Abstract
Background
Birth weight (BW) is a medium- and long-term risk determinant of cardiovascular risk factors.
Objective
To assess the association between BW and cardiovascular risk factors in adolescents of the city of Salvador, Bahia state.
Methods
Cross-sectional study with comparison of BW groups. Sample comprising 250 adolescents classified according to the BMI as follows: high-normal (≥ 50th percentile and < 85th percentile); overweight (≥ 85th percentile and < 95th percentile); and obesity (≥ 95th percentile). The risk variables compared were as follows: waist circumference (WC); arterial blood pressure; lipid profile; glycemia; serum insulin; HOMA-IR; and metabolic syndrome. The BW was informed by parents and classified as follows: low (BW ≤ 2,500g); normal (BW > 2,500g and < 4,000g); and high (BW ≥ 4,000g).
Results
One hundred and fifty-three (61.2%) girls, age 13.74 ± 2.03 years, normal BW 80.8%, low BW 8.0%, and high BW 11.2%. The high BW group as compared with the normal BW group showed a higher frequency of obesity (42.9%, p=0.005), elevated SBP and DBP (42.9%, p=0.000 and 35.7%, p=0.007, respectively), and metabolic syndrome (46.4%, p=0.002). High BW adolescents as compared with normal BW adolescents had a prevalence ratio for high SBP 3.3 (95% CI: 1.7-6.4) and obesity 2.6 (95% CI: 1.3-5.2). The WC of high BW adolescents was 83.3 ± 10.1 (p=0.038). The lipid profile showed no statistically significant differences.
Conclusion
Our findings suggest that obesity, elevated SBP and DBP, and metabolic syndrome during adolescence might be associated with high BW.
Keywords: Birth weight, risk factors, adolescent, obesity, arterial pressure, metabolic syndrome
Introduction
Birth weight (BW) can be a medium- and long-term risk determinant of overweight/obesity, type 2 diabetes, and cardiovascular disease1,2.
Evidence on the impact of intrauterine nutrition on the subsequent cardiovascular risk varies, with some studies having reported a relation to high or low BW2-6. A series of studies has reported a relationship between BW and cardiovascular risk in adolescents and adults with a U or inverted J distribution, showing greater risk factors clustering in individuals with low and high BW7.
Individuals with high BW (BW ≥ 4,000 g) or those large for gestational age, BW > 90th percentile, are at higher risk for obesity measured by use of body mass index (BMI), but with a programming of more lean tissue rather than fat mass7,8. However, infants with low BW have been reported to typically have poor muscle tissue and high fat preservation. This phenotype persists beyond the prenatal period and can be associated with increased central adiposity in childhood, increasing the risk of arterial hypertension and cardiovascular diseases in adulthood3. Thus, the need for early and more efficient treatment in children at higher risk began to be recognized in the last guidelines9,10.
Few studies have been performed on the Brazilian pediatric population, all conducted in the Southern and Southeastern regions11-13 and two in the West Central region5,14, but none in the Northeastern region of Brazil, which had the greater prevalence of low BW and dramatic socioeconomic and cultural differences as compared to the other Brazilian regions. The Brazilian Northeastern region, due to its nutritional transition phase, has shown an increasing prevalence of overweight and obesity15, which can increase the importance of high BW on the population's future health. Thus, this study aimed at assessing the association between BW and cardiovascular risk factors in an adolescent sample of the city of Salvador, Bahia state.
Methods
A cross-sectional study was performed assessing BW by using medical documentation or conducting interviews with parents. Information from the data bank of a previous study on weight status and cardiovascular risk factors was used16. This study population comprised 470 adolescents aged 11 to 18 years and enrolled at public and private schools of the city of Salvador, Bahia state, who participated in the above mentioned study on weight status and cardiovascular risk factors.
The schools, selected according to convenience of the number of students, access to education and structural facilities for determining the variables assessed, comprised three private and four public schools in a middle-class area of the city. After meeting the inclusion criterion of informing the official BW by presenting the live birth certificate and/or vaccine chart and providing written informed consent, the final sample consisted of 250 students, 150 (60%) from public schools and 100 (40%) from private schools, representing 49.9% of the 334 students from public schools and 73.5% of the 136 from private schools.
The exclusion criteria were as follows: adolescents with special needs or pathologies that hindered taking the anthropometric measures and adolescents with no BW document.
The project was approved by the Committee on Ethics and Research of the Climério de Oliveira Maternity of the Universidade Federal da Bahia and by the Committee on Ethics and Research of the Bahia Foundation for the Development of Sciences (Fundação Bahiana para o Desenvolvimento das Ciências).
The primary object of study was the association between cardiovascular risk factors and BW. The cardiovascular risk variables assessed, either isolated or in clusters, were as follows: overweight/obesity; high blood pressure (BP); dyslipidemia; hyperglycemia; hyperinsulinemia; and insulin resistance (IR) measured according to the homeostatic model assessment of insulin resistance (HOMA-IR). The BW was classified according to the WHO criteria as follows: low (BW ≤ 2,500g); normal (BW > 2,500g and < 4,000g); and high (BW ≥ 4,000g)17.
