Abstract
Glioblastoma multiforme is an aggressive form of human astrocytoma, with poor prognosis due to multi-drug resistance to a number of anticancer drugs. The observed multi-drug resistance is primarily due to the efflux activity of ATP Binding Cassette (ABC) efflux transporters such as Pgp, MRP1 and BCRP. The expression of these transporters has been demonstrated in nuclear and cellular membranes of the LN-229 human glioblastoma cell line. Nuclear membrane and cellular membrane fragments from LN229 cells were immobilized on the IAM stationary phase to create nuclear and cellular membrane affinity chromatography columns, (NMAC(LN229)) and (CMAC(LN229)), respectively. Pgp, MRP1and BCRP transporters co-immobilized on both columns was characterized and compared by establishing the binding affinities for estrone-3-sulfate (3.8 vs 3.7μM), verapamil (0.6 vs 0.7μM) and prazosin (0.099 vs 0.033μM) on each column and no significant differences were observed. Since the marker ligands had overlapping selectivities, the selective characterization of each transporter was carried out by saturation of the binding sites of the non-targeted transporters. The addition of verapamil (Pgp and MRP1 substrate) to the mobile phase allowed the comparative screening of 8 compounds at the nuclear and cellular BCRP using etoposide as the marker ligand. AZT increased the retention of etoposide (+15%), a positive allosteric interaction, on the CMAC(LN229) column and decreased it (−5%) on the NMAC(LN229), while the opposite effect was produced by rhodamine. The results indicate that there are differences between the cellular and nuclear membrane expressed BCRP and that NMAC and CMAC columns can be used to probe these differences.
Keywords: Breast Cancer Resistant Protein, bioaffinity chromatography, frontal chromatography, glioblastoma
1. Introduction
Glioblastoma multiforme (GBM) is one of the most aggressive forms of human astrocytoma as only ~10% of patients survive 5 years post diagnosis. Multi-drug resistance to drugs used in cancer treatment carried out by membranes of the ATP Binding Cassette (ABC) efflux transporters has been associated with poor prognosis. The ABC superfamily consists of 49 members [1], of which P-glycoprotein (Pgp), breast cancer resistance protein (BCRP), and multidrug resistance-associated protein 1 and 2 (MRP1 and MRP 2) are the most well-known members. Pgp, MRP1 and BCRP have both individual and overlapping selectivity towards the anticancer agents that are substrates for these efflux transporters. For example, Pgp substrates include vinca alkaloids, anthracyclines, and epipodophyllotoins [2,3], while MRP1 substrates include etoposide, anthracyclines, vincristine, methothrexate, paclitaxel and irinotecan [3], and BCRP plays a role in doxorubicin and methotrexate resistance [4,5].
The role of the ABC transporters in the MDR phenotype have resulted in these proteins being targeted in drug development and drug discovery programs. While ABC transporters are usually considered as cellular membrane proteins, they are also expressed in nuclear and mitochondrial membranes. For example, the expression of BCRP in nuclear and cellular membranes was recently demonstrated in six human-derived glioblastoma and astrocytoma cell lines [5]. The presence of this transporter in two distinct membrane environments raises the potential of differences in selectivity between cellular and nuclear BCRP transporters. While classical binding assays, functional assays and surface plasmon resonance are the most common approaches for binding affinity determination [6,7], differences between the cellular and nuclear BCRP would be difficult to determine using these standard membrane binding techniques. These approaches allow quantitative determination of the binding affinity of a ligand for its receptor, however, are limited in their ability to study allosteric sites and determine conformational changes resulting from changes in the lipid environment. One approach that provides for the direct measurement of multiple binding sites including orthosteric and allosteric sites, multiple conformations as well as subtype ligand interactions is cellular membrane affinity chromatography, where the target transmembrane protein is immobilized onto silica based stationary phase [8].
