Abstract
Periconceptional folic acid can reduce the occurrence of neural tube defects (NTDs) by up to 70%, and autoantibodies for folate receptors (FRs) have been observed in serum from women with a pregnancy complicated by an NTD. This population-based cohort study has examined serum from pregnant mothers for autoantibodies to FRs, antibodies to bovine folate binding protein (FBP), and inhibition of folic acid binding to FR and FBP in association with NTD risk. The mid-gestational maternal serum specimens used for this study were collected during the 15th–18th week of pregnancy. Samples were obtained from the California Birth Defects Monitoring Program; 29 mothers had a pregnancy complicated by spina bifida and 76 mothers had unaffected children. The presence of IgG and IgM antibodies to human FR, bovine FBP, and inhibition of folic acid binding to FR and FBP was determined. Higher activity of IgM to FBP in cases verses controls was observed (P=.04). Higher activity of IgM and IgG autoantibodies to FR was observed (P<0.001 and P=.04, respectively). Risk estimates at two standard deviations above average control antibody concentrations were OR=2.07 (CI=1.02, 4.06) for anti-FBPIgM, OR=2.15 (CI=1.02, 4.69) for anti-FRIgG and OR=3.19 (CI=1.47, 6.92) for anti-FR IgM. These data support the hypothesis that high titers of antibodies and blocking of folic acid binding to FRs by maternal serum should be regarded as risk factors for NTDs.
Keywords: Folate Receptor, Autoantibodies, Pregnancy, Neural Defects
1. Introduction
Over the last two decades, periconceptional folic acid supplementation has been shown to significantly reduce the risk of neural tube defects (NTDs) by as much as 70 percent (Berry et al., 1999; Czeizel and Dudas, 1992; Milunsky et al., 1989; MRC, 1991; Steegers-Theunissen et al., 1994; Werler et al., 1993). However, most pregnant women carrying an NTD-affected fetus do not have serum folate deficiency (Kirke et al., 1993; Yates et al., 1987). No polymorphisms have been observed in folate receptor-α to account for effect of folic acid on NTD risk (Barber et al., 1998). However, multiple polymorphisms for a variety of folate pathway enzymes have been identified, some of which have been proposed as risk factors for NTDs (Botto and Yang, 2000; Shaw et al., 1998; van der Put et al., 1995; Zhu et al., 2003), but they account for only a fraction of the reduction in NTD risk following folate supplementation. The other underlying mechanisms by which folic acid supplementation decreases NTD risk are poorly understood (Cabrera et al., 2004). However, evidence has emerged that maternal immunological responses can have a substantive impact on embryonic development. When antibodies to rat placenta, kidney, heart and other tissues are generated and administered to pregnant rats, they bind to the yolk sac and contribute to congenital abnormalities and embryonic death (da Costa and Rothenberg, 1996; da Costa et al., 2003). It was hypothesized that the presence of antibodies binding to the yolk sac impaired the delivery of critical nutrients to the embryo (da Costa et al., 2003; Rothenberg et al., 2004). Similarly, studies have also indicated that competitive inhibition of proteins such as the FR can result in cellular growth inhibition by blocking the uptake of folate (Ebel et al., 2007; Henderson and Strauss, 1990).
Rothenberg et al. (2004) reported autoantibodies to the FR in 75% of 12 mothers who had given birth to NTD-affected infants, but in only 8.3% of mothers of 24 non-malformed infants.. Unfortunately, this provocative but small study examined only three women during pregnancy, all of whom had a pregnancy complicated by an NTD. Maternal autoantibodies to FR that produce immune responses against, or inhibit folate uptake by, the developing embryo may explain the beneficial effect of periconceptional folic acid supplementation on NTD risk. That is, supplemental folic acid may reduce the level of serum autoantibodies or compensate for blocking of the receptors caused by FR autoantibodies. Consequently nullizygous mutations for, or immunologically targeting of, folate binding protein has been demonstrated to increase risk for folate-responsive congenital anomalies in mouse and rat models (da Costa et al., 2003; Piedrahita et al., 1999; Tang and Finnell, 2003).
Several etiologies have been suggested for the presence of FR autoantibodies in human serum. For example, bovine folate binding protein (bFBP) (GI: 110282963) may be antigenic, and some antibodies cross-react with the endogenous receptor (GI: 544337) due to >80% protein homology as determined by the Protein Basic Local Alignment Search Tool (blastp) (Tatusova and Madden, 1999). It is possible also that degradation or cleavage of FR, or the binding of an antigenic ligand, makes the receptor antigenic. The de novo production of anti-idiotypes by the variable region of an antibody could also explain the presence of maternal autoantibodies to the FR (Schwartz, 2005).
We hypothesized that, during pregnancy, blocking of folic acid binding to FR and serum autoantibodies to FR are risk factors for NTDs. Here, we report the results of anti-FR antibodies and folic acid blocking in serum from expectant mothers. Specifically, two preparations of human placental FR and exogenous bovine milk FBP proteins were assessed for interactions with folic acid and antibodies in maternal serum, and their measure of NTD risk was determined.