The anthropometric measures were as follows: weight; height; BMI; waist circumference (WC). Height was measured with a stadiometer (Leicester) to the nearest 0.1 cm, and weight was measured with a digital scale to the nearest 0.1 kg. The BMI was calculated by using the Quetelet's formula (kg/m2 and classified according to age and gender as follows: high-normal (≥ 50th percentile and < 85th percentile); overweight (≥ 85th percentile and < 95th percentile); and obesity (≥ 95th percentile)6,14. The WC was measured at the end of a normal expiration with a measuring tape placed in the horizontal plane between the lower portion of the last rib and the upper border of the iliac crest. The arithmetic mean of two measures was considered for analysis. Central obesity was defined as WC > 75th percentile for age and gender, according to de Ferranti et al18.
Blood pressure was measured on the upper limb supported at heart level, after a 5 minute rest in the sitting position, with a mercury manometer (Missouri(r). The wide of the cuff used was 40% of the arm circumference, measured at the middle point between the elbow and the acromion, and its length was 80% to 100% of that measure. Three consecutive readings at 60 second intervals were made, and their mean, recorded.
Systolic BP (SBP) and diastolic BP (DBP) were determined during Korotkoff phase I and Korotkoff phase V, respectively, being classified according to the Task Force on "High Blood Pressure in Children and Adolescents from the National High Blood Pressure Education Program" and considering age, gender and height as follows: normal (< 90th percentile); high-normal (≥ 90th percentile and < 95th percentile); and arterial hypertension (≥ 95th percentile). A BP value ≥ 90th percentile was considered elevated.
Samples of venous blood (10 mL) were collected for biochemical analysis after a minimum 12-hour fasting. After centrifuging, the serum was stored in a freezer at -80ºC. Glycemia and the following serum levels were measured: insulin; total cholesterol (TC); HDL cholesterol (HDL-C); and triglycerides (TG).
Glycemia was measured by using the colorimetric enzymatic method. Glycemia ≥ 100mg/dL was considered elevated. Fasting insulinemia was measured by using automated electrochemiluminescence immunoassay (ELISA). Reference insulin serum levels were those recommended by Reavene et al19 as follows: normal, < 15 µU/L; borderline, ≥ 15 and <20 µU/L; and elevated, ≥ 20 µU/L.
The cutoff point used to diagnose insulin resistance was that recommended by Keskin20, HOMA-IR = 3.16. The TC, HDL-C and TG levels were measured by using enzymatic methods at a reference laboratory. LDL cholesterol was calculated with the Friedwald formula for TG < 400mg/dL. The reference values were those recommended by the I Guideline on Atherosclerosis Prevention in Infancy and Adolescence10 and the Third Report of the Adult Treatment Panel (ATP III) modified by de Ferranti18, with a change in the cutoff point of fasting glucose from 110mg/dL to 100mg/dL, as proposed by Grundy et al21. The diagnosis of metabolic syndrome (MS) required meeting at least three of the following five criteria: TG ≥ 100mg/dL; HDL-C < 50mg/dL (< 45mg/dL for boys aged 15 to 19 years); fasting glycemia ≥ 100mg/dL; WC > 75th percentile for age and gender; and SBP > 90th percentile for age, gender and height.
The BW was provided by the families, by presenting the vaccine chart and/or live birth certificate, all official documents provided by the maternity on the day of birth. At the initial interview, the adolescents were asked about their official BW documents. All those with neither a vaccine chart nor a live birth certificate were excluded. The BW distribution in this study sample was compared with data from the Brazilian Unified Health System database (DATASUS) in the 1994-1996 period, for the Salvador metropolitan region, confirming the reliability of our data22. The BW was classified as follows: low (BW ≤ 2,500g); normal (BW > 2,500 and < 4,000g); and high (BW ≥ 4,000g)17.
Statistical Analysis
Continuous variables were expressed as mean and standard deviation. The normality of the distribution of the variables was assessed by use of the Shapiro-Wilk test. Categorical variables were expressed as percentages.
Statistical tests
The means of the following variables were compared in the BW groups by use of analysis of variance (ANOVA): age; BMI; WC; SBP; DBP; glycemia; insulin; and lipid profile. The percentages of the following categorical variables were compared between two BW groups by use of Pearson chi-square test: BMI; WC; SBP; DBP; glycemia; and lipid profile. Multiple logistic regression was used to determine the independence of the influence of the variables associated with the outcome BW, identified by using bivariate analysis. The p-values p< 0.1 and p < 0.05 were adopted for bivariate analysis and logistic regression, respectively. The BW was divided into quartiles for Pearson correlation analysis. P-values lower than 5% (p<0.05) were considered significant. Data were analyzed by using the Statistical Package for the Social Science (SPSS) software, version 12.0.
