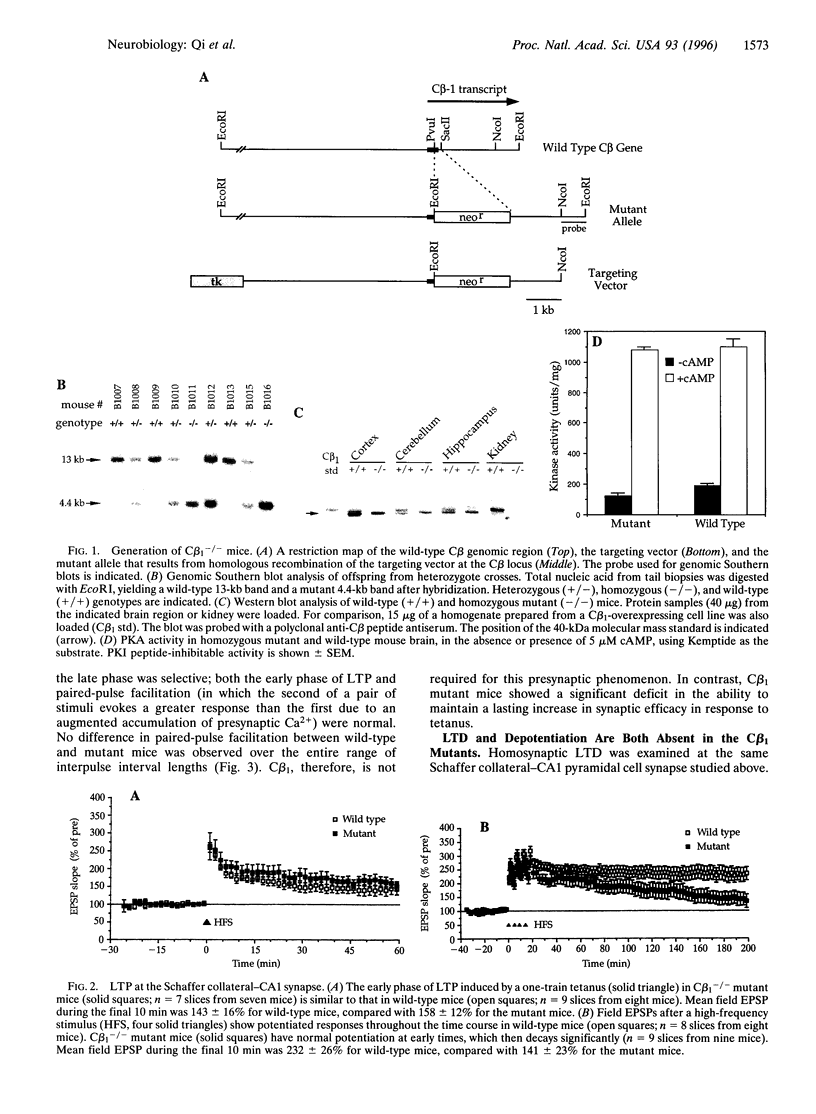
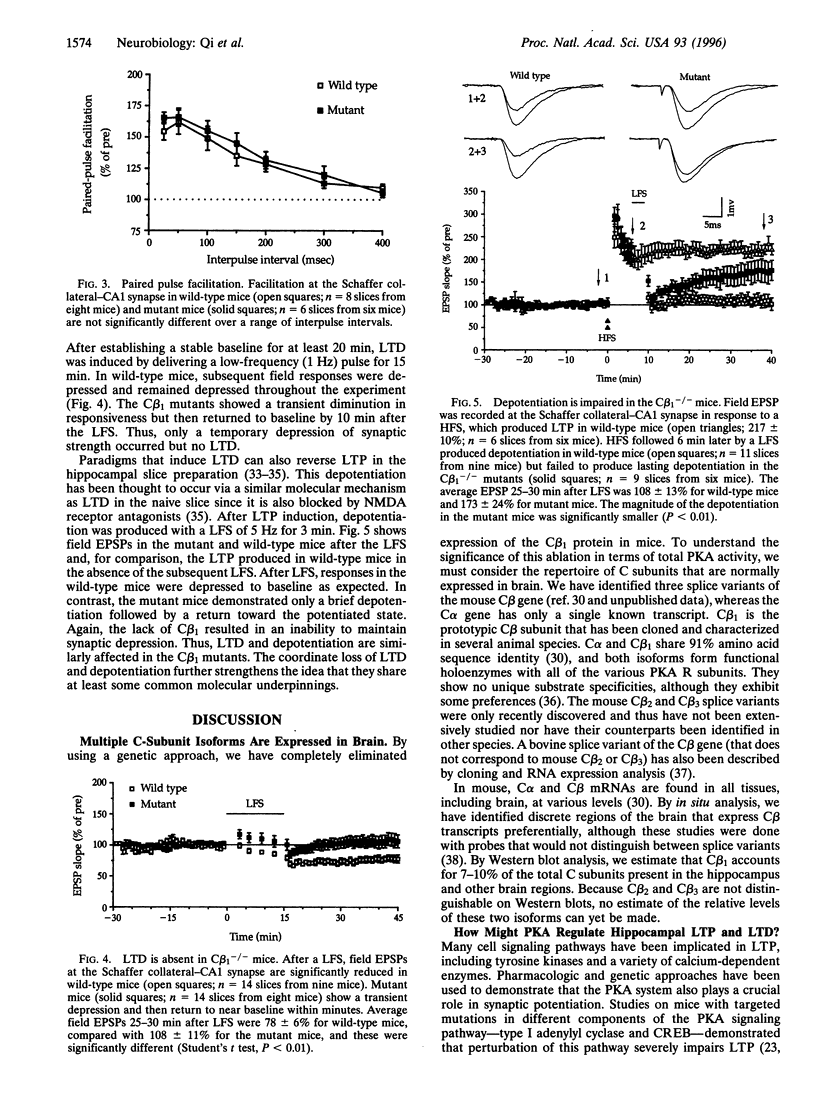
Abstract
Neural pathways within the hippocampus undergo use-dependent changes in synaptic efficacy, and these changes are mediated by a number of signaling mechanisms, including cAMP-dependent protein kinase (PKA). The PKA holoenzyme is composed of regulatory and catalytic (C) subunits, both of which exist as multiple isoforms. There are two C subunit genes in mice, Calpha and Cbeta, and the Cbeta gene gives rise to several splice variants that are specifically expressed in discrete regions of the brain. We have used homologous recombination in embryonic stem cells to introduce an inactivating mutation into the mouse Cbeta gene, specifically targeting the Cbeta1-subunit isoform. Homozygous mutants showed normal viability and no obvious pathological defects, despite a complete lack of Cbeta1. The mice were analyzed in electrophysiological paradigms to test the role of this isoform in long-term modulation of synaptic transmission in the Schaffer collateral-CA1 pathway of the hippocampus. A high-frequency stimulus produced potentiation in both wild-type and Cbeta1-/- mice, but the mutants were unable to maintain the potentiated response, resulting in a late phase of long-term potentiation that was only 30% of controls. Paired pulse facilitation was unaffected in the mutant mice. Low-frequency stimulation produced long-term depression and depotentiation in wild-type mice but failed to produce lasting synaptic depression in the Cbeta1 -/- mutants. These data provide direct genetic evidence that PKA, and more specifically the Cbeta1 isoform, is required for long-term depression and depotentiation, as well as the late phase of long-term potentiation in the Schaffer collateral-CA1 pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Chen C., Goda Y., Silva A. J., Stevens C. F., Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993 Dec 31;75(7):1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- Abeliovich A., Paylor R., Chen C., Kim J. J., Wehner J. M., Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993 Dec 31;75(7):1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Aiba A., Chen C., Herrup K., Rosenmund C., Stevens C. F., Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994 Oct 21;79(2):365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G., Schottler F., Lynch G. The effects of repetitive low frequency stimulation on control and "potentiated" synaptic responses in the hippocampus. Life Sci. 1980 Dec 15;27(24):2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A. J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994 Oct 7;79(1):59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brandon E. P., Gerhold K. A., Qi M., McKnight G. S., Idzerda R. L. Derivation of novel embryonic stem cell lines and targeting of cyclic AMP-dependent protein kinase genes. Recent Prog Horm Res. 1995;50:403–408. doi: 10.1016/b978-0-12-571150-0.50028-7. [DOI] [PubMed] [Google Scholar]
- Brandon E. P., Zhuo M., Huang Y. Y., Qi M., Gerhold K. A., Burton K. A., Kandel E. R., McKnight G. S., Idzerda R. L. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadd G. G., Uhler M. D., McKnight G. S. Holoenzymes of cAMP-dependent protein kinase containing the neural form of type I regulatory subunit have an increased sensitivity to cyclic nucleotides. J Biol Chem. 1990 Nov 15;265(32):19502–19506. [PubMed] [Google Scholar]