The development and use of cellular membrane affinity chromatography (CMAC) columns have been extensively demonstrated [9] and we have recently reported the development of a nuclear membrane affinity chromatographic (NMAC) column obtained using nuclear membranes obtained from the LN-229 cell line [10]. Initial studies using FTC, a selective BCRP inhibitor, as a marker demonstrated that the BCRP transporter was immobilized within the NMAC(LN-229) column and that the protein retained the ability to bind substrates and inhibitors.
Since nuclear and cellular membranes may contain multiple ABC transporters, this study was designed to prepare NMAC(LN-229) and CMAC(LN-229) columns, to examine the expression and function of Pgp, MRP1 and BCRP transporters in both columns, and to determine if any differences exist between ABC transporters contained within nuclear membranes relative to those contained within cellular membranes.
The data from this study demonstrates that Pgp, MRP1 and BCRP were functionally immobilized within both the CMAC(LN-229) and NMAC(LN-229) columns indicating that these were multi-transporter columns. The immobilization of the respective membranes was also carried out in an open-tubular format generating CMAC(LN-229)-OT and NMAC(LN-229)-OT columns. The co-immobilization of the three ABC transporters within the OT columns was also demonstrated. In addition, the OT columns were used for a comparative screening study of eight compounds at the BCRP transporter using the addition of selective Pgp and MRP1 substrates to the mobile phase to selectively study the BCRP transporter. The results indicate that there are differences between the affinity of the BCRP transporter contained within nuclear membranes and the BCRP transporter contained within the cellular membranes.
2. Experimental
2.1. Materials
Ammonium acetate, sodium chloride (NaCl), ethylenediaminetetraacetic acid (EDTA), sodium ortho-vanadate, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), glycerol, 2-mercaptoethanol, benzamidine, protease inhibitor cocktail, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), N-ptosyl-L-phenylalanine chloromethyl ketone (TPCK), phenylmethanesulfonyl fluoride (PMSF), adenosine 5′-triphosphate (ATP), amino propyl trimethoxy silane (APTS), gluteraldehyde aqueous solution, avidin, biotin-X (6-[(biotinoyl)amino]hexanoic acid), etoposide, biochanin A, fumitremorgin C (FTC), verapamil, estrone-3-sulphate, prazosin, zidovudine (AZT), rhodamine 123, quercitin, tamoxifen and sulfasalazine were obtained from Sigma-Aldrich (St. Louis, MO, USA), tris(hydroxymethyl)aminomethane (TRIS) was obtained from Schwarz/Mann Biotech (Cleveland, OH, USA). Dialysis tubing was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Open tubular capillaries (100 μm i.d.) were obtained from Polymicro Technologies (Phoenix, AZ, USA). De-ionized water was obtained from a Milli-Q system (Millipore, Billerica, MA, USA). All other chemicals used were of analytical grade.
2.2. Methods
2.2.1. Cell line
The LN-229 astrocytoma cell line was obtained from American Type Tissue Culture (Manassas, VA, USA) and maintained as previously described [10].
2.2.2. Western blot analysis
Cells were lysed in radioimmunoprecipitation (RIPA) buffer containing EGTA and EDTA (Boston BioProducts, Ashland, MA, USA). The lysis buffer contained protease inhibitor cocktail. Protein concentrations were determined using the BCA assay (Thermo Fisher Scientific). Proteins (20 μg/well) were separated electrophoretically on 4 to 12% Tris-Glycine precast gels (Invitrogen, Grand Island, NY) under reducing conditions and then transferred onto PVDF membrane (Invitrogen). 5% non-fat milk in TBST was used for blocking the non-specific proteins and to incubate with the primary antibody of interest: ABCG2 (M-70), PGP (A-14), and MRP1 (C-20) (Santa Cruz, CA), followed by incubation with a secondary antibody conjugated with the enzyme horseradish peroxidase. The detection of immunoreactive bands was performed by using the ECL Plus Western Blotting Detection System (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The primary antibody for β-actin was from Abcam (Cambridge, MA, USA).