Results
Table 1 shows the major demographic, anthropometric and clinical characteristics of the final sample (250 adolescents) distributed in groups according to gender. In the total sample, the mean age was 13.7±2.0 years and there was a significant predominance of the female gender (61.2% versus 38.8%, p<0.001). Females had a significantly higher age (p=0.026), while males had a higher BW (p=0.046), BMI Z-score (p< 0.001), WC (p=0.010) and SBP (p =0.002). The distribution of skin color was similar between genders, with 53.6% of non-white individuals.
Table 1.
Demographic and anthropometric characteristics and blood pressure of the study adolescents according to gender
| Variables | Total | Male | Female | |
| N(%) | 250 (100) | 97 (38.8) | 153 (61.2)* | |
| Age (mean ± sd years) | 13.7 ± 2.0 | 13.4 ± 2.0 | 14.0 ± 2.1† | |
| Birth weight (BW) | ||||
| BW (mean ± sd kg) | 3292.3 ± 623.8 | 3388.4 ± 569.4 | 3231.3 ± 650.4‡ | |
| BW Z-score | -0.0645 ± 1.35 | -0.0036 ± 1.29 | -0.1031 ± 1.38 | |
| Low BW | 20 (8.0) | 5 (5.2) | 15 (9.8) | |
| Normal BW | 202 (80.8) | 80 (82.5) | 122 (79.7) | |
| High BW | 28 (11.2) | 12 (12.4) | 16 (10.5) | |
| Skin color | ||||
| White | 116 (46.4) | 45 (46.4) | 71 (46.4) | |
| Non-white | 134 (53.6) | 52 (53.6) | 82 (53.6) | |
| School | ||||
| Public | 150 (60) | 52 (53.6) | 98 (64.1) | |
| Private | 100 (40) | 45 (46.4) | 55 (35.9) | |
| Anthropometric data and blood pressure | ||||
| BMI (Kg/m2) | 24.0 ± 3.7 | 24.4±4.0 | 23.8±3.4 | |
| BMI Z-score | 1.98 ±1.04 | 2.29 ±1.18 | 1.78 ± 0.89α | |
| WC (cm) | 79.2±9.6 | 81.1±10.6 | 78.0±8.7 // | |
| SBP (mmHg) | 109.5±12.0 | 112.5±12.8 | 107.5±11.1 # | |
| DBP (mmHg) | 68.7±9.3 | 69.2±9.3 | 68.3 ± 9.3 | |
Data: (N [%]). BMI: body mass index; WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure. Statistics: Male vs Female;*p = 0.001; †p=0.026, ‡ p = 0.046; αp = 0.000; //p = 0.010; # p = 0.002.
Table 2 shows the major characteristics according to the BW status. The sample distribution of BW was as follows: low BW, 20 adolescents (8.0%); normal BW, 202 (80.8%); and high BW, 28 adolescents (11.2%). That proportion is compatible with the global distribution of the population in the Bahia state, based on DATASUS data22.
Table 2.
Demographic and anthropometric characteristics and blood pressure of the study adolescents according to birth weight (BW)
| Variables | Total | Low BW ≤ 2.500g | Normal BW > 2.500g and < 4.000g | High BW ≥ 4.000g |
| N(%) | 250 | 20 (8.0) | 202 (80.8) | 28 (11.2) |
| Age, mean ± sd | 13.7±2.0 | 14.3±2.1 | 13.7±2.1 | 13.7±1.9 |
| BW, mean ± sd | 3.292.3±623.8 | 2.027.0±481.4 | 3.271.1±358.9 | 4.348.8±346.0 |
| Gender n (%) | ||||
| Male | 97 (38.8) | 5(25.0) | 80 (39.6) | 12 (42.9) |
| Female | 153 (61.2)* | 15(75.0) | 122 (60.4)* | 16 (57.1) |
| Skin color n (%) | ||||
| White | 116(46.4) | 8(40.0) | 99(49.0) | 9(32.1) |
| Non-white | 134(53.6) | 12(60.0) | 103(51.0) | 19(67.9) |
| School n (%) | ||||
| Public | 150(60)† | 16(10.7) | 114(76.0) | 20(13.3) |
| Private | 100(40) | 4(4.0) | 88(88.0) | 8(8.0) |
| Anthropometric data and blood pressure (mean ± sd) | ||||
| BMI (Kg/ M²) | 24.0±3.7 | 24.4±3.4 | 24.6±4.0 ‡ | 26.0±4.0 ‡ |
| WC (cm) | 79.2±9.6 | 80.3±9.2 | 78.5±9.4 ¶ | 83.3±10.1¶ |
| SBP (mmHg) | 109.5±12.0 | 112.0±11.1 | 108.6±12.0 # | 113.5±12.3# |
| DBP (mmHg) | 68.7±9.3 | 69.4±9.3 | 68.1±9.0 ** | 72.5±10.8** |
| BMI Z-score | 1.98±1.04 | 1.97±1.01 | 1.90±1.01 | 2.52±1.04 |
BMI: body mass index; WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure. Statistics: *Male vs Female: Total, p<0.001, normal BW, p<0.006; † public vs private school: Total, p=0.003; Clinical data (high BW vs normal BW): ‡ p=0.002; ¶ p=0.013; # p=0.047; ** p=0.017.
