Abstract
Background
Previous studies have shown that coronary plaque composition plays a pivotal role in plaque instability, and imaging modalities and serum biomarkers have been investigated to identify vulnerable plaque. Virtual histology IVUS (VH-IVUS) characterizes plaque components as calcified, fibrotic, fibrofatty, or necrotic core. C-reactive protein (hsCRP) is an independent risk factor and a powerful predictor of future coronary events. However, a relationship between inflammatory response indicated by CRP and plaque characteristics in ACS patients remains not well established.
Objective
To determine, by using VH-IVUS, the relation between coronary plaque components and plasma high-sensitivity CRP levels in patients with acute coronary syndromes (ACS).
Methods
52 patients with ACS were enrolled in this prospective study. Electrocardiographically-gated VH-IVUS were performed in the culprit lesion before PCI. Blood sample was drawn from all patients before the procedure and after 24 hours, and hs-CRP levels were determined.
Results
Mean age was 55.3±4.9 years, 76.9% were men and 30.9% had diabetes. Mean MLA was 3.9±1.3 mm2, and plaque burden was 69±11.3%, as assessed by IVUS. VH-IVUS analysis at the minimum luminal site identified plaque components: fibrotic (59.6±15.8%), fibrofatty (7.6±8.2%), dense calcium (12.1±9.2%) and necrotic core (20.7±12.7%). Plasma hs-CRP (mean 16.02±18.07 mg/L) did not correlate with necrotic core (r=-0.089, p = 0.53) and other plaque components.
Conclusions
In this prospective study with patients with ACS, the predominant components of the culprit plaque were fibrotic and necrotic core. Serum hs C-reactive protein levels did not correlate with plaque composition.
Keywords: Acute Coronary Syndrome; Plaque, Atherosclerosis; C-Reactive Protein; Histological Techniques; Diagnostic Imaging
Introduction
The term acute coronary syndrome encompasses a broad spectrum of clinical presentations and prognostic evolutions, and is related mostly to one pathophysiologic substrate: the rupture or erosion of a vulnerable plaque1,2. Previous studies demonstrate that the main determinant of the atherosclerotic plaque vulnerability is its composition. Thus, lesions characterized by a large lipid core, by the presence of inflammatory cells (instead of smooth muscle cells and fibrous tissue) and thin-cap fibrosis were more subjected to instabilization3,4.
It is postulated that the early detection of thin-cap fibroatheromas would have a profound impact on the prevention of cardiac adverse events. Recently, various cardiovascular imaging methods and laboratorial markers have been investigated with this purpose5,6. Intracoronary ultrasound with radiofrequency, also known as Virtual Histology® (Volcano Therapeutics, EUA), complements anatomic information provided by conventional intracoronary ultrasound, allowing not only the evaluation of plaque dimensions but detailed tissue characterization of the atherosclerotic lesion7-9.
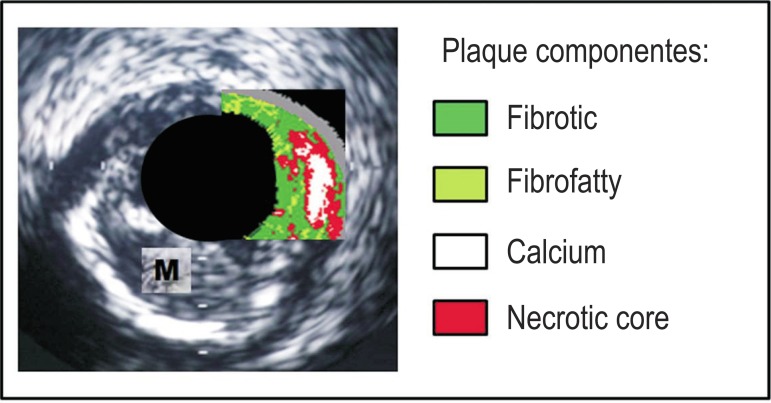
Four basic components of the plaque (fibrous and fibrofatty tissues, calcium and areas with inflammatory activity and necrosis) are determined by the method. In imaging reconstruction (Figure 1), these elements are characterized by different colors: light green (fibrofatty tissue), dark green (fibrotic tissue), white (calcium) and red (necrotic core), which can also be quantified according to their corresponding areas or to the percentage of each component in the total plaque volume10. Among the laboratorial methods that were investigated, C-reactive protein (CRP) is an acute-phase reactant and a sensitive marker of the systemic inflammatory status, and is considered an important predictor of cardiac adverse events11,12. However, its correlation with atheroma plaque characteristics is not well established.
