Abstract
Nanopores can be used to detect and analyse biomolecules. However, controlling and tuning the translocation speed of molecules through a pore is difficult, limiting the wider application of these sensors. Here we show that low-power visible light can be used to control surface charge in solid-state nanopores and can influence the translocation dynamics of DNA and proteins. We find that laser light precisely focused at a nanopore can induce reversible negative surface charge densities as high as 1 C/m2, and that the effect is tuneable on sub-millisecond timescales by adjusting the photon density. By modulating surface charge, we can control the amount of electro-osmotic flow through the nanopore, which affects the speed of translocating biomolecules. In particular, a few mW of green light can reduce the translocation speed of double-stranded DNA by more than an order of magnitude and the translocation speed of small globular proteins such as ubiquitin by more than two orders of magnitude. The laser light can also be used to unclog blocked pores. Finally, we discuss a mechanism to account for the observed optoelectronic phenomenon.
Introduction
The manipulation of electrical surface charge in a rapid and reversible manner is of great importance for a wide variety of biosensing applications, since electrostatic interactions influence the affinity and residence time of biological molecules in the sensing zone.1 Charge manipulation in nanoscale sensors, such as nanopores, nanochannels and nanotubes,2-5 is particularly useful because of the scale of these devices. Solid-state nanopores are a prototypical class of nanoscale sensors, employing electrophoretic forces to thread and translocate charged single nucleic acids or proteins through a nanoscale aperture in an ultrathin membrane.6-8 Nanopore surface charge can affect its direct, Coulombic interactions with charged biomolecules, and can indirectly influence sensing by generating electro-osmotic flow. This net flow can modulate molecular capture rate9 and molecular residence time in the sensing vicinity via hydrodynamic drag.10,11 This effect has previously been characterized using optical tweezers12 and by varying the nanopore’s zeta potential by changing the solution’s pH.13
The ability to modulate the surface charge of a nanopore and, consequently, the associated electro-osmotic flow could allow control of biomolecule translocation speed. This could in turn lead to the development of advanced single molecule, low-cost characterization techniques for a broad spectrum of clinical samples, including genomic DNA, RNAs and proteins.14 This challenge must be met without compromising the ability of the sensor to probe extremely dilute analytes in a reasonable time frame.15 Recent efforts towards this goal have primarily involved the fabrication of metallic electrodes at the nanopore surface to induce an electrical field.16,17 Yet to date, there has been no experimental report of in situ, opto-electrical manipulation of high-density surface charge in nanopores or other nanoscale sensors.
We show here that an off-the-shelf, low-power visible laser beam can be used to directly manipulate the surface charge of a solid-state nanopore. The ability to dynamically increase surface charge has three immediate useful consequences. First, it allows the translocation speed of DNA to be tuned without chemically modifying the nanopore surfaces or altering the buffer properties. Second, it can be used to reliably unblock clogged nanopores, keeping them functional over prolonged periods of time. Third, it permits the detection of extremely low molecular weight proteins, which otherwise are nearly invisible to the resistive pulse sensing technique.
The photo-conductive phenomenon in solid-state nanopores
A silicon nitride membrane containing a single nanopore was scanned in a custom-made confocal microscope with a tightly-focused green laser (532 nm), as illustrated in Figure 1a. The continuously recorded ionic current (I) flowing through the pore at a fixed voltage reveals an increase when the laser spot overlaps with the nanopore location. Figure 1b illustrates this effect with an intensity surface plot of the ionic current I flowing through this pore as a function of laser spot position. A line scan through the image reveals a clear symmetric peak in the ionic current, more than double the baseline current level (FWHM ~500 nm, right panel).
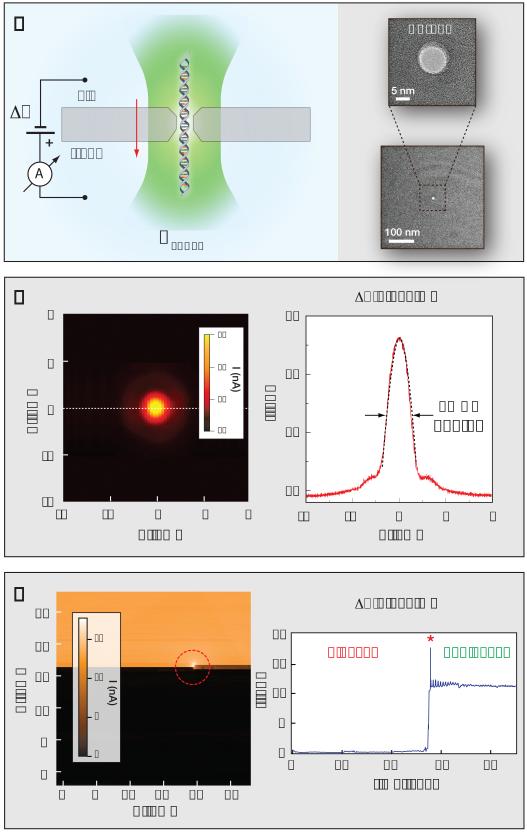
Figure 1. The photo-conductive effect in solid-state nanopores.