2. Materials and Methods
2. 1. Study design
Between January 2003 and December 2004, more than 140,000 serum specimens were collected and banked from women during the 15th–18th week of pregnancy. These sera were collected from women who live in selected regions in California (Orange and San Diego counties, and Central Valley counties). The specimens were collected from women as part of the Expanded Alpha-Fetoprotein (XAFP) Screening Program. Once diagnostic screening was complete, a proportion of the residual serum sample was stored frozen at −80°C in the specimen bank. Each woman’s serum specimen was record-linked with delivery outcome information to determine whether her fetus had an NTD, any other structural malformation ascertained by the California Birth Defects Monitoring Program (Croen et al., 1991), or was born nonmalformed. The study included deliveries that were liveborn, stillborn (fetal deaths at greater than 20 weeks post-conception), or electively terminated based on prenatal diagnoses. We identified specimens for 29 women who had NTD-affected pregnancies. A group of non-malformed controls (n=76) was randomly selected from specimens associated with ‘normal’ birth outcomes. This study was approved by the Committee for the Protection of Human Subjects, California Health and Human Services Agency.
2.2. Serum assays for autoantibodies against folate receptors
The assay procedure used to identify the presence, absence and relative abundance of FR autoantibodies in serum samples was a modification of a microELISA assay (Mendoza et al., (1999). These assays were conducted directly on glass 96-well slides (Precisions Lab Products, Middleton, WI). The slides were rinsed and modified with a fresh 1% solution of (3-glycidoxypropyl) trimethoxysilane in toluene. This method has been shown to produce monolayers of epoxysilane films (Tsukruk et al., 1999). Immediately after drying, slides were utilized for coupling proteins to the surface.
Bovine milk folate-binding proteins (FBPs) bind folates with high affinity (1:1 molar ratio) (Jones and Nixon, 2002). The FBPs used in this study were either kindly provided by Jacob Selhub (FBP), isolated using previously described procedures (Antony et al., 1982), or obtained commercially (FBP.2; Sigma Aldrich, St. Louis, MO). The FRs used in this study, kindly provided by Bart Kamen(FR) and Jacob Selhub (FR.2), were isolated from two different human placentas as previously described (Antony et al., 1981). The proteins were suspended in phosphate-buffered saline (PBS, pH 7.2) with 5mM sodium azide to produce a 1mg/mL stock solution. For printing, this solution was diluted in 50mM NaHCO3 (pH 8.2) at 50µg/mL, mixed 1:1 with Protein Print Buffer (ArrayIt, Sunnyvale, CA) and printed onto the surface in 1.0µL volumes under ambient conditions. The slides were dried under ambient conditions.
Prior to the application of the serum solution, non-bound protein was removed from the wells by two washes with 1xTNT buffer (100mM Tris-HCl pH 7.6, 150mM NaCl, 0.05% Tween-20). All solution volumes were 20µL per well. The amine-reactive surface was then blocked by addition of 1xTNT-methionine (1xTNT, pH 9.0 with 15mM methionine) buffer for five minutes. The wells were washed with 1xTNT thrice, followed by addition of the serum sample to the slide. The slides and serum solutions (1:10 dilution of serum in 1xTNT) were incubated in a polycarbonate cabinet overnight (16–18hrs) under ambient conditions. After incubation, wells were washed five times with 1xTNT. A secondary conjugate labeled with alkaline phosphatase and specific for the detection of human immunoglobulin G or immunoglobulin M (IgG or IgM) was diluted in 1xTNT and then applied (20µL per well) according to the manufacturer’s ELISA recommendations (Sigma Aldrich). The slides and secondary antibody solution were incubated for one hour under ambient conditions. Following incubation, wells were washed seven times with 1xTNT. Negative controls were prepared from antibody-depleted sera (Sigma Aldrich).
Detection of the interaction between FR or FBP, autoantibodies and the alkaline phosphatase IgG secondary conjugate was assayed by using the ELF phosphatase substrate (Molecular Probes, Eugene, OR). Slides with applied substrate were incubated for 30 minutes under ambient conditions. Following this incubation, slides were rinsed once with 1xTBE (10mM Tris-borate 1mM EDTA) followed by a Milli-Q Ultrapure water rinse. Slides were imaged using an 8-bit UV photography workstation (Kodak, New York, NY). Fluorescent signal intensities were determined using ImageJ (NIH, Bethesda, MD) and were reported as foreground signal.
Competitive inhibition of folic acid binding between the serum and the immobilized protein (FR or FBP) was detected via folic acid-labeled HRP (FA-HRP, Ortho-Clinical Diagnostics, Raritan, NJ). Folic acid was removed, as described previously (Zettner and Duly, 1975), by incubation with a solution (500mM citric acid, pH 3.0) of dextran-coated charcoal (Sigma Aldrich) for five minutes. This solution was then passed through PVDF-filtered 0.45µm 96-well multiscreen plates (Millipore, Billerica, MA ) in order to remove the dextran-coated charcoal and endogenous folic acid. The solution was neutralized by addition of 1.2M Tris buffer (pH 9.0) in the well. These conditions were determined to remove up to 100ng/mL serum folic acid, which exceeded the expected normal range 2.9–18ng/mL (Waddell et al., 1976). The peroxidase substrate used for detection was SuperSignal ELISA Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL). Images were collected using a 96-well microplate reader (BMG Labtechnologies, Offenburg, Germany), and intensities were determined from the 16-bit images. Unlabeled folic acid was spiked (0.01–2000ng/mL) into stripped antibody-deplete sera to generate a regression curve. All intensities were extracted, log-transformed and fitted relative to this sigmoid regression curve.