Figure 1.
Ultrasonographic imaging reconstruction by Virtual Histology®; the four components of the plaque are characterized by different colors (green, light green, red and white). The medium layer (according to visualization by conventional intracoronary ultrasound) is identified by the gray color. M: medium layer.
The present study aims to evaluate the constitution of the culprit atherosclerotic lesion in patients with acute coronary syndrome (as characterized by radiofrequency ultrasound) and investigate the relationship of the plaque components and the inflammatory marker C-reactive protein.
Methods
This is a prospective study of 52 consecutive patients with acute coronary syndrome with or without ST segment elevation, admitted at the Dante Pazzanese Cardiology Institute from September 2008 to November 2009, submitted to cinecoronariography according to clinical indication and who were candidates to percutaneous intervention. The study was approved by the Ethics Committee of the Institution involved, in compliance with the Declaration of Helsinki. All patients signed an informed consent document.
The inclusion criteria were: patients ≥18 years old, whose clinical picture was compatible with acute coronary syndrome that had occurred up to 30 days before coronary percutaneous intervention; primary coronary lesion characterized by: a) obstruction ≥ 50 % of the vascular lumen according to visual evaluation, with an extension <18 mm and localized in vessels >2.5 mm, considered the responsible lesion ("culprit") for the clinical picture. Patient with AMI < 72 h, refractory angina, hemodynamic instability or coronary flux TIMI (Thrombolysis in Myocardial Infarction) < 3 requiring immediate coronary intervention and obstructive lesions (≥ 50%) of the left coronary trunk, with total occlusions and thrombotic lesions at the intervention site (according to angiography) were excluded. Patients with current infections, connective tissue diseases or other chronic inflammatory conditions or using corticosteroids were also excluded, since these conditions could also influence the C-reactive protein laboratory results.
Imaging protocol with intracoronary ultrasound with radiofrequency
After characterizing the culprit vessel and before coronary intervention, a 0.014" metallic guide wire was positioned distally to the lesion to be treated. The Eagle Eye® radiofrequency ultrasound probe (Volcano Therapeutics, EUA) was advanced over the metallic guide wire and positioned initially in a segment free of significant atherosclerotic disease; after connection to a specific console (InVision Gold®, Volcano Corporation Inc., Rancho Cordova, EUA), the images were calibrated. The ultrasound probe was then positioned distally to the culprit lesion, and was pulled at the velocity of 0.5 mm/sec with an automatic device (R-100®, Volcano Corporation Inc., Rancho Cordova, EUA) for obtaining image sequences.
After completion of the radiofrequency ultrasound, culprit lesion percutaneous treatment followed. Of note, lesion pre-dilation was performed only after obtaining ultrasound images with Virtual Histology®, so that the plaque characteristics reflected its natural status, before any mechanical intervention.