a) Cartoon illustrating the optical nanopore setup. A solid state nanopore is positioned at a laser beam focus by a nano-positioner, and the ion-current flowing through the pore is measured before and during translocation of DNA molecules. The inset shows a high-resolution Transmission Electron Micrographs of a typical pore (10 nm). b) Left: surface plot showing nanopore ionic current enhancement as a 10 mW focused laser beam (λ = 532 nm) scans the 4 × 4 μm2 SiN membrane at 1 μm/s. When the focused beam reaches the nanopore it produces a significant increase in measured current (2.4-fold increase for this pore). Right: line profile through Y=0, fitted using a Gaussian function with FWHM of ~500 nm, consistent with the diffraction limit. c) Clearing a blocked solid-state nanopore with light. Left: a 5 mW laser intensity raster scan of the entire SiN window (30 × 30 μm2, lower left to top right). The color map represents the current flowing through the pore at ΔV = 300 mV. As soon as the laser beam overlaps with the nanopore location, it clears the pore and thereafter the ionic current stabilizes at the open pore level of 12 nA. Right: time trace of I during the laser scan.
This effect is evident in all tested pores (4-20 nm diameter) even for laser powers of just a few mW. Although a tightly focused IR laser at high power (~1 W) can produce a small increase in current by locally heating the solution,18 laser-induced heating can be ruled out here for two reasons: the electromagnetic absorption of water at 532 nm is extremely small,19 and the laser powers used in our experiments are only a few mW. A temperature increase of ~35°K would be required to produce the observed increase in I, yet heating is estimated to account for a mere 0.005°K per mW of focused light based on measurements performed at 800 nm.20 We thus conclude that direct interaction of light with silicon nitride must cause this increase in the ion current via a photo-conductive effect.
Permanent nanopore blocking by small molecules, tiny air bubbles, or other nanoscale particulates is a common limitation for nanopore sensors, severely reducing their functional lifetime. In some cases, a series of electrical pulses clears the pore,21 but often the system must be disassembled and re-cleaned. We find that in many of these cases a short exposure to a ~5 mW focused laser beam will immediately clear the nanopore. Figure 1c (left) shows a raster scan (bottom left to top right) of a fully blocked nanopore (I ≈ 0 at V = +300 or −300 mV). As soon as the laser beam reaches the nanopore location, the pore is cleared. Thereafter, I stabilizes at the open pore level of 12 nA. This is shown chronologically (Figure 1c, right) as a time trace of I during the laser scan. We have found that light-induced nanopore unblocking is a highly robust and efficient tool to extend the functional lifetime of solid-state nanopores from a few hours to several days, as detailed in the Supporting Information (SI).
Light-induced retardation of DNA and proteins
Over 1,000 translocation events of 10 kbp DNA were acquired in a nanopore (5.4 nm) in the dark (laser power P=0). At t=150 s the laser radiation was switched on (P=2 mW, pre-aligned with nanopore) as the software continued to record translocation events. Representative events before, during, and after illumination are shown in Figure 2a. Three features are immediately apparent: (i) the open pore current (IO) and the current during translocation (IB) both increased when the laser was turned on, (ii) the average event amplitude ΔI = IO − IB remained at the same level of 0.78±0.10 nA under both conditions, and (iii) the mean event dwell-time tD increased by roughly a factor of 10 under illumination, as compared to dark conditions. These features are illustrated in Figure 2b, which shows IO and ΔI as a function of time throughout the experiment (top panel). A 150-event running window average over all translocations (bottom panel) indeed shows a tenfold increase in the translocation time from ~1 ms to ~10 ms under laser illumination. We define the Retardation Factor (RF; here RF=10) as the increase in mean translocation time under laser light relative to darkness. Upon switching off the laser illumination at t=300 s, IO returned to base level (3.6 nA) and the mean translocation dwell time was restored to ~1 ms, suggesting that this effect is completely reversible. This experiment was repeated for nanopores of various sizes, and in all cases we observed a marked retardation of translocation time under laser illumination (see SI for analysis).