Age did not differ between the groups, but there was a significant predominance of the female gender in the normal BW group (60.4% versus 39.6%, p=0.006). In the high BW group, the means of BMI (26.0 ± 4.0 kg/m2 versus 24.6 ± 4.0 kg/m2; p=0.002), WC (83.3 ± 10.1 cm versus 78.5 ± 9.4 cm; p=0.013), SBP (113.5 ± 12.3 mm Hg versus 108.6 ± 12.0 mm Hg; p=0,047) and DBP (72.5 ± 10.8 mm Hg versus 68.1 ± 9.0 mm Hg; p = 0.017) were significantly more elevated than those in the normal BW group, but they did not differ from those in the low BW group. The mean BMI Z-score significantly differed between the groups (p=0.013), being significantly more elevated in the high BW group as compared with the normal BW group (2.52 versus 1.90; p = 0.009).
The students from public schools were characterized by a higher percentage of low and high BW than those from private schools (10.7% versus 4.0% and 13.3% versus 8.0%, respectively), but with no statistical significance.
Table 3 shows the means of the lipid panel, glycemia, insulin and HOMA-IR, which did not significantly differ according to the BW. However, the following aspects are worth noting: the decreasing means of HDL-C (52.4 ± 14.4 mg/dL, 49.2 ± 11.9 mg/dL and 47.2 ± 8.3 mg/dL) from low to high BW, respectively; the mean values of TC (167.5 ± 24.4; 162.4 ± 30.1 and 167.2 ± 36.7) and TG/ HDL-C ratio (2.01 ± 1.27; 1.96 ± 1.37; 1.98 ± 0.85) showing a U curve distribution; and the serum insulin levels (8.8 ± 6.1; 8.1 ± 5.7; 9.7 ± 9.3) and HOMA-IR (1.8 ± 1.0; 1.7 ± 1.2; 2.1 ± 2.2) showing a J curve distribution.
Table 3.
Laboratory characteristics according to birth weight (BW)
| Variables | Total | Low BW ≤ 2.500g | Normal BW > 2.500g e < 4.000g | High BW ≥ 4.000g |
| N | 250 | 20 (8.0)* | 202 (80.8)* | 28 (11.2)* |
| mg/dL | ||||
| Total cholesterol | 163.4 ± 30.5 | 167.5 ± 24.4 | 162.4 ± 30.1 | 167.2 ±36.7 |
| HDL-C | 49.2 ± 11.8 | 52.4 ± 14.4 | 49.2 ± 11.9 | 47.2 ± 8.3 |
| LDL-C | 96.8 ± 26.7 | 96.4 ± 25.7 | 96.0 ± 25.9 | 102.0 ± 33.0 |
| Triglycerides | 89.5 ± 55.5 | 93.7 ± 41.0 | 89.1 ± 59.1 | 89.8 ± 35.4 |
| NHDLC | 109.2 ± 28.1 | 108.8 ± 27.0 | 108.4 ± 27.2 | 114.7 ± 34.6 |
| TG/HDL-C | 1.97 ± 1.31 | 2.01 ± 1.27 | 1.96 ± 1.37 | 1.98 ± 0.85 |
| Glycemia | 86.4 ± 7.15 | 86.05 ± 7.35 | 86.42 ± 7.01 | 86.50 ± 8.26 |
| Insulin(mUI/L) | 8.31 ± 6.10 | 8.82 ± 4.24 | 8.07 ± 5.70 | 9.67 ± 9.27 |
| HOMA-RI | 1.73 ± 1.32 | 1.81 ± 0.95 | 1.67 ± 1.19 | 2.09 ± 2.17 |
*Data: (N [%]). TC: total cholesterol; HDL-C: HDL cholesterol; LDL-C: LDL cholesterol; TG: triglycerides; NHDLC: non-HDL cholesterol; HOMA-IR: homeostatic odel assessment of insulin resistance.
Table 4 shows the proportion of altered clinical and metabolic variables according to BW. Adolescents of the high BW group, as compared with those of the normal BW group, showed the following: a significantly higher prevalence of obesity (42.9% versus 19.3%; p=0.005); BMI Z-score greater than 2 (64.3% versus 42.1%; p=0.027); and high SBP and DBP (> 90th percentile for age, gender and height) (42.9% versus 15.3%; p=0.0001; and 35.7% versus 12.9%, p=0.002, respectively). The SBP of adolescents of the high BW group, as compared with those of the low BW group, showed a trend to be elevated (42.9% versus 20.0%; p =0.082). It is worth noting the increasing percentages of HDL-C, non-HDL cholesterol (NHDL-C), hyperinsulinemia and HOMA-IR in the low BW, normal BW and high BW groups, although lacking significance.