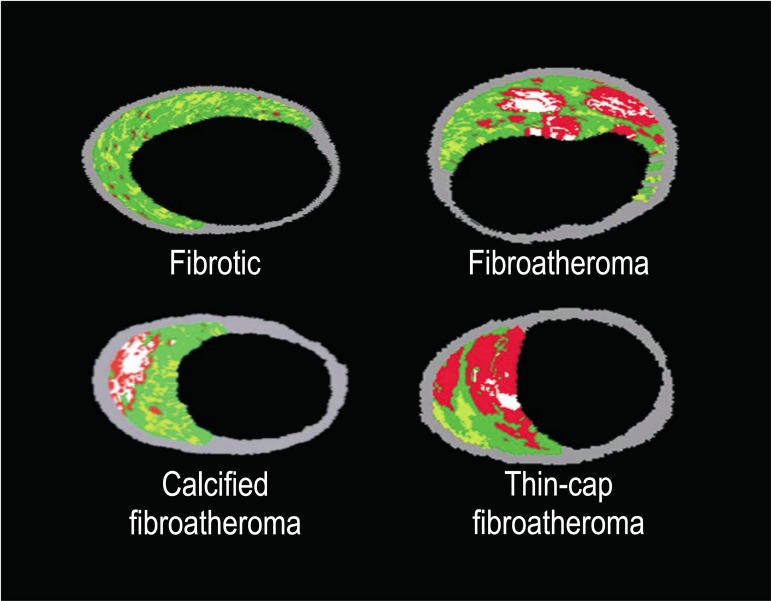
Virtual Histology® image analysis was performed by specific software (pcVH 2.2®, Volcano Corporation Inc.®, Rancho Cordova, EUA). This software allows that the lumen and the external elastic membrane are contoured, providing an automatic classification of the four plaque components in the interest region (fibrous and fibrofatty tissue, calcium and necrotic core). Radiofrequency ultrasound analysis was limited to a 10 mm-segment of the atherosclerotic lesion to be treated. This segment must encompass the major obstruction point of the lumen; thus, taking the minimum lumen area as reference, 5 mm were determined proximally and distally to it. Based on the percent composition of the plaque and the localization of the calcium and necrotic contents in relation to the vascular lumen, the lesions were classified by Virtual Histology® as follows:
• fibrotic plaque: predominantly fibrotic tissue, with no confluent necrotic tissue areas or calcium;
• calcified fibroatheroma: presence of calcium confluent areas (> 10% of the plaque area) in three or more consecutive slices;
• fibroatheroma: presence of necrotic tissue confluent areas (> 10% of the plaque area) in three or more consecutive slices;
• thin-cap fibroatheroma (TCFA): presence of necrotic tissue confluent areas (> 10% of the plaque area) in three or more consecutive slices and in direct contact with the vessel lumen (Figure 2).
Figure 2.
Examples of coronary lesions classified by intracoronary ultrasound with Virtual Histology (adapted from reference 10).
Sample collection and C-reactive protein measurement
Before coronary intervention, all patients were submitted to clinical, electrocardiographic and laboratorial evaluation. Peripheral blood samples for measuring high sensitivity C-reactive protein (an inflammatory marker) were obtained from the arterial access (by means of radial or femoral introducer sheath) immediately before the percutaneous revascularization procedure. The post-procedure samples were obtained 24 hours after the intervention. The serum high sensitivity C-reactive protein measurement was measured with the automatized chemoluminescence test IMMULITE® (DPCMedLab®, Brazil). The variation between this inflammatory marker's basal levels and the one observed 24 hours after the intervention was defined as Δ (delta) CRP, and was calculated for each patient.
Statistical analysis
The categorical variables were expressed in absolute figures and percentages, and presented as relative and absolute frequency tables. The analysis of the differences among the categorical variables was performed by the Pearson's chi-square test and the Fisher exact test, when necessary. The continuous variables were expressed as mean and standard deviation. The differences among continuous variables were determined by the Student t test. When normality was rejected, the variables were compared by the Mann-Whitney non-parametric test. The correlations among the quantitative variables were presented according to the Pearson correlation coefficient. A 5% significance level was considered for all tests (α = 0.05). The software PAWS Statistics (SPSS)® (SPSS Inc, EUA) was employed in the statistical analysis.
Results
Angiographic and clinical characteristics
Mean age was 55.3 ± 4.9 years; 76.9% were male, 67.3% had arterial hypertension, 30.8% were diabetic and 50% were smokers. The predominant clinical picture that motivated the percutaneous coronary intervention was ST segment elevation acute myocardial infarction in its later phase (post-AMI elective intervention). The general clinical characteristics of the selected patients are discriminated on Table 1.
Table 1.
Clinical data of the 52 selected patients
| Variables | ||
| (n = 52) | ||
| Age (mean and standard deviation), years | 55.3 ± 4.9 | |
| Age variation | 39 - 74 | |
| Sex, n (%) | ||
| Male | 40 (76.9) | |
| Risk factors, n (%) | ||
| Arterial Hypertension | 35 (67.3) | |
| Diabetes mellitus | 16 (30.8) | |
| Dyslipidemia | 31 (59.6) | |
| Current smokers | 26 (50.0) | |
| Previous cardiac events, n(%) | ||
| Myocardial Infarction | 7 (13.5) | |
| Cardiovascular history, n (%) | ||
| Percutaneous coronary intervention | 2 (3.8) | |
| Myocardial revascularization | 2 (3.8) | |
| Comorbidities, n (%) | ||
| Renal failure# | 9 (17.3) | |
| Pre-intervention clinical picture, n (%) | ||
| Unstable angina | 16 (30.8) | |
| NSTEMI | 10 (19.2) | |
| STEMI | 26 (50.0) | |
#Creatinine clearance <60 ml/min; STEMI: ST segment-elevation myocardial infarction; NSTEMI: non- ST segment-elevation myocardial elevation
All patients were treated with aspirin (100-200 mg/d); clopidogrel (300-600 mg bolus and 75 mg/d for maintenance); and full-dose enoxaparin (1 mg/kg twice daily) for at least 24 hours before coronary intervention.