2.3. Statistical analysis
The concentrations of maternal autoantibodies to the FR were examined as risk factors for NTDs. The concentration of folic acid blocked from binding FR by maternal serum was also used to estimate risk for NTDs. These risks were estimated by odds ratios (along with 95% confidence intervals) using logistic regression models. The correlation between antibody and folic acid blocking were determined by Pearson Correlation. We performed data management and statistical analyses using Microsoft Excel (Microsoft Corp., Redmond, WA), GraphPad (GraphPad Software, San Diego, CA) and SAS software (SAS Institute, Cary, NC).
3. Results
3.1. Antibodies, autoantibodies, folic acid blocking, and NTD risk
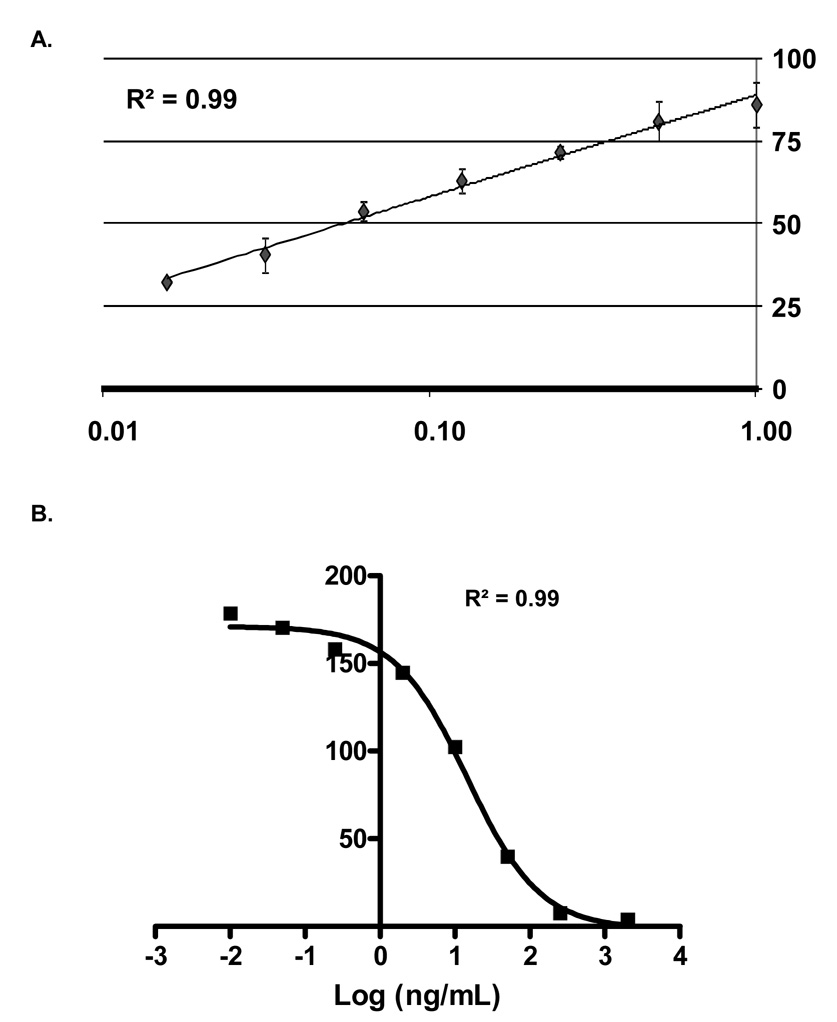
Functional testing of the antibody assay and folate binding assay indicated that both are functional and detect their respective interactions (Figure 1). Data analysis indicated a low coefficient of variance (average CV<10%) for antibody detection, and a minimum detectable fold change of two could accurately be determined for both assays (T-test, P<0.01). The specificity of the assay was confirmed also by using other antigens, in place of folate receptors, with high expected rates of exposure (e.g. Candida albicans and tetanus toxoid) and with no expected exposures (e.g. keyhole limpet hemocyanin, KLH) in this population. Folic acid binding was not detected using these antigens, no serum samples were positive for KLH antibodies, and all samples had positive responses to C. albicans or tetanus toxoid, often both (data not shown).
Figure 1. Antibody detection and folic acid binding inhibition.
Images show the functional testing results of the assay. The vertical axes of the graphs represent detected signal intensities (8-bit). (A) A serial dilution (1:10 to 1:640) of a positive serum sample is shown for IgG antibodies against bovine folate binding protein (FBP). Fluorescent intensities were extracted and plotted against a regression line with standard errors to generate the graph. (B) Results are displayed for folic acid binding to FBP. A dilution series of folic acid was used to quantify interactions. Fluorescent intensities were extracted, log-transformed and fitted against a sigmoid regression curve for the graph (GraphPad Software, San Diego, CA).
