Synopsis
Spirometry is the gold standard for making the diagnosis of COPD. It should be performed in every case of suspected COPD. Other pulmonary functions such as lung volumes can give you insight into physiological consequences of COPD such as hyperinflation. Pulmonary function testing can also aid in assessing the severity of disease and in managing the disease once the diagnosis is made.
Introduction
COPD is defined by airflow limitation caused by chronic bronchitis or emphysema. Spirometry is an essential step in the diagnosis and staging of COPD. Guidelines advise spirometry as the gold standard for COPD diagnosis (1,2). Spirometry is also an important part of monitoring COPD. Despite this, many patients are treated for presumed COPD without ever undergoing pulmonary function testing. In this manuscript, we will review pulmonary function testing, and the abnormalities seen in COPD. We will discuss the role spirometry plays in the diagnosis and management of COPD, as well as quantifying the severity of COPD.
Role of Spirometry in the Diagnosis of COPD
Spirometry is the gold standard for the diagnosis of COPD. In symptomatic patients, spirometry can help determine whether the patient's symptoms are due to respiratory disease or other conditions. Unfortunately, a large proportion of patients with COPD go undiagnosed (3). Frequently the disease will not be diagnosed until it is quite advanced. Targeted screening of symptomatic patients with risk factors for COPD results in a better diagnosis rates and more appropriate therapy (4).
Spirometry is often underutilized in COPD diagnosis. More than a third of patients with a new COPD diagnosis have never had pulmonary function testing, but are given a clinical diagnosis (5). It is important to confirm a clinical diagnosis of COPD with spirometry. Epidemiological data shows that when spirometry is not used, COPD is often underdiagnosed for those with the disease and overdiagnosed for those without the disease (6). If the diagnosis is missed in a patient with COPD, they will not have the benefit of treatment. If the patient is given an incorrect diagnosis of COPD, this also has deleterious consequences. Not only will the patient not be treated for the true cause of their symptom, but they also will likely be given treatment for COPD, which could give them unnecessary side effects. To ensure appropriate diagnosis and treatment, spirometry must be performed.
Introduction to Pulmonary Function Testing
Pulmonary function testing has three basic components: 1) spirometry, 2) lung volumes, and 3) diffusing capacity of the lung for carbon monoxide (DLCO). Each of these components can be affected by COPD. However, only spirometry is necessary to make the diagnosis of COPD.
Spirometry
Spirometry consists of 1) forced vital capacity (FVC), 2) forced expiratory volume in one second (FEV1) and 3) FEV1/FVC ratio. To perform spirometry, the patient takes the biggest breath possible and blows it out as fast as they can. This is called a forced exhalation maneuver. During this procedure, the total volume of air that the patient can exhale in one breath is measured; this is the FVC. The amount of air that the patient can exhale in the first second is also measured; this is the FEV1. Each is measured in liters and is reported as percent of predicted for that patient. The percent predicted is based on reference values that take into account the age, sex and race of the patient. The FVC is a measure of the amount of air the lungs hold. The FEV1 is a measure of how easily air flows through the lungs. COPD patients often have narrowing or inflammation of the airways. This hinders how fast air can leave the lungs. This leads to a decrease in the FEV1. If the FEV1 is decreased disproportionately to the FVC, a diagnosis of COPD is made. To determine if the decrease is disproportionate, the FEV1/FVC ratio is calculated. An FEV1/FVC ratio of <0.70 after bronchodilator is typically considered diagnostic of COPD. However, the use of a fixed cut-off has created some controversy, which is described later in the manuscript.
Along with spirometry, a flow-volume loop is also typically generated. A flow-volume loop plots flow on the y axis and volume on the x axis. A normal flow volume loop (Figure 1a) has a characteristic shape. In obstructive lung disease such as COPD, the expiratory limb takes on a coved shape (Figure 1b).
Figure 1.