The angiographic characteristics of the included patients are shown on Table 2. Most patients presented single-artery obstruction, and the artery most frequently treated was the anterior descendent artery. Regarding anatomic complexity, 57.7% of the lesions were classified as B2 or C according to ACC/AHA classification. Left ventricular function, according to global ejection fraction, was 57.8 ± 5.3%.
Table 2.
Angiographic characteristicsa
| (n = 52) | ||
| [mean and standard deviation or n(%)] | ||
| Arterial disease extension | ||
| Single artery | 34 (65.4) | |
| Two arteries | 17 (32.7) | |
| Three arteries | 1 (1.9) | |
| Treated vessel | ||
| Anterior descendent artery | 39 (75) | |
| Circumflex artery | 10 (19.2) | |
| Right coronary artery | 13 (25) | |
| Lesion localization | ||
| Ostium | 2 (3.8) | |
| Proximal | 21 (40.4) | |
| Medium | 28 (53.8) | |
| Distal | 1 (1.9) | |
| Lesion classification* | ||
| A/B1 | 22 (42.3) | |
| B2/C | 30 (57.7) | |
| LV ejection fraction, % | 57.8 ± 5.3 | |
art.: artery LV: left ventricle; *According to the classification proposed by the American Heart Association and American College of Cardiology
The mean period of time between initial clinical presentation of the acute coronary syndrome and cinecoronariography performance was 6.1 ± 1.8 days, and 8.2 ± 2.5 days for the percutaneous coronary intervention. Regarding the procedure's technical characteristics, 38% of the lesions were dilated previously to stent implantation. Stent post-dilation was performed in 90% of the patients.
Hospital and 30-day outcomes
All the 52 included patients were submitted to coronary intervention with clinical and angiographic success, and were discharged after a mean period of 1.32 ± 0.86 days. Thirty days after the procedure, the patients were submitted to the planned clinical, laboratorial and electrocardiographic evaluations. There were no cases of stent thrombosis, major cardiac events (death, infarct or stroke) or myocardial ischemia symptom recurrence in this period.
Virtual Histology® ultrasound evaluation
Radiofrequency ultrasound results (Virtual Histology®) are shown in table 3. According to the proposed classification, fibroatheroma and calcified fibroatheroma lesions predominated (61.5% of the total). Nine (17.3%) of the lesions were defined as thin-cap fibroatheromas.
Table 3.
Characteristics of the atherosclerotic plaques at Virtual Histology®
| [mean and standard deviation or n(%)] | (n = 52) | |
| Plaque type10 | ||
| Fibrotic | 8 (15.4) | |
| Fibrocalcified | 3 (5.8) | |
| Fibroatheroma | 19 (36.5) | |
| Calcified fibroatheroma | 13 (25) | |
| Thin-cap fibroatheroma | 9 (17.3) | |
| Percentage (%) | ||
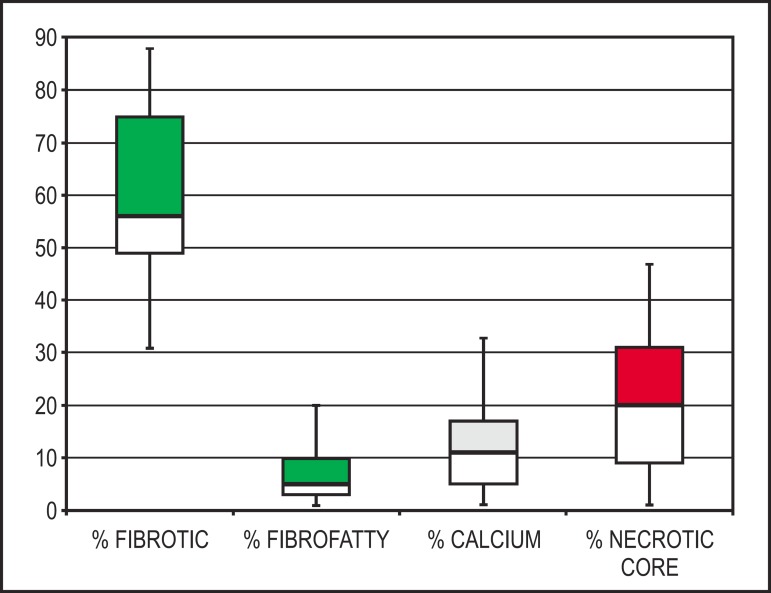
| Fibrotic | 59.6 ± 15.8 | |
| Fibrofatty | 7.6 ± 8.2 | |
| Calcium | 12.1 ± 9.2 | |
| Necrotic | 20.7 ± 12.7 | |
Regarding the plaque components mean percentage, the fibrotic tissue was the predominant component (59.6 ± 15.8% of the total analyzed plaque volume); approximately 20% of the total lesion volume was composed by necrotic core. Figure 3 shows the median and interquartile intervals of the volumetric percentage of each one of the four atherosclerotic plaque components.