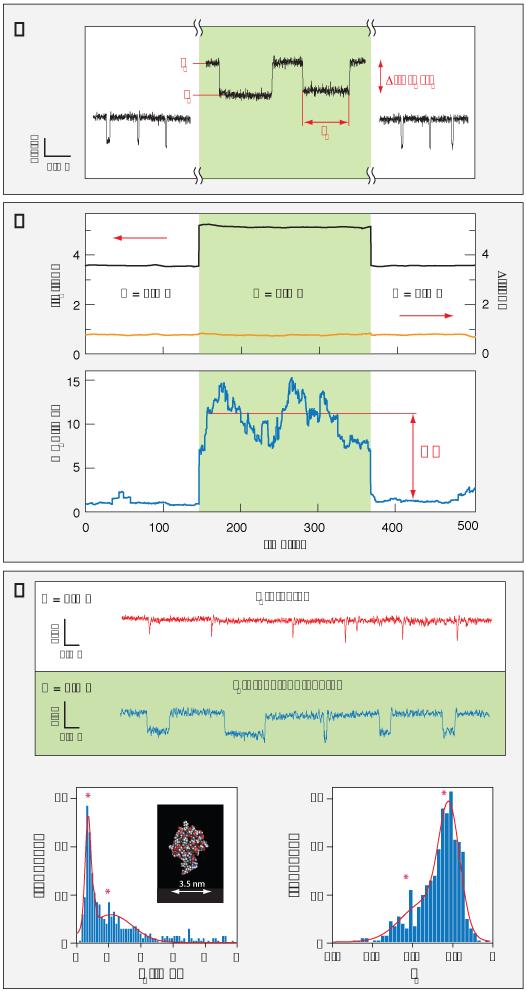
Figure 2. Slowing down DNA and protein translocation speed with light.
a) Representative translocation events with and without illumination, showing the increase in IO, IB and tD. b) Time traces showing the open pore current IO, the blocked current amplitude ΔI (top panel), and the mean translocation time <tD> (lower panel). The net effect of the laser illumination is to increase IO and tD while keeping ΔI constant. All data points in <tD> represent a running average over 150 translocation events, initialized at the moment the laser is switched on/off. The Retardation Factor, RF, defined as the mean tD with light divided by the mean tD in darkness. In this example a factor of ~10 is obtained with P = 2 mW. c) Detection of the small molecular weight protein ubiquitin in its native state using a 5 nm nanopore, enabled by illumination of the chip with laser light. Typical translocation time traces are shown at P = 0 (red) and P = 4 mW (blue). With P = 4 mW we observe at least two orders of magnitude increase in the events’ dwell times. A typical translocation time distribution and fractional blockade current (IB) of the ubiquitin under 4 mW of focused light are shown at the bottom (N > 500). Under these conditions, we typically observe two prominent timescales for ubiquitin translocation (340±5 μs and 890±70 μs), as well as two peaks in the blockade currents (0.88 and 0.78), approximated by a sum of two Gaussians (black lines). The inset is a cartoon of the crystallographic structure of wild type human ubiquitin (PDB 1d3z).
We next tested the photo-conductive effect for analytes other than DNA. Previous studies have sensed proteins such as bovine β-lactoglobulin or avidin13,22 in solid-state nanopores, and antibodies such as anti-biotin Fab fragments or anti-biotin in lipid coated nanopores.23 However, the detection of small proteins (MW < 10 kDa) in their native state remains a major challenge, since these proteins translocate through the pore too quickly to produce a resolvable signal given current bandwidth limitations.24
We selected ubiquitin (MW ~ 8.5 kDa, 4 nm diameter) as a representative small protein, relevant to a broad range of biological processes,25 to demonstrate that the photo-conductive effect can slow small protein translocations and enable their detection and characterization. Under dark conditions, purified wild-type ubiquitin (see SI) translocating through a ~5 nm pore yielded sporadic, brief downward spikes (red trace, Figure 2c). The dwell time of these spikes was estimated to be <12 μs, but neither this nor the current amplitude of ubiquitin translocations could be accurately determined due to bandwidth constraints even at 100 kHz,26 suggesting that the true translocation dwell time of these proteins is shorter than estimated.
Laser illumination of the same nanopore at P=4 mW resulted in a marked increase in both the frequency and average dwell time of detected ubiquitin events (blue trace, Figure 2c). The average translocation time increased by at least two orders of magnitude, allowing full characterization of both amplitude and dwell times for ubiquitin translocations. The increase in event rate under illumination may be attributed to events that were previously undetectable under dark conditions due to bandwidth limitations. As with DNA translocations, this effect was completely reversible.
A typical dwell time distribution and fractional blockade current (IB) for illuminated ubiquitin translocations are shown in Figure 2c (N > 500). Two prominent timescales are observed for ubiquitin translocation (340±5 and 890±70 μs), as well as two peaks in the blockade currents (0.88 and 0.78), approximated by a sum of two Gaussians. We hypothesize that the two peaks observed in these distributions may be associated with different orientations of the asymmetric “mushroom-like” ubiquitin (inset) during translocation. This hypothesis is consistent with previous reports for other protein/DNA complexes.27,28
Light-induced modulation of electro-osmotic flow
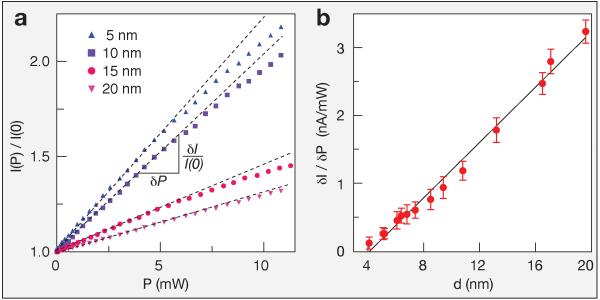
To elucidate the physical origin of light-induced retardation of translocation, we first characterized the pore current I at varying laser intensity P for a range of nanopore sizes (4-20 nm diameter, d). Figure 3a displays ionic current enhancement (I(P)/I(0)), for four representative pores (d = 5, 10, 15, 20 nm). In all cases I is linear for P<5 mW, above which I deviates slightly from linearity. Linear fits for P<5 mW characterize the response of each pore to light as the initial slope δI/δP. A plot of δI/δP as a function of diameter for 14 pores (4-20 nm) exhibits a clear linear dependence on d (Figure 3b). This suggests that when the SiNx thickness L and nanopore drilling conditions are maintained, the pores response to light per their surface area (πdL) is a constant. From Figure 3b we obtain a value of 2.2×10−3 nA mW−1 nm−2.