Table 4.
Percentages of altered clinical and metabolic variables according to birth weight (BW)
| Variables | Total | Baixo Peso≤2.500g | Peso Normal> 2.500g e < 4.000g | Alto Peso≥ 4.000g |
| n (%) | 250 | 20 (8.0)* | 202 (80.8)* | 28 (11.2)* |
| Anthropometric data, n (%) | ||||
| WC > 75th percentile | 117 (46.8) | 11 (55.0) | 89 (44.1) | 17 (60.7) |
| Current BMI | ||||
| High-normal | 113 (45.2) | 9 (45.0) | 97 (48.0) | 7 (25.0) |
| Overweight | 82 (32.8) | 7 (35.0) | 66 (32.7) | 9 (32.1) |
| Obese | 55 (22.0) | 4 (20.0) | 39 (19.3) | 12 (42.9) † |
| BMI Z-score ≥1 | 197 (78.8) | 16 (80.0) | 156 (77.2) | 25 (89.3) |
| BMI Z-score ≥2 | 112 (44.8) | 9 (45.0) | 85 (42.1) | 18 (64.3)// |
| Clinical data | ||||
| SBP > 90th percentile | 46 (18.4) | 3(15.0) | 31 (15.3) | 12 (42.9) ‡# |
| DBP > 90th percentile | 40(16.0) | 4(20.0) | 26(12.9) | 10(35.7) α |
| Metabolic profile, n (%) | ||||
| TC > 170 mg/dl | 96(38.4) | 9(45.0) | 76(37.6) | 11(39.3) |
| Low HDL-C* | 135(54.0) | 9(45.0) | 109(54.0) | 17(60.7) |
| LDL-C > 110mg/dl | 72(28.8) | 7(35.0) | 57(28.2) | 8(28.6) |
| TG ≥ 100mg/dl | 69(27.6) | 7(35.0) | 51(25.2) | 11(39.3) |
| NHDLC > 160mg/dl | 8(3.2) | 0(0.0) | 6(3.0) | 2(7.1) |
| Glycemia ≥ 100mg/dl | 5(2.0) | 1(5.0) | 2(1.0) | 2(7.1) |
| Insulin > 15µUI/L | 26(10.4) | 1(5.0) | 20(10.0) | 5(17.9) |
| HOMA-IR > 3.16 | 25(10.0) | 1(5.0) | 19(9.5) | 5(17.9) |
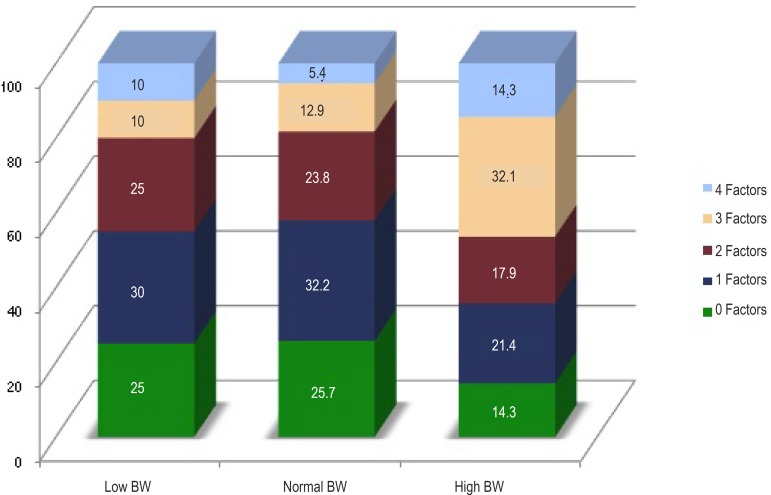
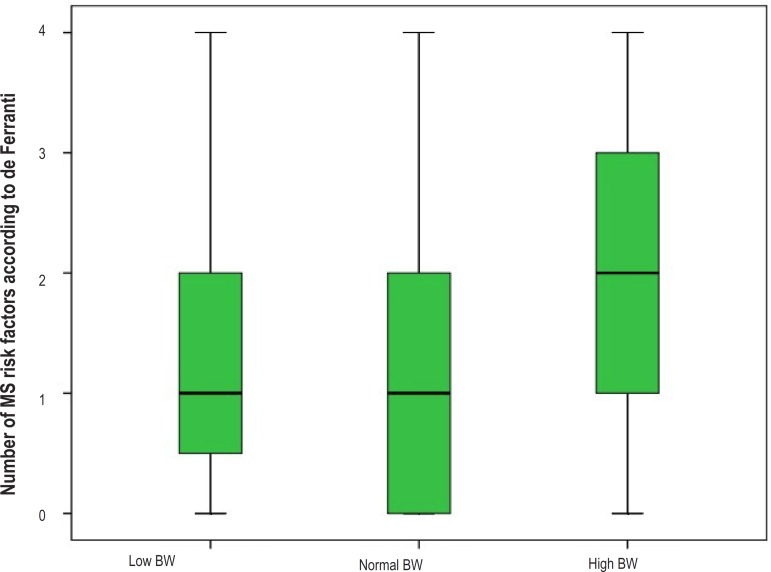
Table 5 shows the behavior of the diagnostic criteria for MS proposed by de Ferranti18 and modified by Grundy21, according to BW. All five criteria were more prevalent in the high BW group; however, only high SBP was significantly more frequent as compared with that in the normal BW group (42.9% versus 15.3%; p=0.001) and showed a tendency to significance as compared with that in the low BW group (42.9% versus 15.0%; p=0.082). In addition, Table 5 shows a significantly elevated prevalence of MS criteria clustering in the high BW group as compared with that in the normal BW group (46.4% versus 18.3%; p=0.002), with a significant difference in the percentage of clustering of three cardiometabolic risk factors in the former. Chart 1 illustrates the proportion of MS criteria clustering per BW group. Chart 2, listing the mean number of MS criteria per BW group, illustrates clearly the clustering power of those with high BW as follows: low BW group, 1.50 ± 1.28; normal BW group, 1.40 ± 1.16; and high BW group, 2.11 ± 1.32 (p=0.014).