Figure 3.
Graphic representation (median and interquartile intervals) of the volumetric percentages of each component of the treated atherosclerotic plaques.
Association of the atherosclerotic lesion characteristics according to Virtual Histology® and C-reactive protein levels
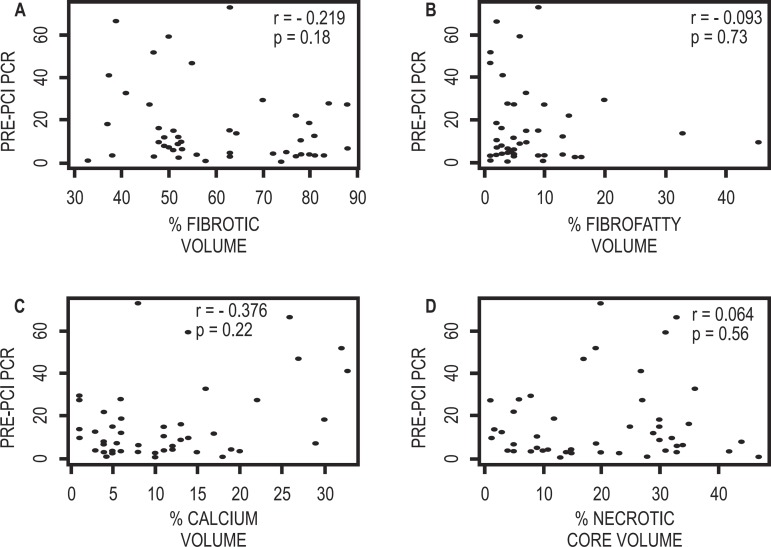
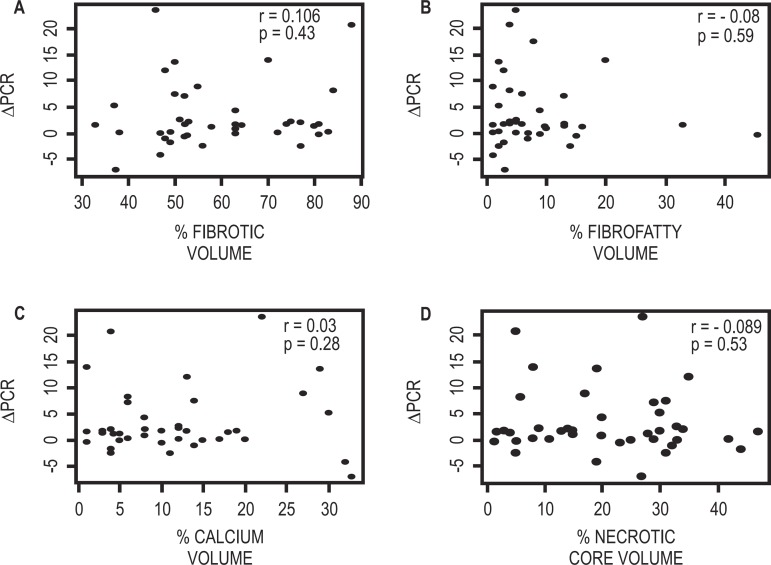
C-reactive protein basal levels of the patients included in this study were 16.02 ± 18.07 mg/l (reference value, < 5 mg/l). No association was observed between the atherosclerotic lesion component percentage according to Virtual Histology® findings and the inflammatory marker high sensitivity C-reactive protein measured before percutaneous coronary intervention. Figure 4 show the correlation graphics between different plaque components and pre-procedure C-reactive protein levels.