2.2.3. Preparation of nuclear membranes (NM)
The LN-229 nuclear membranes were prepared as previously described [10]. Briefly, 10×106 LN-229 cells were re-suspended in CER-1 reagent from NE-PER Kit (Thermo Fisher Scientific) supplemented by 1:100 dilution of protease inhibitor cocktail, 1:100 dilution of Halt™ Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific) and 1:1000 dilution of 1 mM sodium ortho-vanadate. After incubation on ice, 110 μL CER-II reagent from NE-PER Kit was added. The cell-suspension was centrifuged for 5 min at 4°C at 16,000 ×g. The cytoplasmic extract was removed and the resultant pellet was re-suspended in NER. After incubation on ice, the solution was centrifuged at 16,000 ×g for 10 min at 4°C and the resulting supernatant contained the nuclear membranes.
2.2.4. Preparation of cellular membrane (CM)
The cellular membranes were prepared following a previously described protocol with slight modifications [11]. Briefly, 10×106 LN-229 frozen cell pellet was washed once with PBS and centrifuged for 5 min at 1000 rpm. The cell pellet was re-suspended in 10 mL of Tris buffer [10 mM, pH 7.4], supplemented with 500 mM NaCl, 5 mM 2-mercaptoethanol, 100 μM benzamidine, 1:100 dilution of protease inhibitor cocktail, 50μg/mL TPCK, 100 μM PMSF and 100 μM ATP. The cell suspension was homogenized 3 times for 30 sec at 3000 rpm using PRO200 homogenizer (PRO Scientific, Oxford, CT, USA). The homogenized cell suspension was centrifuged for 10 min at 700 ×g at 4°C. The supernatant containing the cellular membranes was collected and centrifuged for 30 min at 100,000 ×g at 4°C. The supernatant was discarded and the pellet contained the cellular membranes.
2.2.5. Preparation of LN-229 nuclear membrane affinity chromatography (NMAC) and cellular membrane affinity chromatography (CMAC) columns
Nuclear and cellular membranes were suspended in 10 mL of solubilization buffer (Tris buffer [10 mM, pH 7.4], supplemented with 2% (w/v) CHAPS, 10% glycerol, 500 mM NaCl, 5 mM 2-mercaptoethanol, 100 μM benzamidine, 1:100 dilution of protease inhibitor cocktail, 50μg/mL TPCK, 100 μM PMSF and 100 μM ATP) for the IAM columns and 3 mL of solubilization buffer for the open tubular (OT) columns. The resulting mixture was mixed for 18 h using a tube roller (Stovall Life Science, Inc., Peosta, IA, USA) at 150 rpm at 4°C.
IAM columns
The solubilized NM or CM was mixed with 150 mg Immobilized Artificial Membranes (IAM) particles (Regis Technologies, Morton Grove, IL) and rotated at room temperature (RT) using an orbital shaker for 1 h at 150 rpm. The suspended particles were then dialyzed against Tris buffer [10 mM, pH 7.4] containing 500 mM NaCl, 1 mM EDTA and 100 nM of benzamidine (cellular membranes) and against HEPES buffer [10 mM, pH 8.0] containing 500 mM NaCl and 1 mM EDTA (nuclear membranes), for 1 day, and repeated. Next, the suspension was centrifuged for 3 min at 4 °C at 700 ×g. The pellet obtained was then washed two times with ammonium acetate [10 mM, pH 7.4] by centrifuging 3 min at 4 °C at 700 ×g. Final pellet was re-suspended in 2 mL ammonium acetate [10 mM, pH 7.4] and packed into an HR 5/2 column (Amersham Pharmacia Biotech, Uppsala, Sweden) to yield a 150 × 5-mm (i.d.) chromatographic bed.