Figure 3. Ionic current enhancement as a function of laser power and pore diameter.
a) Plots of the ionic current enhancement I(P)/I(0) as a function of laser power for a 5, 10, 15, and 20 nm-diameter pores, as indicated. b) Pore response to light, δI/δP, as obtained from linear fits to plots of I(P), as a function of pore diameter. The dependences on the current enhancement shown in a) and of the pore response to light shown in b) on pore size suggest that the origin of the photo-conductance effect resides on the pore’s surface (i.e. pore walls) and not on its volumetric content.
The linear dependence of δI/δP on nanopore diameter suggests that photo-conductance current enhancement is a surface phenomenon rather than a volumetric one (which would exhibit ∝ d2 dependence). Solid-state nanopores possess weak surface charge, which can dominate pore conductivity at salt concentrations lower than ~100 mM.29-31 However, it has been theorized that high charge densities on nanopore walls can affect electrical conductivity even under high salt conditions.32,33 Surface charges induce a diffuse double layer containing an excess of oppositely charged ions, of thickness comparable to the Debye screening length κ−1. Ionic current is enhanced by the electrical double layer in two ways: (i) by creating a local imbalance of counter ions within κ−1, which (ii) drags water molecules with its motion, creating an electro-osmotic flow (EOF) which in turn pulls along additional current (see Figure 4a). The total ionic current for a nanopore with a charged surface is:
| (1) |
where Ibulk is the current due to ions outside the electrical double layer, IDL is the current due to ions forming the double layer, and IEOF is the current due to net water flow induced by net movement of ions within the double layer. IEOF is proportional to the product of the flow velocity field and the net charge density ρ(r), which is only nonzero within κ−1. For small κ−1 compared to the pore diameter, (i.e. κ−1≈0.3 nm at 1M KCl at 21°C), only Ibulk scales with cross sectional area (Ibulk ∝ πd2) whereas IDL and IEOF scale with circumference (IDL, IEOF ∝ πdκ−1). Thus, surface charges can, in principle, explain increased I as well as its linear scaling with d. Moreover, if the analyte remains mostly within the bulk pore volume where there is no charge imbalance, κ−1 distance away from the pore walls, we expect ΔI to be independent of surface charge – and thus independent of P. This is consistent with our observations (Figure 2).
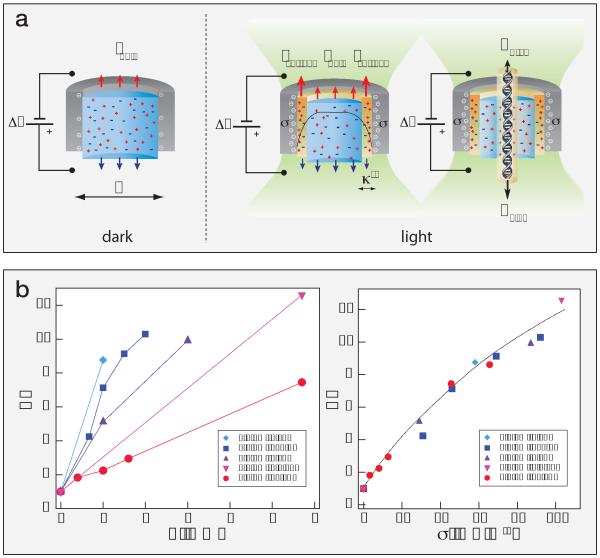
Figure 4. Light-induced surface charge density modulates the EOF and DNA translocation speed.
a) Cartoons showing the bulk and surface ionic current terms discussed in the text, and the origin of the electro osmotic force acting to retard the DNA translocation for the same nanopore in darkness (left) and under laser illumination (right). Here Isurface = IDL + IEOF (Eq.1) b) Left, the Retardation Factor (RF) as a function of laser intensity measured for different nanopore sizes: 4.3, 5.4, 5.6, 6.1, and 7.4 nm (diamonds, squares, upward triangles, circles and downward triangles, respectively) and DNA lengths: 400 bp (squares), 3.5 kbp (downward triangles), 5 kbp (upright triangles and diamonds), and 10 kbp (circles). Each data point was calculated from at least 1,000 events per laser intensity. Right, the RF as a function of the nanopore surface charge density calculated using the open pore current versus laser intensity for each pore (Eq. 2). Remarkably, all data points collapse onto a single curve, regardless of DNA length or nanopore diameter.