Figure 4.
Correlation graphics of the culprit plaque components and the basal high sensitivity C-reactive protein level (PCR as PRE-PCI). A) Fibrotic component. B) Fibrofatty component. C) Calcium D) Necrotic core. PCI: Percutaneous coronary intervention.
The variation between this inflammatory marker basal levels and the levels observed 24 hours after the intervention (s Δ CRP) was calculated as 3.18 ± 6.26 mg/L. As for the pre-intervention measurement, no correlation was detected between individual C-reactive protein variations and treated plaque components (Figure 5).
Figure 5.
Correlation graphics of the culprit plaque components and the high sensitivity C-reactive protein level variation (pre- and post-intervention). A) Fibrotic component. B) Fibrofatty component. C) Calcium D) Necrotic core.
Discussion
In our prospective study with acute coronary syndrome patients, the plaque composition according to Virtual Histology® ultrasound was predominantly fibrotic, with a high percentage of the necrotic component. No correlation was found between the basal C-reactive protein levels (or the pre- and post-intervention level range) and the culprit atherosclerotic plaque composition in this series.
Pathology studies demonstrate that the rupture of plaques with large necrotic and inflammatory cores and subsequent thrombosis are the main mechanisms that determine the occurrence of acute coronary syndrome. These findings correlate with Virtual Histology® results. Using this method, Pundziute et al13 observed that the mean percentage of necrotic cores in the observed coronary lesions was significantly greater in acute coronary syndrome patients than in stable angina patients (11.2 ± 6.1% versus 9.1 ± 4.6%, p = 0.02). In an analysis of 318 cases, Hong et al14 report that the culprit plaque morphology of patients with unstable clinical picture is distinct of the target lesions in stable patients: the necrotic core at the major lumen reduction point is larger (33% versus 29%, p = 0.015) and the fibrofatty tissue percentage is smaller (5% versus 7%, p = 0.020), respectively. Rodriguez-Granillo et al15 demonstrated that the mean percentage of necrotic content is larger in the non-culprit lesions of patients with unstable syndromes than in the atherosclerotic plaques in stable patients (12.26% ± 7.0% versus 7.4 ± 5.5%), p < 0.006), suggesting that the systemic pro-inflammatory status can influence not only the destabilization of culprit lesions but also the activation of quiescent lesion in other coronary sites. Surmely et at16 report that, at least in the site with the smaller lumen diameter, the fibrotic component area can be larger in the plaques of patients with acute syndrome than in stable individuals (6.0 ± 10.7 versus 61.4 ± 8.9%, p = 0.034). In our study, most culprit lesions were classified as fibroatheromas or calcified fibroatheromas; great variation in plaque types was observed, and 17.3% were defined as thin-cap fibroatheromas. The fibrotic component (59.6% ± 15.8%) predominated in the analyzed lesions, which also showed a large percentage of necrotic cores (20.7% ± 12.7%).
C-reactive protein, a laboratorial marker of the systemic inflammatory status, is also synthesized at the site of the atherosclerotic plaque, and would reflect the destabilization process7,8. Various studies searched to evaluate the association between C-reactive protein levels and atherosclerotic plaque characteristics at monochromatic intracoronary ultrasound. In a study with post-AMI patients, Sano et al17 found a higher prevalence of lesions with rupture ultrasonographic changes, with positive remodeling or lipid lakes in individuals with high C-reactive protein levels, as compared to patients with normal levels of this marker. Hong et al18, on the other side, in a intracoronary ultrasound performed on the three main vessels, found that high C-reactive protein levels were the only predictors of ruptured plaques. In our investigation with radiofrequency intracoronary ultrasound, however, no association was found between the component percentage in the culprit plaque and the serum C-reactive protein levels. It is possible that, since only the lesions that had already suffered erosion or rupture were analyzed, part of the necrotic core had already embolized; consequently, the observation of a smaller percentage of this component may have led to the classification of some lesions as fibrotic and influenced the correlation with C-reactive protein levels.