Table 5.
Percentage distribution of the metabolic syndrome diagnostic criteria1 according to birth weight (BW)
| N=250 | Total | Low BW | Normal BW | High BW | |||
| Criteria | (250[100])* | (20[8])* | (202[80,8])* | (28[11,2])* | |||
| SBP > 90th percentile | 46(18.4) | 3 (15) | 31 (15.3) | 12(42.9)† | |||
| WC > 75th percentile | 117(46.8) | 11(55) | 89 (44.1) | 17 (60.7) | |||
| HDL-C < 50 mg/dL | 135(54) | 9 (45) | 109(54.2) | 17 (60.7) | |||
| TG ≥ 100 mg/dL | 69(27.6) | 7 (35) | 51 (25.4) | 11 (39.3) | |||
| Glycemia ³ 100 mg/dL | 5(2) | 1 (5) | 2 (1) | 2 (7.1) | |||
| Number of metabolic syndrome criteria** | |||||||
| No criterion | 61(24.4) | 5 (25) | 52 (25.7) | 4 (14.3) | |||
| 1 criterion | 77(30.8) | 6(30) | 65(32.2) | 6(21.4) | |||
| 2 criteria | 58(23.2) | 5(25) | 48(23.8) | 5(17.9) | |||
| 3 ≥ criteria | 54(21.6) | 4 (20) | 37 (18.3) | 13 (46.4)α | |||
*Data: (N [%]); † p=0.001; α p=0.002 (high BW vs low BW) ** Diagnosis of metabolic syndrome according to de Ferranti criteria modified by Grundy. WC: waist circumference; SBP: systolic blood pressure; HDL-C: HDL cholesterol; TG: triglycerides; 1-Ferranti (2004) modified by Grundy (2005).
Chart 1.
Proportional number of metabolic syndrome risk factors according to birth weight (BW).
Chart 2.
Median and standard deviation of the diagnostic criteria of metabolic syndrome (MS) according to de Ferranti, according to birth weight (BW).
The high BW group adolescents as compared with the normal BW group had a prevalence ratio for high SBP and obesity as follows: DBP ≥ 90th percentile: 2.99 (95% CI: 1.51-5.95; p=0.001); SBP ≥ 90th percentile: 3.26 (95% CI: 1.67-6.38; p=0.001); BMI ≥ 95th percentile: 2.63 (95% CI: 1.33-5.20; p=0.013); MS: 3.12 (95% CI: 1.59-6.11; p=0.002); WC > 75th percentile: 1.77 (95% CI: 0.87-3.60; p=0.109).
Because of clinical relevance and statistical significance, the following variables were included in the multivariate logistic regression model: SBP ≥ 90th percentile, DBP ≥ 90th percentile, BMI ≥ 95th percentile, MS and WC > 75th percentile. The multivariate logistic regression indicated that high BW group adolescents have a 3.21 probability (95% CI: 1.3-7.9; p=0.011) of having SBP ≥ 90th percentile and a 2.20 probability of tendency to central obesity (95% CI: 0.90-5.36; p=0.082) as compared with normal BW group adolescents.
Because the correlations between BW and cardiovascular risk factors are not linear, but have a U or inverted J form, the sample was divided into quartiles to undergo Pearson correlation analysis. The first and second quartiles were grouped, as were the third and fourth quartiles, for the analyses. The fourth quartile was also independently analyzed, due to its greater number of significant correlations (Table 6).
Table 6.