Previous theoretical work has predicted that surface charge on a nanopore will affect (i) translocation times10,11 and (ii) polymer capture rate.9,34 A negatively charged nanopore produces an EOF towards the cathode (cis chamber, Figure 4a), increasing drag on a negatively charged translocating polymer, resulting in longer translocation times and reduced polymer capture rates. Conversely, positive surface charges would enhance capture rate and reduce translocation times. Our results (Figure 2) indicate that the RF for DNA translocation grows with laser intensity. Moreover, DNA capture rate was reduced by 30%, from 11 s−1 to 8 s−1, for 2 mW of laser light (see SI). These observations lead us to hypothesize that visible light can induce negative surface charges on silicon nitride, which in turn slows DNA translocation through the drag created by EOF moving in the opposite direction of DNA.
To validate this hypothesis we approximate that for low laser powers the surface charge density (σ) grows linearly with laser intensity, such that σ = γ P where γ is the photo-reactivity of the pore (C m−2 W−1). The total ionic current I can be obtained as a function of surface charge density using the following three individual contributions (see SI for full derivation): (i) Ibulk is the electrophoretic movement of each ion species through the nanopore; (ii) IDL may be determined from the net charge distribution ρ for a given wall potential, derived analytically from the Poisson-Boltzmann equation and from the Boltzmann distribution, assuming a cylindrical nanopore and using the Debye-Hückel approximation;9-11 (iii) IEOF is a product of ρ and the EOF velocity profile described by the Navier-Stokes equation. The Grahame equation may be substituted to give σ as a function of wall potential.35 The contributions to the ion current are:
| (2.1) |
| (2.2) |
| (2.3) |
for elementary charge e, permittivity of aqueous solution ε, parameter β = 1/kBT (Boltzmann constant kB, absolute temperature T), pore length L, applied voltage clamp ΔV, and solution viscosity η. The number density of potassium or chloride ions is nKCl, with electrophoretic mobilities μK and μCl (see SI for numerical values). Nanopore diameter d can be determined from TEM images, enabling a quantitative estimation of the nanopore photo-reactivity γ via Eq.2 (see SI Figure S7). We additionally measured the dependence of I on KCl concentration (0.01 M to 1 M) at different P and found it consistent with the model (see SI Figure S8).
Additional DNA translocations were performed using a range of DNA lengths, nanopores, and laser powers to determine the role of surface charge in slowing translocation speed. The results are summarized in Figure 4b (left), where RF is plotted against P for five nanopores (4.3, 5.4, 5.6, 6.1, and 7.4 nm diameters) and four DNA lengths (0.4, 3.5, 5, and 10 kbp). tD was measured for >1,000 events per laser intensity, and the tD histograms were fitted with exponential functions as previously described.26 These measurements suggest that RF per mW of light widely varies with pore size and DNA length.
Fitting Eq. 2 as described above to obtain γ for each of the pores, we then transform the DNA RF into surface charge density σ using the relationship σ = γ P. Remarkably, all data points collapse onto a single curve (Figure 4b, right). Thus, light-induced slowing is dependent only on the induced surface charge density σ. Controlling the RF value can be achieved either by selecting a highly optically reactive pore (high γ) under low laser intensity, or using a less optically reactive pore under higher laser intensity.
Nanopore photo-reactivity depends on e-beam exposure during the formation of the nanopore
Drilling nanopores in SiNx with an electron beam reduces the N/Si ratio around the pore due to preferential ablation of N atoms.36 Moreover, the principal defects in silicon-rich CVD amorphous SiNx materials are Si dangling bonds, which can trap electrons or holes to become charged.37-39 When the N/Si ratio approaches 0.8, the band gap is within visible light energies.39,40 When the N/Si ratio of the material surrounding the pore is low enough, we propose that visible light can excite electrons from the ground state across the band gap, trapping them in Si dangling bonds.41 Eventually, trapped electrons would recombine with holes, but a high density of arriving photons could maintain a steady state of negatively charged Si dangling bonds, creating a net surface charge density.
We TEM-drilled four additional nanopores, all with similar diameters, in a freshly deposited SiNx membrane. Two were exposed to the e-beam for 60 s, and two were exposed for 500 s, producing different local N/Si stoichiometry (see SI).42 As expected, the pores drilled with a low e-beam dose had relatively low photo-reactivity (γ=27 C m−2 W−1), whereas the pores exposed to a high e-beam dose had much higher photo-reactivity (γ=70 C m−2 W−1), establishing a direct correlation between e-beam dosage and optical reactivity.