It is known that distal embolization of necrotic content and atherosclerotic plaque debris during percutaneous coronary intervention is associated to adverse clinical and angiographic outcomes. Virtual Histology® is being investigated in this setting for the identification of lesions with higher complication risk. Various studies with STEMI AMI demonstrated that a higher percentage of the necrotic core in the treated plaques is associated to the development of electrocardiographic signs suggestive of non-reperfusion19, to no-reflow occurrence20 and to the appearance of transient and high frequency signals at intracoronary Doppler21 - indicating distal embolization. These complications may be accompanied by an increase in C-reactive protein levels. In our series, no ischemic complications related to the revascularization procedure occurred, and no association was detected between the component percentages in the culprit lesion and the C-reactive protein increase. Drug therapy started before the intervention (dual antiplatelet therapy, anti-thrombin drugs and statins) may explain the discrepancy between our study's findings and the ones previously reported.
Limitations
Some limitations of this study are related to the imaging method itself. First, since it is a technology based on ultrasound principles, Virtual Histology® has a 100-µm axial resolution and 250-µm lateral resolution, which prevents the detection of plaques with thinner fibrotic plaques (< 65 µm), which have been pointed out as precursors of acute ischemic events. Moreover, not all plaque components are identified by the method: intraluminal thrombus, for example, is not included in contemporary classification algorithms. Thus, thrombotic tissue is automatically classified as a fibrous or fibrofatty component12. Due to it, the observation of images suggestive of thrombus at angiography (common in patients with acute syndromes) is one our study's exclusion criteria. Similarly to conventional intracoronary ultrasound, non-confluent calcium deposition does not lead to difficulties in the evaluation of the most profound plaque tissues. On the other side, due to the appearance of acoustic shadow, its confluent and organized disposition may reduce method accuracy for the recognition of other components.
Naturally, this investigation's findings do not eliminate the potential indication of the imaging method in the numerous other possibilities for which it is being studied, among which we highlight: better understanding of coronary disease natural evoulaiton22,23; effects of drug therapies aiming for regression or stabilization of atherosclerotic plaques24-26; and correlation between the type and composition of lesions with percutaneous intervention results19-21,27.
Conclusions
In our study, the composition of the plaques according to Virtual Histology® ultrasound was predominantly fibrotic and had large necrotic core content. No correlation was found between the basal C-reactive protein levels (or the pre- and post-intervention level range) and the culprit atherosclerotic plaque composition in patients with acute coronary syndrome.
Author contributions
Conception and design of the research and Statistical analysis: Siqueira DAA, Costa Junior JR; Acquisition of data: Siqueira DAA, Costa Junior JR, Costa RA, Staico R, Tanajura LFL, Centemero MP, Feres F, Abizaid AAC; Analysis and interpretation of the data: Siqueira DAA, Sousa AGMR, Costa Junior JR; Obtaining funding: Siqueira DAA, Sousa AGMR, Abizaid AAC; Writing of the manuscript: Siqueira DAA; Critical revision of the manuscript for intellectual content: Siqueira DAA, Sousa AGMR, Costa RA, Staico R, Tanajura LFL, Abizaid AAC, Sousa JEMR.
Footnotes
Potential Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Sources of Funding: This study was funded by CAPES.
Study Association: This article is part of the thesis of doctoral submitted by Dimytri Alexandre de Alvim Siqueira, from Instituto Dante Pazzanese de Cardiologia an organization associated with Universidade de São Paulo - USP.