Correlation between cardiovascular risk factors and birth weight (BW) quartiles
| BW quartiles (g) | 1st quartile + 2nd quartile | 3rd quartile + 4th quartile | 4th quartile | |||
| BW ≤ 3,299g | BW ≥ 3,300g | BW ≥ 3,657g | ||||
| R | p | R | p | R | p | |
| BMI Z-score | -0.031 | 0.737 | 0.287 | 0.001 | 0.254 | 0.046 |
| Nº of MS risk factors | -0.059 | 0.517 | 0.206 | 0.022 | 0.285 | 0.025 |
| DBP | -0.231 | 0.011 | 0.166 | 0.067 | 0.306 | 0.015 |
| SBP | -0.252 | 0.005 | 0.141 | 0.119 | 0.247 | 0.053 |
BW: birth weight; BMI: body mass index; MS: metabolic syndrome; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Discussion
According to literature review, this is the first evidence of the association of intrauterine growth and clustering of cardiovascular risk factors in a sample of adolescents in the Northern-Northeastern region of Brazil.
The present study showed that, in the high BW group of adolescents, the probability of high BP, obesity and MS was two to three times greater than that in the normal BW group, suggesting that excessive fetal weight, by itself, can predispose to metabolic disorders in adolescence. In addition, it showed the increase in SBP as an independent variable associated with high BW, suggesting that its elevation can be influenced by changes in the BP regulatory mechanism that occurs in the uterus or during infancy.
A significant difference in the percentage of abnormalities of some risk variables could not be established between low and normal BW adolescents, as reported in other studies1-3,5-7. A Brazilian study found no relationship between low BW and BP elevation14. However, the analysis of those risk variables shows that some of them were distributed in the three BW groups, in a U or J curve or linearly. Thus, WC, DBP > 90th percentile, TC and TG showed an abnormality percentage distributed in a U or J curve, while the percentages of low HDL-C, serum insulin and HOMA-IR showed increasing values from low to high BW, suggesting metabolic effects resulting from high BW. Those data clearly suggest that high BW adolescents are prone to have more severe metabolic disorders23, a fact supported by the finding of an elevated mean number of MS criteria in high BW adolescents, approximately 1.8-fold greater than in normal BW adolescents and 1.6 fold greater than in low BW adolescents.
In this sample of adolescents, a contributing factor to the low frequency of metabolic disorders regarding low BW might be related to the greater difficulty in obtaining the information about BW from mothers of public school students. That information was only provided by 44.9% of them, probably from families with a better socioeconomic level, and, thus, with a lower chance of having intrauterine fetal restriction. In the initial study population of 470 adolescents, the ratio of public school to private school students was 2.5, dropping to 1.5 after BW information. Another limiting factor was the lack of information regarding maternal characteristics, mainly gestational age and prenatal conditions related to maternal weight.
This study did not include nutritional data from infancy, because, although observation studies have suggested that programming extends throughout the first years of life, with breastfeeding representing a protective effect, their results are controversial10,24-26. In addition, as the dietary data of the infants were not registered in their medical records and their collection would happen approximately one decade after the period of interest, the information would be strongly biased.
The validity of this study data is reinforced by the similar mean age of the adolescents in the three BW groups and by the proportionality of the BW groups to that of the population of the city of Salvador in the 1990s22. The global predominance of the female gender (F/M=1.6) is expected in a volunteer selection study, in which a more effective participation of females is frequent. The frequency distribution of the racial group is in accordance with the ethnical-social stratification of the population of the city of Salvador, with a predominance of non-white individuals.
Conclusion
This study reveals that, in a certain sample of adolescents, those with a BW ≥ 4,000g can have a high prevalence of MS in adolescence, with elevated SBP as the major cardiovascular risk factor. No increase in the prevalence of cardiovascular risk factors was observed in adolescents whose BW was ≤ 2,500g. Prospective studies initiating in the prenatal period are important to clarify the complex mechanism of adaptation to fetal growth restrictions or excesses, as the most effective way to ensure a healthy metabolic development.
Author contributions
Conception and design of the research: Sousa MACA, Guimarães AC; Acquisition of data: Sousa MACA, Guimarães ICB; Analysis and interpretation of the data: Sousa MACA, Daltro C, Guimarães AC; Statistical analysis: Sousa MACA, Daltro C; Obtaining funding: Sousa MACA, Guimarães ICB, Guimarães AC; Writing of the manuscript: Sousa MACA; Critical revision of the manuscript for intellectual content: Sousa MACA, Guimarães ICB, Daltro C, Guimarães AC.
Footnotes
Potential Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Sources of Funding: This study was partially funded by FAPESB.
Study Association: This article is part of the thesis of master submitted by Maria Amenaide Carvalho Alves de Sousa, from Escola Bahiana de Medicina e Saúde.
