google Scholar]
- 12.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72(5):624–631. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 13.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14(5):1158–1162. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 14.Zanardo G, Michielon P, Paccagnella A, Rosi P, Calo M, Salandin V, et al. Acute renal failure in the patient undergoing cardiac operation: Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg. 1994;107(6):1489–1495. [PubMed] [Google Scholar]
- 15.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128(3):194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Machado MN, Miranda RC, Takakura IT, Palmegiani E, Santos CA, Oliveira MA, et al. Acute kidney injury after on-pump coronary artery bypass graft surgery. Arq Bras Cardiol. 2009;93(3):247–252. doi: 10.1590/s0066-782x2009000900008. [DOI] [PubMed] [Google Scholar]
- 17.de Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26(7):915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R, Kellum J, Ronco C. Acute renal failure: time for consensus. Intensive Care Med. 2001;27(11):1685–1688. doi: 10.1007/s00134-001-1120-6. [DOI] [PubMed] [Google Scholar]
- 19.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15(1):R16–R16. doi: 10.1186/cc9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert AM, Kramer RS, Dacey LJ, Charlesworth DC, Leavitt BJ, Helm RE, et al. Northern New England Cardiovascular Disease Study Group Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg. 2010;90(6):1939–1943. doi: 10.1016/j.athoracsur.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 21.D'Onofrio A, Cruz D, Bolgan I, Auriemma S, Cresce GD, Fabbri A, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. 2010 Jul;16(Suppl 1):S32–S36. doi: 10.1111/j.1751-7133.2010.00170.x. [DOI] [PubMed] [Google Scholar]
- 22.Ho J, Reslerova M, Gali B, Nickerson PW, Rush DN, Sood MM, et al. Serum creatinine measurement immediately after cardiac surgery and prediction of acute kidney injury. Am J Kidney Dis. 2012;59(2):196–201. doi: 10.1053/j.ajkd.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Kubal C, Srinivasan AK, Grayson AD, Fabri BM, Chalmers JA. Effect of risk-adjusted diabetes on mortality and morbidity after coronary artery bypass surgery. Ann Thorac Surg. 2005;79(5):1570–1576. doi: 10.1016/j.athoracsur.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 24.Tuttle KR, Worrall NK, Dahlstrom LR, Nandagopal R, Kausz AT, Davis CL. Predictors of ARF after cardiac surgical procedures. Am J Kidney Dis. 2003;41(1):76–83. doi: 10.1053/ajkd.2003.50025. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues AJ, Evora PR, Bassetto S, Alves L, Junior, Scorzoni A, Filho, Araujo WF, et al. Risk factors for acute renal failure after heart surgery. Rev Bras Cir Cardiovasc. 2009;24(4):441–446. doi: 10.1590/s0102-76382009000500003. [DOI] [PubMed] [Google Scholar]
- 26.Boldt J, Brenner T, Lehmann A, Suttner SW, Kumle B, Isgro F. Is kidney function altered by the duration of cardiopulmonary bypass. Ann Thorac Surg. 2003;75(3):906–912. doi: 10.1016/s0003-4975(02)04559-9. [DOI] [PubMed] [Google Scholar]
- 27.Beauford RB, Saunders CR, Niemeier LA, Lunceford TA, Karanam R, Prendergast T, et al. Is off-pump revascularization better for patients with non-dialysis-dependent renal insufficiency. Heart Surg Forum. 2004;7(2):E141–E146. doi: 10.1532/HSF98.200330203. [DOI] [PubMed] [Google Scholar]
- 28.Ascione R, Nason G, Al-Ruzzeh S, Ko C, Ciulli F, Angelini GD. Coronary revascularization with or without cardiopulmonary bypass in patients with preoperative nondialysis-dependent renal insufficiency. Ann Thorac Surg. 2001;72(6):2020–2025. doi: 10.1016/s0003-4975(01)03250-7. [DOI] [PubMed] [Google Scholar]
- 29.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16(1):162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 30.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297(16):1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 31.Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Society of Thoracic Surgeons National Cardiac Surgery Database Investigators Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]