The age and ethnic background of the 29 case and the 76 control women providing serum samples during the 15th–18th week of conception are shown in Table 1. No significant differences were observed between case and control samples. Two variables approached significance, age and ethnicity, but both reflect actual differences in the case and control populations and, in order to avoid overmatching, no corrections were taken. The immunological characterization of antibodies, folic acid blocking, and NTD risk are shown in Table 2. It was determined by serial dilution of a positive sample that a 10-unit change in mean signal equaled a halving of the antibody concentration. Significantly higher mean concentrations of IgM antibodies against FBP (P=0.04), IgG (P=0.02) and IgM (P<0.001) autoantibodies to the human placental FR, and IgM autoantibodies to FR.2 (P=.05) were detected in serum of case mothers. In addition to mean differences, the potential continuous effect on NTD risk of these measures was estimated. We estimated risks associated with a two standard deviation increase in antibody concentrations and observed the following: OR=2.07 (CI=1.02, 4.06) for anti-FBP IgM, OR=2.15 (CI=1.02, 4.69) for anti-FR IgG, and OR=3.19 (CI=1.47, 6.92) for anti-FR IgM.
Table 1.
Demographics of serum samples from case and control mothers
| Controls (n=76) | NTD cases (n=29) | |
|---|---|---|
| No. (%) | No. (%) | |
| Race/ethnicity | ||
| Hispanic | 34 (44.7) | 20 (69.0) |
| White non-Hispanic | 29 (38.2) | 5 (17.2) |
| Asian | 9 (11.8) | 2 (6.9) |
| Black | 2 (2.6) | 1 (3.5) |
| Other | 2 (2.6) | 1 (3.5) |
| Age (years) | ||
| Less than 25 | 22 (29.0) | 13 (44.8) |
| 25–29 | 21 (27.6) | 9 (31.0) |
| 30–34 | 27 (35.5) | 3 (10.3) |
| Older than 34 | 6 (7.9) | 4 (13.8) |
Table 2.
Characterization of serum samples from case and control mothers
| Controls | NTD cases | p-value* | Unadjusted OR (95% CI) | ||
|---|---|---|---|---|---|
| Assay type | Mean signal† (SD) | Mean signal† (SD) | Per mean difference§ | Per 2 SD¶ | |
| FBP_IgG | 76.4 (35.5) | 88.7 (39.4) | 0.12 | 1.12 (0.96–1.31) | 1.89 (0.81–4.77) |
| FBP_IgM | 50.4 (28.0) | 66.1 (46.3) | 0.04 | 1.23 (1.00–1.48) | 2.07 (1.02–4.06) |
| FR_IgG | 5.7 (7.8) | 12.5 (18.5) | 0.02 | 1.40 (1.01–1.96) | 2.15 (1.02–4.69) |
| FR_IgM | 59.0 (21.5) | 79.5 (39.3) | < 0.001 | 1.74 (1.2–2.52) | 3.19 (1.47–6.92) |
| FBP.2_IgG | 29.8 (21.5) | 34.8 (26.1) | 0.32 | 1.05 (0.96–1.15) | 1.47 (0.68–3.33) |
| FBP.2_IgM | 38.5 (28.3) | 51.5 (40.6) | 0.07 | 1.17 (0.99–1.38) | 1.97 (0.94–4.12) |
| FR.2_IgG | 5.2 (14.5) | 10.7 (22.1) | 0.14 | 1.10 (0.96–1.26) | 1.64 (0.79–3.38) |
| FR.2_IgM | 18.7 (14.1) | 28.6 (36.7) | 0.05 | 1.21 (0.97–1.52) | 1.71 (0.92–3.27) |
| FBP - folic acid (ng/mL) blocked | 1.0 (3.6) ‡ | 3.8 (10.4) ‡ | 0.04 | 1.24 (0.95–1.66) | 1.74 (0.88–3.71) |
| FR - folic acid (ng/mL) blocked | 2.6 (5.5) ‡ | 8.2 (12.4) ‡ | 0.002 | 1.57 (1.12–2.25) | 2.44 (1.25–4.93) |
FBP denotes folate binding protein from bovine milk (laboratory preparation), FBP.2, commercial preparation (Sigma); FR, folate receptor from human placenta (laboratory preparation contained autoantibodies, IgG); FR.2 folate receptor from human placenta (laboratory preparation).
Significant differences between means were determined by unpaired two-tailed t test.
Relative concentrations are presented as mean values and standard deviations (SD); units are fluorescent signal intensity (8-bit). For antibody detection, it was determined by titer dilutions that a decrease of 10 fluorescent units equals a halving of antibody concentration.
Fluorescent signal was transformed to ng/mL folic acid blocked.
The odds ratio, OR, is presented as a continuous measure of risk per the difference between mean case and mean control concentrations.
Using the control group as a reference, the OR is presented as 2 SD above the mean control concentration.