OT columns
The NMAC-OT and CMAC-OT columns were synthesized using a previously described protocol [9,12]. Briefly, the open tubular capillary (25 cm × 100 μm i.d.) was primed and then a 10% aqueous solution of APTS was passed through the capillary followed by 30 min incubation at 95°C twice. After 18 h, a 1% aqueous solution of glutaraldehyde was passed through the capillary for 1h followed by water and 25 mM avidin. Both tips of the capillary were submerged in the avidin solution for 4 days at 4°C. Then 14 mM biotin-X was run through the capillary for 1 h. The solubilized NM and CM were recycled through the column for 30 min. The open tubular capillary was then dialysed against Tris buffer [10 mM, pH 7.4] containing 500 mM NaCl and 1 mM EDTA for 1 day, and repeated.
2.3. Chromatographic studies
The CMAC and NMAC columns were attached to the chromatographic system Series 1100 Liquid Chromatography/Mass Selective Detector (Agilent Technologies, Palo Alto, CA, USA) equipped with a vacuum de-gasser (G 1322 A), a binary pump (1312 A), a mass selective detector (G1946 B) supplied with atmospheric pressure ionization electrospray. The chromatographic system was interfaced to a 2.80 GHz Pentium(R) 4 computer (Hewlett-Packard, Palo Alto, CA, USA) running ChemStation software (Rev B.10.00, Hewlett-Packard).
In the chromatographic studies, mobile phase consisted of ammonium acetate [10 mM, pH 7.4] delivered at 0.4 mL/min for the IAM columns and 0.05 mL/min for the OT columns. Pumps A, C and D were used to apply a series of ligands: etoposide (1 – 20 μM), FTC (0.125 – 7 μM), biochanin A (1 – 20 μM), verapamil (1 – 5 μM), estrone-3-sulphate (1 – 20 μM) and prazosin (0.025 – 1 μM). Etoposide, estrone-3-sulphate and prazosin were monitored in the negative ion mode using single ion monitoring at m/z = 587.20, 349 and 384 [MW − H]−, respectively, with the capillary voltage at 3000 V, the nebulizer pressure at 35 psi, and the drying gas flow at 11 L/min at a temperature of 350°C. FTC, biochanin A and verapamil were monitored in the positive ion mode using single ion monitoring at m/z = 380.50, 288.0 and 455 [MW + H]+ ion, respectively, with the capillary voltage at 3000 V, the nebulizer pressure at 35 psi, and the drying gas flow at 11 L/min at a temperature of 350°C.
2.4. Data Analysis
The dissociation constants, Kd's, for the displacer ligands were determined using a previously reported approach [13]. The experimental paradigm is based upon the effect of escalating approach of a competitive binding ligand on the retention volume. For example, the displacer ligands (D) dissociation constant, Kd, as well as the number of the active binding sites of the immobilized BCRP, Bmax, can be calculated using equation (1):
| (1) |
where: V is the retention volume of ligand, Vmin is the retention volume of ligand when the specific interaction is completely suppressed and P is the product of the Bmax and (Kd/KdM). The Kd for D is obtained from the plot of [D] (V−Vmin) versus [D]. The data was analysed by nonlinear regression with a sigmoidal response curve using Prism 4 software (Graph pad Software, Inc., San Diego, CA, USA) running on a personal computer.
2.5. Selective Determination of Kds for BCRP, Pgp and MRP1
BCRP
The mobile phase consisted of ammonium acetate [10 mM, pH 7.4] containing 2 μM verapamil delivered at 0.4 mL/min. Frontal chromatographic studies were run with a series of concentrations of etoposide ranging from 1 – 20 μM.
Pgp
The mobile phase consisted of ammonium acetate [10 mM, pH 7.4] containing 8 μM estrone-3-sulfate delivered at 0.4 mL/min. Frontal chromatographic studies were run with a series of concentrations of etoposide ranging from 1 – 20 μM.
MRP1
The mobile phase consisted of ammonium acetate [10 mM, pH 7.4] containing 2 μM prazosin delivered at 0.4 mL/min. Frontal chromatographic studies were run with a series of concentrations of etoposide ranging from 1 – 20 μM.