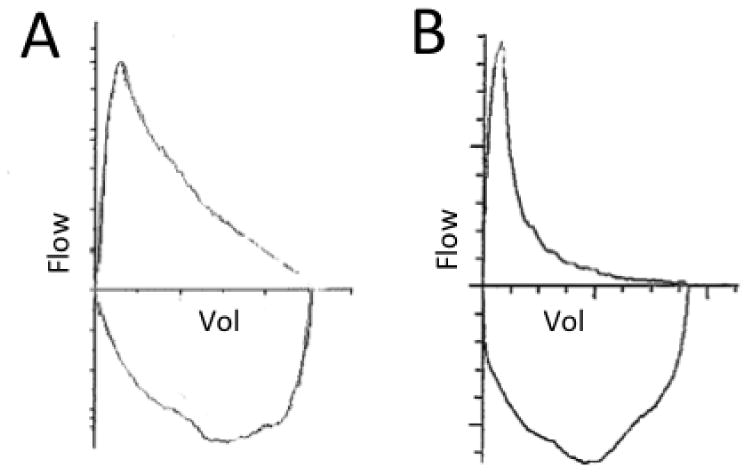
A) A normal flow volume loop. B) A flow volume loop of a COPD patient showing coving of the expiratory limb.
Lung Volumes
In addition to spirometry, lung volumes can also be measured. Lung volumes consist of: total lung capacity (TLC), residual volume (RV) and functional residual capacity (FRC). The TLC is the volume of air contained in the lung after a full inhalation. The RV is the volume of air left in the lung after a full exhalation. The FRC is the volume of gas left in the lungs after a tidal breath.
Lung volumes are measured by helium dilution, nitrogen washout or body plethysmography. Body plethysmography has come to be the gold standard in COPD, because both helium dilution and nitrogen washout can underestimate total lung capacity in COPD. Helium dilution and nitrogen washout can only measure air that communicates with the airways. Bullae, which are common in COPD, are not measured by these methods.
Emphysema destroys lung tissue, leading to loss of elastic recoil. Loss of elastic recoil allows the lungs to be stretched to abnormally large volumes, resulting in an increased TLC. RV can also be increased in COPD when disease progression destroys the elastic tethers that help hold small airways open during exhalation. This leads to premature closing of the airways, which causes abnormal amounts of air to be trapped in the lung. In some patients, there is also inflammation of the small airways, which causes narrowing, further contributing to air trapping and the increase in RV.
Air trapping can lead to hyperinflation. There is both a static and dynamic component of hyperinflation. Static hyperinflation refers to the baseline level of air trapping seen at rest. This is due to the loss of elastic recoil properties of the lung and fixed airway obstruction. Dynamic hyperinflation occurs during exercise or times of rapid respiratory rate. In these situations, the patient is unable to finish exhaling before the next breath starts. With each breath, the patient becomes progressively more hyperinflated. This puts the respiratory muscles at a disadvantage, and increases the work of breathing.
DLCO
The diffusing capacity of the lung for carbon monoxide (DLCO) is a measure of how easily carbon monoxide (CO) molecules transfer from the alveolar gas to the hemoglobin of the red cells in the pulmonary circulation. To measure the DLCO, the patient inhales a single breath containing a minute amount of CO and holds it for 10 seconds. The breath is then exhaled and the exhaled breath is analyzed for CO. The change in the concentration of the CO is then multiplied by the single breath TLC to calculate the DLCO. Some patients with severe COPD may have difficulty performing the breath hold required to measure DLCO.
In COPD, the DLCO decreases with increasing severity of disease. This is because in emphysema, the lung has lost alveoli, resulting in a lower surface area available for diffusion. In addition, there is also a loss of capillary bed, which can also decrease DLCO. When DLCO falls below 55% of predicted, the patient should undergo oximetry during exercise to determine if oxygen is required (7).
Diagnosis of COPD Based on Spirometry
A diagnosis of COPD is confirmed by spirometry when the FEV1/FVC ratio is < 0.70. Although there is some controversy about what the cutoff should be, both the GOLD guidelines (2) and the combined American College of Physicians, American College of Chest Physicians, American Thoracic Society and the European Respiratory Society COPD guidelines (8) recommend using the fixed cutoff of <0.70. This criterion is set regardless of age and gender in an attempt to simplify the diagnosis of COPD. However, the fixed ratio cutoff is not perfect. The FEV1/FVC ratio is known to decline with normal aging. Using this cutoff may lead to an overdiagnosis of COPD in the elderly (9, 10), and an underdiagnosis in young adults (11). This is due to age-related changes in FEV1/FVC ratio.