The mean concentration of folic acid blocked from binding to FBP in serum from case women samples (3.8ng/mL ± 10.4) was significantly higher (P=0.04) than control women (1.0ng/mL ± 3.6). The mean concentration of folic acid blocked from binding to FR (8.2ng/mL ± 12.4) was also significantly higher (P=0.002) in case women than in controls (2.6ng/mL ± 5.5) and was associated with an increase in NTD risk (OR= 1.08, CI=1.02, 1.15). For folic acid binding, the OR is based on a 1-unit change, where the unit is ng/mL. We estimated also the risk associated with a two standard deviation decrease in folic acid binding to FR and observed an OR=2.44 (CI=1.25, 4.93).
Higher background IgG signals were observed in the antibody detection assay using antibody depleted serum against FR. Autoantibodies, IgG, were detected also by Western and ELISA of the FR preparation (data not shown); however, no additional placenta remained to confirm that antibodies were bound to it prior to FR purification.
3.2. Correlations
The Pearson correlation coefficients among controls (Table 3) indicated that 13 of the 28 correlations were not significant. The highest correlations found in controls (0.78, P<0.0001), also found in cases (0.94, P<0.0001), were between anti-FBP IgM and anti-FBP.2 IgM measurements. The Pearson correlation coefficients among cases (Table 4) indicated that 25 of 28 antibody measurements were correlated; the three exceptions were with IgG to FBP (anti-FBP IgG). Specifically, anti-FBP IgG was not significantly correlated with IgM to FR (anti- FR IgM), nor was it correlated with IgM or IgG autoantibodies against FR.2 (anti-FR.2 IgM, anti-FR.2 IgG). Other highly significant correlations in both groups included case (0.80, P<0.001) and control (0.68, P<0.001) anti-FBP.2 IgM and anti-FR.2 IgM, case (0.85, P<0.001) and control (0.49, P<0.001) anti-FBP IgM and anti-FR IgM, case (0.68, P<0.001) and control (0.67, P<0.001) anti-FBP IgM and anti-FR.2 IgM, and case (0.88, P<0.001) and control (0.51, P<0.001) anti-FBP.2 IgM and anti-FR IgM. In regard to folic acid, the only significant correlation in both cases (0.51, P=0.006) and controls (0.27, P=0.02) was between the blocking of folic acid from binding of FBP (FBP FA) and antibodies against FBP (anti-FBP IgM).
Table 3.
Pearson correlation coefficients: controls (N=76), cases (N=29)
| FBP_IgG | FBP_IgM | FR_IgG_ | FR_IgM | FR.2_IgG | FR.2_IgM | FBP.2_IgG | FBP.2_IgM | FBP_FA blocked | FR_FA blocked | |
|---|---|---|---|---|---|---|---|---|---|---|
| FBP_IgG | 1 | 0.53 | 0.40 | 0.32 | 0.34 | 0.30 | 0.72 | 0.53 | 0.30 | −0.23 |
| p-value | 0.003 | 0.03 | 0.09 | 0.07 | 0.11 | <.0001 | 0.003 | 0.12 | 0.23 | |
| FBP_IgM | 0.28 | 1 | 0.60 | 0.85 | 0.60 | 0.68 | 0.56 | 0.94 | 0.51 | −0.01 |
| p-value | 0.01 | 0.0005 | <.0001 | 0.0007 | <.0001 | 0.002 | <.0001 | 0.006 | 0.95 | |
| FR_IgG | 0.22 | 0.10 | 1 | 0.73 | 0.88 | 0.88 | 0.61 | 0.68 | 0.83 | 0.14 |
| p-value | 0.11 | 0.48 | <.0001 | <.0001 | <.0001 | 0.0004 | <.0001 | <.0001 | 0.49 | |
| FR_IgM | 0.24 | 0.49 | 0.19 | 1 | 0.71 | 0.84 | 0.46 | 0.88 | 0.63 | −0.01 |
| p-value | 0.04 | <.0001 | 0.18 | <.0001 | <.0001 | 0.01 | <.0001 | 0.0003 | 0.97 | |
| FR.2_IgG | 0.03 | 0.05 | 0.31 | 0.08 | 1 | 0.93 | 0.62 | 0.72 | 0.90 | 0.04 |
| p-value | 0.79 | 0.66 | 0.02 | 0.49 | <.0001 | 0.0003 | <.0001 | <.0001 | 0.86 | |
| FR.2_IgM | 0.16 | 0.67 | 0.16 | 0.45 | 0.13 | 1 | 0.56 | 0.80 | 0.87 | 0.03 |
| p-value | 0.17 | <.0001 | 0.25 | <.0001 | 0.28 | 0.001 | <.0001 | <.0001 | 0.89 | |
| FBP.2_IgG | 0.40 | 0.26 | 0.56 | 0.13 | 0.61 | 0.28 | 1 | 0.62 | 0.57 | −0.12 |
| p-value | 0.0004 | 0.03 | <.0001 | 0.28 | <.0001 | 0.02 | 0.0004 | 0.001 | 0.56 | |
| FBP.2_IgM | 0.16 | 0.78 | 0.11 | 0.51 | 0.04 | 0.68 | 0.32 | 1 | 0.64 | −0.03 |
| p-value | 0.17 | <.0001 | 0.42 | <.0001 | 0.72 | <.0001 | 0.005 | 0.0003 | 0.86 | |
| FBP_FA blocked | 0.05 | 0.27 | 0.06 | 0.05 | 0.07 | 0.09 | 0.01 | 0.18 | 1 | 0.15 |
| p-value | 0.68 | 0.02 | 0.67 | 0.69 | 0.57 | 0.45 | 0.96 | 0.11 | 0.45 | |
| FR_FA blocked | −0.02 | 0.22 | 0.32 | 0.17 | 0.07 | 0.13 | 0.18 | 0.17 | 0.39 | 1 |
| p-value | 0.89 | 0.06 | 0.02 | 0.14 | 0.57 | 0.28 | 0.12 | 0.15 | 0.0005 |
The p-value indicates the probability of observing this correlation coefficient under the null hypothesis (H0) that the correlation (Rho) is 0 (Prob greater than |r| under H0: Rho=0). Significant p-values, less than 0.05, are presented in bold. The lower left half of the Table gives the correlation coefficients for controls, and the upper right half provides the correlation coefficients for cases.