Prior to the run the NMAC-IAM column was equilibrated with the mobile phase for 4h.
2.6. Screening of BCRP ligands
The mobile phase consisted of ammonium acetate [10 mM, pH 7.4] containing 2 μM verapamil. Both NMAC-OT and CMAC-OT were equilibrated with the mobile phase for 4h prior to analysis. The change in the retention volume of 0.75 μM etoposide in the presence of 4.25 μM of the following compounds was determined: biochanin A, estrone-3-sulfate, AZT, rhodamine 123, quercitin, tamoxifen and sulfasalazine and etoposide. The data was normalized to the change in retention volume observed in 5 μM etoposide.
3. Result and discussion
The presence of BCRP on the nuclear membrane of the LN-229 cell line was previously established using western blot analysis, confocal microscopy and frontal affinity chromatography [5,10]. We also demonstrated that nuclear membrane fragments from this cell line could be immobilized on an IAM chromatographic support to produce a nuclear membrane affinity chromatography (NMAC(LN-229)) column. Ligand binding to the immobilized BCRP was determined on the NMAC(LN-229) using frontal displacement chromatography with etoposide as a marker ligand, and the results demonstrated that this column could be used to characterize the immobilized nuclear BCRP [10].
BCRP is also present in the cellular membranes of LN-229 and it is unknown if the different membrane environments produce differences in the selectivity and function of the BCRP transporter. The development of the NMAC(LN-229) column suggested that the binding selectivity and affinity of nuclear and cellular membrane BCRP could be compared using data obtained from affinity columns produced using nuclear and cellular membranes. To that end, cellular membrane fragments obtained from LN-229 cells were immobilized onto the surface of the IAM stationary phase to create a cellular membrane affinity (CMAC) column. The CMAC(LN-229) column was characterized by frontal displacement chromatography using three established BCRP ligands, etoposide, FTC and biochanin A (Table 1). The calculated binding affinities, expressed as Kd values, for etoposide, FTC and biochanin A on the CMAC(LN-229) column were similar to previously reported Kd values obtained using the NMAC(LN-229) column, 1.9 vs 4.5, 1.1 vs 1.6 and 1.4 vs 1.6 μM, respectively, (Table 2) [10].
Table 2.
Binding affinities (μM) of etoposide determined on the NMAC(LN-229)-IAM column using frontal affinity chromatography with ammonium acetate [10mM, pH 7.4] containing 8 μM of estrone-3-sulfate to block the BCRP and MRP1 binding, 2 μM of verapamil to block Pgp and MRP1 and 2 μM prazosin to block BCRP and PgP binding with a flow rate of 0.4 mL/min.
| Etoposide | |
|---|---|
| All receptors | 3.75 ± 0.97 |
| Pgp (Estrone-3-sulphate) | 5.83 ± 0.68 |
| BCRP (Verapamil) | 1.41 ± 0.54 |
| MRP1 (Prazosin) | 4.39 ± 1.93 |
It has been previously established that etoposide and biochanin A bind to multiple ABC transporters, BCRP, Pgp and MRP1, with micromolar affinities [14], which raised the prospect that the calculated Kd values obtained on the NMAC(LN-229) and CMAC(LN-229) columns actually represented multiple binding affinities and did not accurately reflect specific binding to BCRP. This possibility was supported by the data from Western blot studies, which established the presence of BCRP, Pgp and MRP1 in both cellular and nuclear membranes of the LN-229 cells (Figure 1).
Figure 1.

Western blot analysis of nuclear (NE), cellular (CE) and whole cell (WCE) extracts of LN-229 cells. Expression of BCRP, Pgp and MRP1 was observed in all the samples.