Because of these imperfections in the FEV1/FVC ratio, there have been several alternate methods proposed to diagnose obstruction. One proposal is to use the lower limit of normal (LLN) for the cutoff of FEV1/FVC ratio. The LLN takes into consideration the age, height and gender for each individual. This minimizes the age-related changes in the FEV1 /FVC ratio, and may reduce the misclassification of airway obstruction (10). However, it also may be more difficult for primary care providers to perform and interpret. In addition, using this cutoff may not improve clinical care. Despite the proposed ‘overdiagnosis’ in the elderly, the FEV1/FVC ratio still correlates better with COPD exacerbations and mortality than using the LLN (12).
It may be difficult for some patients to perform a forced exhalation maneuver for several reasons, including poor mental status and coughing. Proposed alternatives include using slow vital capacity (SVC) or forced expiratory volume in 6 seconds (FEV6) instead of FVC. Slow vital capacity measures the vital capacity while breathing in and out in a slow and steady manner rather than the forced maneuver used to measure the FVC. SVC was found to be useful in elderly patients who are unable to perform an FVC maneuver without coughing (13). However, it was not easier for patients with poor mental status to perform (13). Another option is the FEV1 /FEV6 ratio. The FEV1/FEV6 ratio has been shown to be an acceptable surrogate for the FEV1 / FVC ratio (14). It is also thought to be an easier test for patients to perform than the FVC maneuver.
Differentiating COPD from Asthma with Bronchodilator Reversibility Testing
Asthma and COPD have many features in common, including symptoms of shortness of breath. Bronchodilator reversibility testing can be used to help differentiate asthma from COPD. In both diseases, spirometry can show an obstructive pattern. However, the airway obstruction caused by asthma is typically completely reversed (FEV1/FVC ratio is normal) with bronchodilator therapy. In contrast, in patients with COPD the obstruction remains post-bronchodilator treatment (FEV1/FVC ratio remains <0.7). Although the airway obstruction remains in COPD, there may be improvement in the FEV1 after bronchodilator therapy. Significant improvement in FEV1 after bronchodilator is defined as an increase in FEV1 by 12% AND 200 ml.
Although bronchodilator reversibility testing can be helpful in distinguishing between COPD and asthma, it is not a perfect test. Some patients with COPD have significant reversibility of their FEV1 after bronchodilator. Likewise, some patients with severe, long-standing asthma may not have complete reversibility after bronchodilator. The test must be interpreted along with the clinical context.
Quantifying COPD Severity with Spirometry
Spirometry can be used to quantify COPD severity. Several scales have been used in the past and COPD staging guidelines have been criticized because they do not accurately reflect severity of disease. Data is emerging that indicates that COPD stage correlates with other important measures of severity. For instance, COPD stage correlates well with patient reports of dyspnea (15). In addition, COPD severity is associated with a higher rate of severe exacerbations requiring hospitalization.
The GOLD guidelines ratings of severity have gone through several changes. The initial guidelines in 2001 included a stage 0, “at risk” which included people with normal spirometry that had a history of smoking, exposure to pollutants, respiratory symptoms or a family history of respiratory disease. The current GOLD guidelines have removed stage 0 (2). These changes are summarized in Table 1. The percent predicted FEV1 is used to grade the severity of COPD. It should be noted that the post-bronchodilator FEV1 should be used to determine severity stage. It is more reproducible than the pre-bronchodilator FEV1 (16). Stage I or “mild” COPD is defined as a FEV1/FVC ratio of <0.7 and an FEV1 >80% predicted; Stage II is an FEV1 of 50-79% predicted; Stage III 30-49% predicted, Stage IV <30% predicted.
Table 1. FEV1 : Forced expiratory volume in one second. FVC: Forced Vital Capacity.
| GOLD 2001 | GOLD 2011 | FEV1/FVC ratio | FEV1 (% predicted) |
|---|---|---|---|
| 0: “At risk” | Not applicable | ||
| I: Mild | I: Mild | ≤ 0.7 | ≥ 80 |
| II: Moderate | II: Moderate | ≤ 0.7 | 50-79 |
| III: Severe | III: Severe | ≤ 0.7 | 30-49 |
| IV: Very Severe | IV: Very Severe | ≤ 0.7 | <30 |
Role of Spirometry in the Management of COPD
Spirometry plays an essential role in not only the diagnosis, but also the management of COPD. Spirometry can guide therapy for COPD, enable monitoring of disease progression, help determine prognosis and estimate long-term survival.