- Cadd G., McKnight G. S. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989 Jul;3(1):71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega L. E., Stevens C. F. Modulation of synaptic efficacy in field CA1 of the rat hippocampus by forskolin. Brain Res. 1992 Mar 6;574(1-2):85–92. doi: 10.1016/0006-8993(92)90803-h. [DOI] [PubMed] [Google Scholar]
- Chrivia J. C., Uhler M. D., McKnight G. S. Characterization of genomic clones coding for the C alpha and C beta subunits of mouse cAMP-dependent protein kinase. J Biol Chem. 1988 Apr 25;263(12):5739–5744. [PubMed] [Google Scholar]
- Clegg C. H., Correll L. A., Cadd G. G., McKnight G. S. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem. 1987 Sep 25;262(27):13111–13119. [PubMed] [Google Scholar]
- Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., Scott J. D. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995 Jan 6;267(5194):108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- Dahl D., Sarvey J. M. Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4776–4780. doi: 10.1073/pnas.86.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Butcher S. P., Morris R. G. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992 Jan;12(1):21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek S. M., Bear M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Taylor M., Heginbotham L. R., Proctor W. R. Long-term increases in excitability in the CA1 region of rat hippocampus induced by beta-adrenergic stimulation: possible mediation by cAMP. J Neurosci. 1992 Feb;12(2):506–517. doi: 10.1523/JNEUROSCI.12-02-00506.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U., Huang Y. Y., Kandel E. R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993 Jun 11;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Fujii S., Saito K., Miyakawa H., Ito K., Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991 Jul 26;555(1):112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Bonni A., Greenberg M. E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994 Jun 3;77(5):713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Silva A. J. Targeting learning. Trends Neurosci. 1994 Feb;17(2):71–75. doi: 10.1016/0166-2236(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Johnston D. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J Neurophysiol. 1988 Feb;59(2):667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Kandel E. R. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994 May-Jun;1(1):74–82. [PubMed] [Google Scholar]
- Huang Y. Y., Kandel E. R., Varshavsky L., Brandon E. P., Qi M., Idzerda R. L., McKnight G. S., Bourtchouladze R. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995 Dec 29;83(7):1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Li X. C., Kandel E. R. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994 Oct 7;79(1):69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Johnson B. D., Scheuer T., Catterall W. A. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., DeCola J. P., Landeira-Fernandez J., Fanselow M. S. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behav Neurosci. 1991 Feb;105(1):126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Connor J. A. Long-term synaptic depression. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvig N., Ribak C. E., Scott J. D., Rubin C. S. Immunocytochemical localization of the neural-specific regulatory subunit of the type II cyclic AMP-dependent protein kinase to postsynaptic structures in the rat brain. Brain Res. 1990 Jun 18;520(1-2):90–102. doi: 10.1016/0006-8993(90)91694-c. [DOI] [PubMed] [Google Scholar]