Conclusions
We have shown that just a few mW of visible laser light focused on a nanopore can induce surface charge densities up to ~1 C m−2, much larger than the weak native charge of SiNx. Increased σ produces net charge near the pore walls, which, under external voltage, creates an electro-osmotic flow moving in opposition to an anionic translocating molecule. This flow slows translocating DNA and proteins, allowing detection and characterization of small analytes, such as ubiquitin. Previous approaches to slowing analyte translocation have introduced permanent modifications to the nanopore walls, inducing stronger interactions of analytes with the pore surface during translocation.13,23,43,44 By contrast, the optoelectronic effect allows completely reversible, in-situ control of σ and translocation speed without permanently altering the surface properties of the nanopore or buffer.45,46 This is particularly useful for detection and characterization of molecules in their native form. Moreover, ultra-fast tuning of surface charge by light completely decouples the capture process from subsequent translocation. Solid-state lasers, as well as other light sources, can be readily switched on/off in sub-μs timescales, and hence induce current jumps in the nanopores within milliseconds (see SI). The dynamic range of the photoconductive effect is determined by local N/Si stoichiometry, which in turn depends on e-beam exposure during nanopore drilling. We predict that this method will facilitate a broad range of emerging nanopore sensing applications, including genotyping using Peptide Nucleic Acid (PNA) probes47 and quantification of epigenetic markers.48,49
Ultra-fast and reversible modulation of surface charge is fundamentally important for the development of novel nanosensors. By exploiting the intrinsically high sensitivity of the nanopore ionic current to small modulations of surface charge, we were able to explore and characterize a new optoelectronic effect, model the increase in electric conductance under visible laser illumination, and propose an underlying mechanism. We have shown here three useful applications of the technique: (i) clearing blocked nanopores and thus significantly extending their useful lifetime; (ii) slowing and tuning the translocation speed of DNA; and (iii) detecting and characterizing translocation events of very small proteins, such as ubiquitin. Together, these milestones broaden the range of molecules that can be probed by solid-state nanopores, enhance nanopore sensitivity, and extend the lifetime and throughput of individual pores.
Methods
Nanopores were drilled on low-stress (silicon-rich), amorphous LPCV-deposited SiNx membranes 30 nm thick. Chips were then cleaned using Piranha solution and kept in water until mounted on the Teflon holder, rendering two independent chambers connected by the nanopore and filled with an electrolytic solution (1M KCl, 20 mM TRIS, pH 8.0, 21° C). A pair of Ag/AgCl electrodes was used to apply an electrostatic potential difference across the chambers separated by the insulating SiNx membrane. The resulting ionic current through the nanopore was recorded using a patch-clamp amplifier (Axopatch 200B, Molecular Devices Cooperation). The whole apparatus was shielded from external electromagnetic noise by a Faraday cage. The electrical readout from the amplifier was filtered with a 10 kHz low-pass Bessel filter and digitized using a 16 bit A/D card (National Instruments) controlled by a custom-written LabView (National Instruments) program (see SI for a detailed diagram of the apparatus).
The nano-chip was secured to a closed loop nano-positioner (Physik Instrumente) with sub-nm accuracy, also controlled by the LabView program. The nano-positioner was mounted in a custom-built confocal setup, in order to illuminate the nanopore with a focused laser beam. A 532 nm line from a laser diode (New Focus) was cleaned by a Glan-Thompson polarizer (Thorlabs), with the final power adjusted on demand by a half-wave plate mounted on a motorized rotating holder. This resulted in a final dynamic range of 2 μW to 17 mW with 500 intermediate values. The laser beam was expanded to completely fill the back aperture of a 60×, 1.2 NA water immersion objective (Olympus UPlanApo). The emission side contained a long-pass filter (Chroma HQ560LP) to remove any elastically scattered light, followed by a motorized mirror that acted as a shutter for the APDs and allowed direct visualization of the membrane using a CCD camera (Thorlabs DC111).
Supplementary Material
Acknowledgements
We acknowledge support for this work by NIH (NHGRI) grant number R01 HG-005871, from the Marie Curie People award GA-2010-277060 (ERC) and by the Israeli Centers of Research Excellence (I-CORE) program (Center #1902/12). We thank the staff at the Harvard University Center for Nanoscale Sciences (CNS) and the Technion Electron Microscopy Center for dedicated support.
Footnotes
Author Contributions
N.D.F. and A.M. conceived and designed the experiments; N.D.F., D.B. and T.G. performed the experiments; A.S. drilled all pores; N.D.F., A.S., D.B., T.G. and A.M. analyzed the data; N.D.F., T.M. and A.M. developed the model; N.D.F., A.S., D.B., T.G., T.M. and A.M. co-wrote the paper.
Supplementary information is available in the online version of the paper.
Reprints and permission information is available online at www.nature.com/reprints.