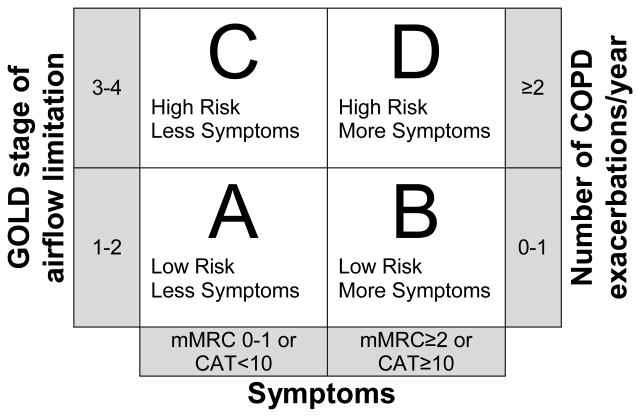
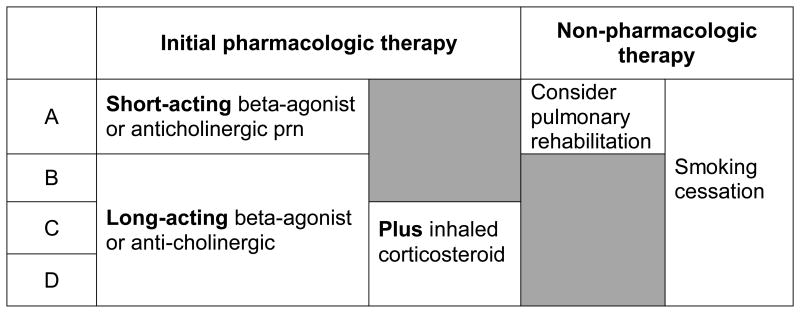
Spirometry has been shown to prompt changes in therapy for COPD patients (17). Several guidelines make recommendations for treatment based on severity as measured by FEV1. The ACP, ACCP, ATS and ERS guidelines recommend treatment with inhaled bronchodilators for those with an FEV1 of <60% predicted, and pulmonary rehabilitation for those with an FEV1 of < 50% predicted. The 2011 GOLD guidelines take this one step further, using more than just the patient's FEV1 to help guide treatment (2). Of course, spirometry alone cannot give us an accurate picture of the severity of an individual patient's disease. Symptoms and number of exacerbations must also be considered. The 2011 GOLD guidelines propose a method to combine spirometry, symptoms and numbers of exacerbations to produce a more comprehensive picture. In this classification, patients are grouped into Group A-D, with group A having the lowest severity and group D having the highest severity. In order to calculate the patient's group, you must have 1) Spirometry, 2) A measurement of symptoms, and 3) The number of COPD exacerbations the patient has a year. The patient's symptoms can be quantified using either the COPD assessment test (CAT) (www.catestonline.org) or the Modified British Medical Research Council (mMRC) questionnaire (Table 2). You can then use Figure 2 to determine which category the patient falls into. The GOLD guidelines also make recommendations for initial therapy based on the patient's group. Group A should be treated with a short-acting beta agonist or a short-acting anticholinergic. Group B should be treated with a long-acting beta-agonist or anticholinergic. Group C&D should be treated with a inhaled corticosteroid and a long-acting beta-agonist or long-acting anticholinergic. These recommendations are summarized in Table 3.
Table 2. Modified Medical Research Council Questionnaire for Assessing the Severity of Breathlessness.
| Grade 0 | I only get breathless with strenuous exercise. |
| Grade 1 | I get short of breath when hurrying on the level or walking up a slight hill. |
| Grade 2 | I walk slower than people of the same age on the level because of breathlessness, or I have to stop for breath when walking on my own pace on the level. |
| Grade 3 | I stop for breath after walking about 100 meters or after a few minutes on the level. |
| Grade 4 | I am too breathless to leave the house or I am breathless when dressing or undressing. |
Figure 2.
The combined COPD Assessment. (Modified from the 2011 GOLD guidelines (2)) Patients are assigned lettered groups A-D based on spirometry, symptoms and number of exacerbations per year. If there is a discrepancy between which group the patient would fall into, always choose the higher risk group. For example, a patient who is GOLD stage II, with a CAT of 22 and 2 COPD exacerbations a year would fall into Group D rather than group B.