References
- 1.Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83(3):361–366. doi: 10.1136/heart.83.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104(3):365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 3.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15(6):439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 5.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient. A call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 6.Maehara A, Mintz GS, Weissman NJ. Advances in intravascular imaging. Circ Cardiovasc Interv. 2009;2(5):482–490. doi: 10.1161/CIRCINTERVENTIONS.109.868398. [DOI] [PubMed] [Google Scholar]
- 7.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106(17):2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 8.Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47(12):2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Garcia HM, Gonzalo N, Regar E, Serruys PW. Virtual histology and optical coherence tomography: from research to a broad clinical application. Heart. 2009;95(16):1362–1374. doi: 10.1136/hrt.2008.151159. [DOI] [PubMed] [Google Scholar]
- 10.García-García HM, Mintz GS, Lerman A, Vince DG, Margolis MP, Morel MA, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5(2):177–189. doi: 10.4244/eijv5i2a29. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 12.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy AS, Rifai N, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105(15):1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 13.Pundziute G, Schuijf JD, Jukema JW, Decramer I, Sarno G, Vanhoenacker PK, et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J. 2008;29(19):2373–2381. doi: 10.1093/eurheartj/ehn356. [DOI] [PubMed] [Google Scholar]
- 14.Hong MK, Mintz GS, Lee CW, Lee JW, Park JH, Park DW, et al. A three-vessel virtual histology intravascular ultrasound analysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. Am J Cardiol. 2008;101(5):568–572. doi: 10.1016/j.amjcard.2007.09.113. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Granillo GA, McFadden EP, Valgimigli M, van Mieghem CA, Regar E, de Feyter PJ, et al. Coronary plaque composition of nonculprit lesions, assessed by in vivo intracoronary ultrasound radio frequency data analysis, is related to clinical presentation. Am Heart J. 2006;151(5):1020–1024. doi: 10.1016/j.ahj.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Surmely JF, Nasu K, Fujita H, Terashima M, Matsubara T, Tsuchikane E, et al. Coronary plaque composition of culprit/target lesions according to the clinical presentation: a virtual histology intravascular ultrasound analysis. Eur Heart J. 2006;27(24):2939–2944. doi: 10.1093/eurheartj/ehl285. [DOI] [PubMed] [Google Scholar]
- 17.Sano T, Tanaka A, Namba M. C-reactive protein and lesion morphologyin patients with acute myocardial infarction. Circulation. 2003;108(3):282–285. doi: 10.1161/01.CIR.0000079173.84669.4F. [DOI] [PubMed] [Google Scholar]
- 18.Hong MK, Mintz GS, Lee CW, Kim YH, Lee SW, Song JM, et al. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation. 2004;110(8):928–933. doi: 10.1161/01.CIR.0000139858.69915.2E. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi R, Oshima S, Jingu M, Tsurugaya H, Toyama T, Hoshizaki H, et al. Usefulness of virtual histology intravascular ultrasound to predict distal embolization for ST-segment elevation myocardial Infarction. J Am Coll Cardiol. 2007;50(17):1641–1646. doi: 10.1016/j.jacc.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Kubo N, Ako J, Momomura S. Angiographic no-reflow phenomenon and plaque characteristics by virtual histology intravascular ultrasound in patients with acute myocardial infarction. J Interv Cardiol. 2007;20(5):335–339. doi: 10.1111/j.1540-8183.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto T, Okura H, Koyama Y, Toda I, Taguchi H, Tamita K, et al. The relationship between coronary plaque characteristics and small embolic particles during coronary stent implantation. J Am Coll Cardiol. 2007;50(17):1635–1640. doi: 10.1016/j.jacc.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. PROSPECT Investigators A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 23.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 24.Nasu K, Tsuchikane E, Katoh O, Tanaka N, Kimura M, Ehara M, et al. Effect of fluvastatin on progression of coronary atherosclerotic plaque evaluated by virtual histology intravascular ultrasound. JACC Cardiovasc Interv. 2009;2(7):689–696. doi: 10.1016/j.jcin.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Hong MK, Park DW, Lee CW, Lee SW, Kim YH, Kang DH, et al. Effects of statin treatments on coronary plaques assessed by volumetric virtual histology intravascular ultrasound analysis. JACC Cardiovasc Interv. 2009;2(7):679–688. doi: 10.1016/j.jcin.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Samady H, McDaniel MC. Can statins alter coronary plaque composition assessed by radiofrequency backscatter intravascular ultrasound? JACC Cardiovasc Interv. 2009;2(7):697–700. doi: 10.1016/j.jcin.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Sangiorgi GM, Clementi F, Cola C, Biondi-Zoccai G. Plaque vulnerability and related coronary event prediction by intravascular ultrasound with virtual histology: "it's a long way to tipperary"? Catheter Cardiovasc Interv. 2007;70(2):203–210. doi: 10.1002/ccd.21134. [DOI] [PubMed] [Google Scholar]