References
- 1.Zhang X, Ju H, Wang J. Electrochemical Sensors, Biosensors and their Biomedical Applications. Elsevier Inc.; 2008. [Google Scholar]
- 2.Venkatesan BM, Bashir R. Nanopore sensors for nucleic acid analysis. Nature Nanotech. 2011;6:615–624. doi: 10.1038/nnano.2011.129. [DOI] [PubMed] [Google Scholar]
- 3.Tegenfeldt JO, et al. The dynamics of genomic-length DNA molecules in 100-nm channels. Proc. Nat. Acad. Sci. USA. 2004;101:10979–10983. doi: 10.1073/pnas.0403849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang P, He J, Park J, Krstic PS, Lindsay S. Origin of Giant Ionic Currents in Carbon Nanotube Channels. ACS Nano. 2011;5:7277–7283. doi: 10.1021/nn202115s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W, Fan R, Reed MA. Field-effect reconfigurable nanofluidic ionic diodes. Nat Commun. 2011;2:506. doi: 10.1038/ncomms1514. [DOI] [PubMed] [Google Scholar]
- 6.Dekker C. Solid-state nanopores. Nature Nanotech. 2007;2:209–215. doi: 10.1038/nnano.2007.27. [DOI] [PubMed] [Google Scholar]
- 7.Healy K. Nanopore-based single-molecule DNA analysis. Nanomedicine. 2007;2:459–481. doi: 10.2217/17435889.2.4.459. [DOI] [PubMed] [Google Scholar]
- 8.Wanunu M, Meller A. In: Laboratory Manual on Single Molecules. Ha T, Selvin P, editors. 395-420. Cold Spring Harbor Press; 2008. [Google Scholar]
- 9.Wong CTA, Muthukumar M. Polymer capture by electro-osmotic flow of oppositely charged nanopores. J. Chem. Phys. 2007;126:164903. doi: 10.1063/1.2723088. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Tsutsui M, Fan C, Taniguchi M, Kawai T. Controlling DNA translocation through gate modulation of nanopore wall surface charges. ACS Nano. 2011;5:5509–5518. doi: 10.1021/nn201883b. [DOI] [PubMed] [Google Scholar]
- 11.Kejian D, Weimin S, Haiyan Z, Xianglei P, Honggang H. Dependence of zeta potential on polyelectrolyte moving through a solid-state nanopore. App. Phys. Lett. 2009;94:014101. [Google Scholar]
- 12.Van Dorp S, Keyser UF, Dekker NH, Dekker C, Lemay SG. Origin of the electrophoretic force on DNA in solid-state nanopores. Nature Physics. 2009;5:347–351. [Google Scholar]
- 13.Firnkes M, Pedone D, Knezevic J, Döblinger M, Rant U. Electrically facilitated translocations of proteins through silicon nitride nanopores: conjoint and competitive action of diffusion, electrophoresis, and electroosmosis. Nano Lett. 2010;10:2162–2167. doi: 10.1021/nl100861c. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal SM, Bashir R, editors. Nanopores, Sensing and foundamental biological interactions. Springer; 2011. [Google Scholar]
- 15.Wanunu M, Morrison W, Rabin Y, Grosberg AY, Meller A. Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt gradient. Nature Nanotech. 2010;5:160–165. doi: 10.1038/nnano.2009.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam S-W, Rooks MJ, Kim K-B, Rossnagel SM. Ionic Field Effect Transistors with Sub-10 nm Multiple Nanopores. Nano Lett. 2009;9:2044–2048. doi: 10.1021/nl900309s. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z, Stein D. Charge regulation in nanopore ionic field-effect transistors. Phys. Rev. E. 2011;83:031203. doi: 10.1103/PhysRevE.83.031203. [DOI] [PubMed] [Google Scholar]
- 18.Keyser UF, et al. Nanopore tomography of a laser focus. Nano Lett. 2005;5:2253–2256. doi: 10.1021/nl051597p. [DOI] [PubMed] [Google Scholar]
- 19.Svoboda K, Block SM. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- 20.Peterman EJG, Gittes F, Schmidt CF. Laser-induced heating in optical traps. Biophys. J. 2003;84:1308–1316. doi: 10.1016/S0006-3495(03)74946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beamish E, Kwok H, Tabard-Cossa V, Godin M. Precise control of the size and noise of solid-state nanopores using high electric fields. Nanotechnology. 2012;23:405301. doi: 10.1088/0957-4484/23/40/405301. [DOI] [PubMed] [Google Scholar]
- 22.Talaga DS, Li J. Single-Molecule Protein Unfolding in Solid State Nanopores. Journal of the American Chemical Society. 2009;131:9287–9297. doi: 10.1021/ja901088b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yusko EC, et al. Controlling protein translocation through nanopores with bio-inspired fluid walls. Nature Nanotech. 2011;6:253–260. doi: 10.1038/nnano.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plesa C, et al. Fast Translocation of Proteins through Solid State Nanopores. Nano Lett. 2013;13:658–663. doi: 10.1021/nl3042678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell. Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 26.Wanunu M, Sutin J, McNally B, Chow A, Meller A. DNA translocation governed by interactions with solid state nanopores. Biophys. J. 2008;95:4716–4725. doi: 10.1529/biophysj.108.140475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soni GV, Dekker C. Detection of Nucleosomal Substructures using Solid-State Nanopores. Nano Lett. 2012;12:3180–3186. doi: 10.1021/nl301163m. [DOI] [PubMed] [Google Scholar]