Spirometry also is an objective way of monitoring for disease progression. It is often helpful to determine whether an increase of shortness of breath is due to worsening COPD or other etiologies such as heart disease. This is especially important given the fact that many patients with COPD also have concomitant heart disease.
Spirometry can also be used to help determine the long-term survival and prognosis of a COPD patient. Classically, the FEV1 has been used to determine prognosis (18). With decreasing FEV1, mortality increases. However, the FEV1 alone does not give us the entire picture of the patient's status. Other combinations of criteria, which include the FEV1 are being studied such as the BODE index (19).
Conclusion
Spirometry is the gold standard for making the diagnosis of COPD. It should be performed in every case of suspected COPD. Other pulmonary functions such as lung volumes can give you insight into physiological consequences of COPD such as hyperinflation. Pulmonary function testing can also aid in assessing the severity of disease and in managing the disease once the diagnosis is made.
Figure 3.
Initial pharmacologic therapy and Non-pharmacologic therapy based on GOLD group A-D. Adapted from the 2011 GOLD guidelines.
References
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007 Sep 15;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Global Strategy for the Diagnosis, Management and Prevention of COPD. 2011 doi: 10.1183/09031936.03.00063703. Available at: Available from: http://www.goldcopd.org/ [DOI] [PubMed]
- 3.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000 Jun 12;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 4.Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J. 2006 Nov;28(5):945–952. doi: 10.1183/09031936.06.00019306. [DOI] [PubMed] [Google Scholar]
- 5.Han MK, Kim MG, Mardon R, Renner P, Sullivan S, Diette GB, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007 Aug;132(2):403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 6.Joo MJ, Au DH, Lee TA. Use of spirometry in the diagnosis of chronic obstructive pulmonary disease and efforts to improve quality of care. Transl Res. 2009 Sep;154(3):103–110. doi: 10.1016/j.trsl.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Owens GR, Rogers RM, Pennock BE, Levin D. The diffusing capacity as a predictor of arterial oxygen desaturation during exercise in patients with chronic obstructive pulmonary disease. N Engl J Med. 1984 May 10;310(19):1218–1221. doi: 10.1056/NEJM198405103101903. [DOI] [PubMed] [Google Scholar]
- 8.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002 Nov;20(5):1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 10.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008 Dec;63(12):1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 11.Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Anto JM, et al. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008 Dec;63(12):1040–1045. doi: 10.1136/thx.2008.095554. [DOI] [PubMed] [Google Scholar]
- 12.Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007 Mar;62(3):237–241. doi: 10.1136/thx.2006.068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen SC, Charlton C, Backen W, Warwick-Sanders M, Yeung P. Performing slow vital capacity in older people with and without cognitive impairment--is it useful? Age Ageing. 2010 Sep;39(5):588–591. doi: 10.1093/ageing/afq084. [DOI] [PubMed] [Google Scholar]
- 14.Jing JY, Huang TC, Cui W, Xu F, Shen HH. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest. 2009 Apr;135(4):991–998. doi: 10.1378/chest.08-0723. [DOI] [PubMed] [Google Scholar]
- 15.Mahler DA, Ward J, Waterman LA, McCusker C, Zuwallack R, Baird JC. Patient-reported dyspnea in COPD reliability and association with stage of disease. Chest. 2009 Dec;136(6):1473–1479. doi: 10.1378/chest.09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SH, Kuo PH, Kuo SH, Yang PC. Severity staging of chronic obstructive pulmonary disease: differences in pre- and post-bronchodilator spirometry. Yonsei Med J. 2009 Oct 13;50(5):672–676. doi: 10.3349/ymj.2009.50.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavannes N, Schermer T, Akkermans R, Jacobs JE, van de Graaf G, Bollen R, et al. Impact of spirometry on GPs' diagnostic differentiation and decision-making. Respir Med. 2004 Nov;98(11):1124–1130. doi: 10.1016/j.rmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986 Jan;133(1):14–20. doi: 10.1164/arrd.1986.133.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Cote CG, Pinto-Plata VM, Marin JM, Nekach H, Dordelly LJ, Celli BR. The modified BODE index: validation with mortality in COPD. Eur Respir J. 2008 Nov;32(5):1269–1274. doi: 10.1183/09031936.00138507. [DOI] [PubMed] [Google Scholar]