- Malenka R. C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994 Aug 26;78(4):535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Malenka R. C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992 Nov;9(5):967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Nguyen P. V., Abel T., Kandel E. R. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994 Aug 19;265(5175):1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994 Jul-Aug;1(2):129–139. [PubMed] [Google Scholar]
- Roche K. W., Tingley W. G., Huganir R. L. Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol. 1994 Jun;4(3):383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Carr D. W., Bergeson S. E., Nilaver G., Scott J. D., Westbrook G. L. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994 Apr 28;368(6474):853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- Sakimura K., Kutsuwada T., Ito I., Manabe T., Takayama C., Kushiya E., Yagi T., Aizawa S., Inoue Y., Sugiyama H. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995 Jan 12;373(6510):151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Scott J. D., McCartney S. Localization of A-kinase through anchoring proteins. Mol Endocrinol. 1994 Jan;8(1):5–11. doi: 10.1210/mend.8.1.8152430. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Paylor R., Wehner J. M., Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Slack J. R., Pockett S. Cyclic AMP induces long-term increase in synaptic efficacy in CA1 region of rat hippocampus. Neurosci Lett. 1991 Sep 2;130(1):69–72. doi: 10.1016/0304-3940(91)90229-m. [DOI] [PubMed] [Google Scholar]
- Soriano P. Gene targeting in ES cells. Annu Rev Neurosci. 1995;18:1–18. doi: 10.1146/annurev.ne.18.030195.000245. [DOI] [PubMed] [Google Scholar]
- Staubli U., Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1-5 Hz stimulation. Brain Res. 1990 Apr 9;513(1):113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- Thompson M. A., Ginty D. D., Bonni A., Greenberg M. E. L-type voltage-sensitive Ca2+ channel activation regulates c-fos transcription at multiple levels. J Biol Chem. 1995 Mar 3;270(9):4224–4235. doi: 10.1074/jbc.270.9.4224. [DOI] [PubMed] [Google Scholar]
- Uhler M. D., Chrivia J. C., McKnight G. S. Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Nov 25;261(33):15360–15363. [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]
- Walsh D. A., Van Patten S. M. Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB J. 1994 Dec;8(15):1227–1236. doi: 10.1096/fasebj.8.15.8001734. [DOI] [PubMed] [Google Scholar]
- Weisskopf M. G., Castillo P. E., Zalutsky R. A., Nicoll R. A. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994 Sep 23;265(5180):1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Wiemann S., Kinzel V., Pyerin W. Isoform C beta 2, an unusual form of the bovine catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1991 Mar 15;266(8):5140–5146. [PubMed] [Google Scholar]
- Wu Z. L., Thomas S. A., Villacres E. C., Xia Z., Simmons M. L., Chavkin C., Palmiter R. D., Storm D. R. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]