- 28.Raillon C, et al. Nanopore Detection of Single Molecule RNAP-DNA Transcription Complex. Nano Lett. 2012;12:1157–1164. doi: 10.1021/nl3002827. [DOI] [PubMed] [Google Scholar]
- 29.Ho C, et al. Electrolytic transport through a synthetic nanometer-diameter pore. Proc. Nat. Acad. Sci. USA. 2005;102:10445–10450. doi: 10.1073/pnas.0500796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogerheide DP, Garaj S, Golovchenko JA. Probing surface charge fluctuations with solid-state nanopores. Phys. Rev. Lett. 2009;102 doi: 10.1103/PhysRevLett.102.256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smeets R, et al. Salt dependence of ion transport and DNA translocation through solid-state nanopores. Nano Lett. 2006;6:89–95. doi: 10.1021/nl052107w. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Ni Z, Wang G. Electroosmotic flow in nanotubes with high surface charge densities. Nano Lett. 2008;8:42–48. doi: 10.1021/nl0718566. [DOI] [PubMed] [Google Scholar]
- 33.Plecis A, Schoch RB, Renaud P. Ionic transport phenomena in nanofluidics: experimental and theoretical study of the exclusion-enrichment effect on a chip. Nano Lett. 2005;5:1147–1155. doi: 10.1021/nl050265h. [DOI] [PubMed] [Google Scholar]
- 34.He Y, Tsutsui M, Fan C, Taniguchi M, Kawai T. Gate manipulation of DNA capture into nanopores. ACS Nano. 2011;5:8391–8397. doi: 10.1021/nn203186c. [DOI] [PubMed] [Google Scholar]
- 35.Behrens SH, Grier DG. The charge of glass and silica surfaces. J. Chem. Phys. 2001;115:6716–6721. [Google Scholar]
- 36.Wu M, et al. Control of shape and material composition of solid-state nanopores. Nano Lett. 2009;9 doi: 10.1021/nl803613s. [DOI] [PubMed] [Google Scholar]
- 37.Deshpande SV, Gulari E. Optical properties of silicon nltride films deposited by hot filament chemical vapor deposition. J. App. Phys. 1995;77:6534–6541. [Google Scholar]
- 38.Moustakas TD. The role of extended defects on the performance of optoelectronic devices in nitride semiconductors. Phys. Status Solidi A. 2012 doi: 10.1002/pssa.201200561. [Google Scholar]
- 39.Robertson J, Warren WL, Kanicki J. Nature of the Si and N dangling bonds in silicon nitride. J. Non-Cryst. Solids. 1995;187:297–300. [Google Scholar]
- 40.Robertson J, Powell MJ. Gap States in Silicon Nitride. App. Phys. Lett. 1984;44:415–417. [Google Scholar]
- 41.Singh R, J MR, Unlu MS, Moustakas TD. Intensity dependence of photoluminescence in gallium nitride thin films. Appl. Phys. Lett. 1994;64 [Google Scholar]
- 42.Wu MY, Krapf D, Zandbergen M, Zandbergen H, Batson PE. Formation of nanopores in a SiN/SiO2 membrane with an electron beam. App. Phys. Lett. 2005;87:113106. [Google Scholar]
- 43.Venkatesan BM, Shah AB, Zuo J-M, Bashir R. DNA Sensing Using Nanocrystalline Surface-Enhanced Al2O3 Nanopore Sensors. Advanced Functional Materials. 2010;20:1266–1275. doi: 10.1002/adfm.200902128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson BN, Muthukumar M, Meller A. pH Tuning of DNA Translocation Time through Organically Functionalized Nanopores. ACS nano. 2013;7:1408–1414. doi: 10.1021/nn3051677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fologea D, Uplinger J, Thomas B, McNabb DS, Li J. Slowing DNA translocation in a solid-state nanopore. Nano Lett. 2005;5:1734–1737. doi: 10.1021/nl051063o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kowalczyk SW, Wells DB, Aksimentiev A, Dekker C. Slowing down DNA Translocation through a Nanopore in Lithium Chloride. Nano Lett. 2012;12:1038–1044. doi: 10.1021/nl204273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer A, Rapireddy S, Ly DH, Meller A. Electronic barcoding of a viral gene at the single-molecule level. Nano Lett. 2012;12:1722–1728. doi: 10.1021/nl300372a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shim J, et al. Detection and Quantification of Methylation in DNA using Solid-State Nanopores. Scientific reports. 2013;3:1389. doi: 10.1038/srep01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wanunu M, et al. Discrimination of Methylcytosine from Hydroxymethylcytosine in DNA Molecules. Journal of the American Chemical Society. 2011;133:486–492. doi: 10.1021/ja107836t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.