4. Discussion
This study of mid-gestational serum samples from NTD-affected pregnancies compared to pregnancies involving non-malformed fetuses indicates that high concentrations of IgG or IgM antibodies to FRs or FBPs are risk factors for NTDs. During the fourth week of gestation, the neural tube closes and the human embryo is interstitially implanted into the uterine wall, and migration of syncitiotrophoblasts and cytotrophoblasts has formed chorionic villi that cover the entire surface of the blastocyst (Cross et al., 1994). The cytotrophoblasts of these villi have been observed to function as a barrier to the transport of IgG during this stage of development (Bright and Ockleford, 1995). These observations suggest that, during neural tube closure, IgM and IgG antibodies are prohibited from reaching the embryo. However, autoantibodies may indirectly influence neural tube closure and NTD risk. These indirect influences would include IgG or IgM immune responses at the maternal-embryonic trophoblast interface and inhibition of nutrient transport to the blastocyst. In support of inhibition of nutrient transport, we observed also that the serum from mothers who delivered an NTD-affected child significantly blocked higher concentrations of folic acid from binding to FBP and FR versus serum from mothers of an unaffected child. However, several samples with high titers of antibodies allowed FR and FBP still to bind folic acid. This may indicate that the antibodies can bind FR and FBP at different locations, but they must interact with specific epitopes in order to inhibit folic acid binding. In support of direct and indirect influences, binding to or blocking of folic acid to FR increased NTD risk estimates to the greatest extent. Specifically, IgM autoantibodies, folic acid blocking and IgG autoantibodies against FR had the highest estimates of NTD risk at two standard deviations (Table 2).
The IgM and IgG concentrations are highly correlated in all case samples and in FBP.2 in controls (Table 3). This may indicate an ongoing primary immune response, which begins with IgM production and is followed by IgG (Perelson et al., 1980). With regard to the positive association of antibodies for FRs and FBPs, the >80% homology between these proteins (determined by blastp (Tatusova and Madden, 1999); FBP, GI: 110282963; FR, GI: 544337) may allow cross-reaction with most serum IgM and some IgG antibodies, resulting in significant correlations between the different proteins tested. However, the similarities of these proteins does not explain the difference in the number of significant correlations observed in controls (N=18) versus cases (N=32). This difference in significant correlations may be caused by a decrease in antibody specificity, more cross-reactivity or a more concerted immune response in cases relative to controls.
The forbidden clone hypothesis indicates that autoimmunity is mitigated by clonal selection (Burnet, 1972) but, if proteins expressed by developmental tissues undergo modification during periods of inflammation or metabolic stress, an immune response may be activated against the modified forms (McCully, 1993,1994; Undas et al., 2004). Folic acid supplementation has been reported to lower homocysteine, homocysteine thiolactone and autoantibodies against homocysteinylated serum proteins in a majority of patients with coronary artery disease (Undas et al., 2006). Based on these observations, we suspect that the etiology of FR autoantibodies may be due in part to post-translation modification of proteins by homocysteine thiolactone in vivo and that autoantibodies produced against homocysteinylated FRs may cross-react with native FR and increase the risk for folate responsive birth defects, such as NTDs. As proposed and observed previously, these post-translation modification may also be part of other autoimmune phenomenon (Cabrera et al., 2004; Perla-Kajan et al., 2007). Specifically, the production of antigenic proteins by homocysteine thiolactone may influence the etiology of autoantibodies to synovial cells in arthritis, antiphospholipids in lupus erythematosus, thyroid stimulating hormone receptor in Grave’s disease and intrinsic factor in pernicious amemia (Lazzerini et al., 2007; McCully, 1994).