References
- 1.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C. Infant mortality, childhood nutrition and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/ FAO Expert Consultation. Geneva: 2003. ((WHO Technical Report Series, 916)). [PubMed] [Google Scholar]
- 4.Wang X, Liang L, Junfen FU, Lizhong DU. Metabolic syndrome in obese children born large for gestacional age. Indian J Pediatr. 2007;74(6):561–565. doi: 10.1007/s12098-007-0108-9. [DOI] [PubMed] [Google Scholar]
- 5.Salgado CM, Jardim PC, Teles FB, Nunes MC. Baixo peso ao nascer como marcador de alterações na monitorização ambulatorial da pressão arterial. Arq Bras Cardiol. 2009;92(2):113–121. [Google Scholar]
- 6.Mehta SH, Kruger M, Sokol RJ. Being too large for gestational age precedes childhood obesity in AfricanAmericans. Am J Obstet Gynecol. 2011;204(3):265.e1–265.e5. doi: 10.1016/j.ajog.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditionson adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass:a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77(3):726–730. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- 9.McCrindle BW, Urbina EM, Dennison BA, Jacobson MS, Steinberger J, Rocchini AP, et al. American Heart AssociationAtherosclerosis, Hypertension, and Obesity in Youth Committee. American Heart Association Council of Cardiovascular Disease in the Young. American Heart Association Council on Cardiovascular Nursing Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement fromthe American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, With the Council on Cardiovascular Nursing. Circulation. 2007;115(14):1948–1967. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 10.Sociedade Brasileira de Cardiologia. Departamento de Aterosclerose I Diretriz de prevenção da aterosclerose na infância e na adolescência da Sociedade Brasileira de Cardiologia. Arq Bras Cardiol. 2005;85(supl 6):1–35. [Google Scholar]
- 11.Araújo CL, Hallal PC, Nader GA, Neutzling MB, de Fátima Vieira M, Menezes AM, et al. Effect of birth size and proportionality on BMI and skinfold thickness in early adolescence: prospective birth cohort study. Eur J Clin Nutr. 2009;63(5):634–639. doi: 10.1038/ejcn.2008.20. [DOI] [PubMed] [Google Scholar]
- 12.Menezes AM, Hallal PC, Horta BL, Araújo CL, Vieira M de F, Neutzling M, et al. Size at birth and blood pressure in early adolescence: a prospective birth cohort study. Am J Epidemiol. 2007;165(6):611–616. doi: 10.1093/aje/kwk031. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro PO, Victora CG, Barros FC, Monteiro LM. Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27(10):1274–1282. doi: 10.1038/sj.ijo.0802409. [DOI] [PubMed] [Google Scholar]
- 14.Naghettini AV, Belem JM, Salgado CM, Vasconcelos HM, Júnior, Seronni EMX, Junqueira AL, et al. Avaliação dos fatores de risco e proteção associados à elevação da pressão arterial em crianças. Arq Bras Cardiol. 2010;94(4):486–491. doi: 10.1590/s0066-782x2010005000020. [DOI] [PubMed] [Google Scholar]
- 15.Batista M, Filho, Rissin A. A transição nutricional no Brasil: tendências regionais e temporais. Cad Saúde Pública. 2003;19(Suppl 1):S181–S191. doi: 10.1590/s0102-311x2003000700019. [DOI] [PubMed] [Google Scholar]
- 16.Guimarães IC, Almeida AM, Santos AS, Barbosa DB, Guimarães AC. Pressão arterial: efeito do índice de massa corporal e da circunferência abdominal em adolescentes. Arq Bras Cardiol. 2008;90(6):393–399. doi: 10.1590/s0066-782x2008000600007. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization(WHO) Expert group on prematurity: final report. Geneva: 1950. ((Technical report series27)). [PubMed] [Google Scholar]
- 18.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Healthand Nutrition Examination Survey. Circulation. 2004;110(16):2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM. Banting lecture 1988: Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 20.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–e503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. American Heart Association. National Heart, Lung, andBloodInstitute Diagnosis and management of the metabolic syndrome: an American Heart Association/ National Heart, Lung and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 22.Ministério da Saúde DATASUS.Indicadores e dados básicos 2007. [Acesso em 2008 nov 20]. Disponível em: http://tabnet.datasus.gov.br/cgi/idb2007/matriz.htm.
- 23.Dabalea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22(6):944–950. doi: 10.2337/diacare.22.6.944. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Huffman SL. Nutrition in pregnancy and early childhood and associations withobesityin developing countries. Matern Child Nutr. 2013;9(Suppl 1):105–119. doi: 10.1111/mcn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plagemann A, Harder T, Schellong K, Schulz S, Stupin JH. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract Res Clin Endocrinol Metab. 2012;26(5):641–653. doi: 10.1016/j.beem.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Parsons TJ, Power C. Breast feeding and obesity in childhood: cross sectional study. BMJ. 2003;327(7420):904–905. doi: 10.1136/bmj.327.7420.904. [DOI] [PMC free article] [PubMed] [Google Scholar]