A previous study identified an association (relative risk of 27.0) between FR autoantibodies and NTDs, with a reported frequency of 10% for the presence of autoantibodies among controls (Rothenberg et al., 2004). We observed continuous distributions of antibodies amongst cases and controls, all mean antibody concentrations for cases were higher than controls, and several concentrations differed significantly (Table 2). The major difference between these experiments were serum collection times (i.e. only three mid-gestational serum samples from women with a pregnancy complicated by a neural tube defect and no mid-gestational serum controls were used in the other study). We utilized also smaller testing volumes (2–10µL), 96-well processing and enzymatic detection for the presence or absence of the autoantibodies (anti-IgG-AP or anti-IgM-AP) and folic acid (FA-HRP). Additionally, we observed that the source of the FRs or FBPs can modify the detected interactions with serum antibodies (Table 2 and 3).
This study was conducted during a time period when the food supply was fortified with folic acid, so this may explain the low ~1/4700 incidence of NTDs and also makes it unlikely that all of the observed NTDs are folate-responsive. However, if folic acid supplementation reduces the homocysteinylation of other proteins critical for embryonic development, antibodies against these proteins may also modify birth defect risk. Untangling these complex interactions and investigating methods that reduce the level of pathogenic autoantibodies will serve as the subject for future studies.
Acknowledgments
The authors are grateful to Drs. Jacob Selhub and Bart Kamen for providing folate receptors for these experiments. This work was partially supported by the NIH/NHLBI P30 HL66398, and by funds from the Centers of Disease Control and Prevention, Centers of Excellence Award no. U50/CCU913241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antony AC, Utley C, Van Horne KC, Kolhouse JF. Isolation and characterization of a folate receptor from human placenta. J. Biol. Chem. 1981;256:9684–9692. [PubMed] [Google Scholar]
- Antony AC, Utley CS, Marcell PD, Kolhouse JF. Isolation, characterization, and comparison of the solubilized particulate and soluble folate binding proteins from human milk. J. Biol. Chem. 1982;257:10081–10089. [PubMed] [Google Scholar]
- Barber RC, Shaw GM, Lammer EJ, Greer KA, Biela TA, Lacey SW, Wasserman CR, Finnell RH. Lack of association between mutations in the folate receptor-alpha gene and spina bifida. Am. J. Med. Genet. 1998;76:310–317. [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler J, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention [corrected; erratum to be published] N. Engl. J. Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am. J. Epidemiol. 2000;151:862–877. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- Bright NA, Ockleford CD. Cytotrophoblast cells: a barrier to maternofetal transmission of passive immunity. J. Histochem. Cytochem. 1995;43:933–944. doi: 10.1177/43.9.7642966. [DOI] [PubMed] [Google Scholar]
- Burnet FM. A reassessment of the forbidden clone hypothesis of autoimmune disease. Aust. J. Exp. Biol. Med. Sci. 1972;50:1–9. doi: 10.1038/icb.1972.1. [DOI] [PubMed] [Google Scholar]
- Cabrera RM, Hill DS, Etheredge AJ, Finnell RH. Investigations into the etiology of neural tube defects. Birth Defects Res. C. Embryo Today. 2004;72:330–344. doi: 10.1002/bdrc.20025. [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Jensvold NG, Harris JA. Birth defects monitoring in California: a resource for epidemiological research. Paediatr. Perinat. Epidemiol. 1991;5:423–427. doi: 10.1111/j.1365-3016.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- da Costa M, Rothenberg SP. Purification and characterization of folate binding proteins from rat placenta. Biochim. Biophys. Acta. 1996;1292:23–30. doi: 10.1016/0167-4838(95)00180-8. [DOI] [PubMed] [Google Scholar]
- da Costa M, Sequeira JM, Rothenberg SP, Weedon J. Antibodies to folate receptors impair embryogenesis and fetal development in the rat. Birth Defects Res. A Clin. Mol. Teratol. 2003;67:837–847. doi: 10.1002/bdra.10088. [DOI] [PubMed] [Google Scholar]
- Ebel W, Routhier EL, Foley B, Jacob S, McDonough JM, Patel RK, Turchin HA, Chao Q, Kline JB, Old LJ, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6. [PMC free article] [PubMed] [Google Scholar]
- Henderson GB, Strauss BP. Growth inhibition by homofolate in tumor cells utilizing a high-affinity folate binding protein as a means for folate internalization. Biochem. Pharmacol. 1990;39:2019–2025. doi: 10.1016/0006-2952(90)90624-t. [DOI] [PubMed] [Google Scholar]
- Jones ML, Nixon PF. Tetrahydrofolates are greatly stabilized by binding to bovine milk folate-binding protein. J. Nutr. 2002;132:2690–2694. doi: 10.1093/jn/132.9.2690. [DOI] [PubMed] [Google Scholar]
- Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q. J. Med. 1993;86:703–708. [PubMed] [Google Scholar]
- Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun. Rev. 2007;6:503–509. doi: 10.1016/j.autrev.2007.03.008. [DOI] [PubMed] [Google Scholar]
- McCully KS. Chemical pathology of homocysteine. I. Atherogenesis. Ann. Clin. Lab. Sci. 1993;23:477–493. [PubMed] [Google Scholar]
- McCully KS. Chemical pathology of homocysteine. III. Cellular function and aging. Ann. Clin. Lab. Sci. 1994;24:134–152. [PubMed] [Google Scholar]
- Mendoza LG, McQuary P, Mongan A, Gangadharan R, Brignac S, Eggers M. High-throughput microarray-based enzyme-linked immunosorbent assay (ELISA) Biotechniques. 1999;27:778–780. 782–786, 788. doi: 10.2144/99274rr01. [DOI] [PubMed] [Google Scholar]
- Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, Rothman KJ, Willett W. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA. 1989;262:2847–2852. doi: 10.1001/jama.262.20.2847. [DOI] [PubMed] [Google Scholar]
- MRC; MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Perelson AS, Goldstein B, Rocklin S. Optimal strategies in immunology III. The IgM-IgG switch. J. Math. Biol. 1980;10:209–256. doi: 10.1007/BF00276984. [DOI] [PubMed] [Google Scholar]
- Perla-Kajan J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007;32:561–572. doi: 10.1007/s00726-006-0432-9. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, Finnell RH. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat. Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Rothenberg SP, da Costa MP, Sequeira JM, Cracco J, Roberts JL, Weedon J, Quadros EV. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N. Engl. J. Med. 2004;350:134–142. doi: 10.1056/NEJMoa031145. [DOI] [PubMed] [Google Scholar]
- Schwartz RS. Autoimmune folate deficiency and the rise and fall of "horror autotoxicus". N. Engl. J. Med. 2005;352:1948–1950. doi: 10.1056/NEJMp058034. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Rozen R, Finnell RH, Wasserman CR, Lammer EJ. Maternal vitamin use, genetic variation of infant methylenetetrahydrofolate reductase, and risk for spina bifida. Am. J. Epidemiol. 1998;148:30–37. doi: 10.1093/oxfordjournals.aje.a009555. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RPM, Boers GHJ, Trijbels FJM, Finkelstein JD, Blom HJ, Thomas CMG, Borm GF, Wouters MGAJ, Eskes TK. Maternal hyperhomocysteinemia: a risk factor for neural tube defects? Metabolism. 1994;43:1475–1480. doi: 10.1016/0026-0495(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Tang LS, Finnell RH. Neural and orofacial defects in Folp1 knockout mice [corrected] Birth Defects Res A Clin. Mol. Teratol. 2003;67:209–218. doi: 10.1002/bdra.10045. [DOI] [PubMed] [Google Scholar]
- Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- Tsukruk VV, Luzinov I, Julthongpiput D. Sticky Molecular Surfaces: Epoxysilane Self-Assembled Monolayers. Langmuir. 1999;15:3029–3032. [Google Scholar]
- Undas A, Perla J, Lacinski M, Trzeciak W, Kazmierski R, Jakubowski H. Autoantibodies against N-homocysteinylated proteins in humans: implications for atherosclerosis. Stroke. 2004;35:1299–1304. doi: 10.1161/01.STR.0000128412.59768.6e. [DOI] [PubMed] [Google Scholar]
- Undas A, Stepien E, Glowacki R, Tisonczyk J, Tracz W, Jakubowski H. Folic acid administration and antibodies against homocysteinylated proteins in subjects with hyperhomocysteinemia. Thromb. Haemost. 2006;96:342–347. doi: 10.1160/TH06-04-0228. [DOI] [PubMed] [Google Scholar]
- van der Put NMJ, Steegers-Theunissen RPM, Frosst P, Trijbels FJM, Eskes TK, van den Heuvel LP, Mariman ECM, den Heyer M, Rozen R, Blom HJ. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet. 1995;346:1070–1071. doi: 10.1016/s0140-6736(95)91743-8. [DOI] [PubMed] [Google Scholar]
- Waddell CC, Domstad PA, Pircher FJ, Lerner SR, Brown JA, Lawhorn BK. Serum folate levels. Comparison of microbiologic assay and radioisotope kit methods. Am. J. Clin. Pathol. 1976;66:746–752. doi: 10.1093/ajcp/66.4.746. [DOI] [PubMed] [Google Scholar]
- Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269:1257–1261. [PubMed] [Google Scholar]
- Yates JR, Ferguson-Smith MA, Shenkin A, Guzman-Rodriguez R, White M, Clark BJ. Is disordered folate metabolism the basis for the genetic predisposition to neural tube defects? Clin. Genet. 1987;31:279–287. doi: 10.1111/j.1399-0004.1987.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Zettner A, Duly PE. Relative efficacy of separation of "free" and "bound" (3′,5′-3H) pteroylglutamate by charcoal coated with various materials. Clin. Chem. 1975;21:1927–1931. [PubMed] [Google Scholar]
- Zhu H, Wicker NJ, Shaw GM, Lammer EJ, Hendricks K, Suarez L, Canfield M, Finnell RH. Homocysteine remethylation enzyme polymorphisms and increased risks for neural tube defects. Mol. Genet. Metab. 2003;78:216–221. doi: 10.1016/s1096-7192(03)00008-8. [DOI] [PubMed] [Google Scholar]