The isolation and characterization of the binding of ligands to just one of the three immobilized ABC transporters was approached through the addition to the mobile phase of compounds chosen to block interactions with one or more of the immobilized transporters. The selected compounds were estrone-3-sulfate (with affinity for BCRP and MRP1), verapamil (Pgp and MRP1) and prazosin (BCRP and PgP) (Table 1b). In order to determine the saturating concentrations required to block the target transporters, the Kd values of estrone-3-sulfate, verapamil and prazosin were determined on the NMAC(LN-229) and CMAC(LN-229) columns, and similar values were obtained on both columns, 3.7 vs 3.8 μM, 0.7 vs 0.6 μM and 0.033 vs 0.099 μM, respectively (Table 1b). Based on the calculated binding affinities, 8 μM of estrone-3-sulfate was used as a saturating concentration for BCRP and MRP1, 2 μM of verapamil was used to block Pgp and MRP1 and 2 μM prazosin was used to block BCRP and PgP.
Table 1 B.
Binding affinities (μM) of estrone-3-sulfate, verapamil and prazosin determined by frontal affinity chromatography on the NMAC(LN-229)-IAM and CMAC(LN-229)-IAM columns.
| Estrone-3-sulphate | Verapamil | Prazosin | |
|---|---|---|---|
| NMAC(LN-229)-IAM | 3.78 ± 0.64 | 0.62 ± 0.36 | 0.099 ± 0.007 |
| CMAC(LN-229)-IAM | 3.67 ± 1.28 | 0.71 ± 0.32 | 0.033 ± 0.009 |
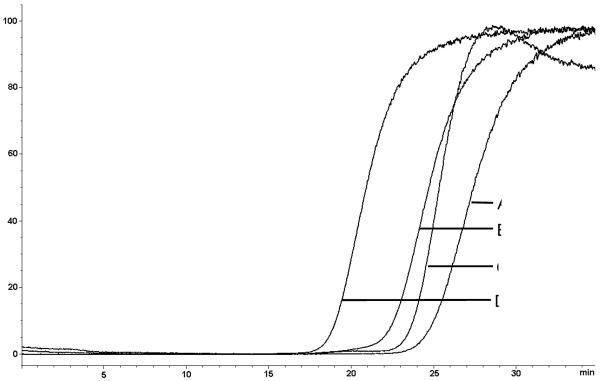
The ability to determine binding to one of the transporters in the presence of the other two was explored using etoposide as the marker ligand as this compound interacts with each of the three transporters (Table 2). In these studies, the binding affinity of etoposide was initially determined on the NMAC(LN-229) column alone and then in the presence of saturating concentrations of the blocking compounds. The elution profiles of etoposide at two sub-saturating concentrations, 0.75 μM and 5 μM, are presented Figure 2, curve A and B, respectively. These studies were carried out without the addition of a blocking compound and the midpoints of the breakthrough curves occurred at 27.73 min (0.75 μM) and 24.12 min (5 μM) representing breakthrough volumes of 11.09 mL and 9.65 mL, respectively. The concentration-dependent decrease in the breakthrough volumes is the expected reflection of the binding to the 3 immobilized transporters and indicates that competitive displacement experiments can be used to calculate binding affinities. When 2 μM verapamil was added to the mobile phase, the breakthrough volumes were reduced to 25.48 min (0.75 μM) and 21.01 min (5 μM) representing ~10% and ~15% reductions. The results indicate that the saturation of the Pgp and MRP1 transporters reduced the affinity of etoposide on the NMAC(LN-229) column but did not eliminate specific binding to the immobilized BCRP nor the ability to determine binding affinities.
Figure 2.
Frontal elution profiles 0.75 μM etoposide (A), 5 μM etoposide (B), 0.75 μM etoposide + 2 μM verapamil (C), 5 μM etoposide + 2 μM verapamil (D), on the NMAC (LN-229)-IAM column (0.531 cm × 2 cm) on the Agilent LC–MSD by frontal affinity chromatography using ammonium acetate [10 mM, pH 7.4] at 0.4 ml min−1 as the mobile phase.
The binding affinity of etoposide was then determined in the presence of each of the blocking compounds (Table 2). The binding of etoposide for BCRP was the strongest at 1.41 ± 0.54 μM, while its affinity for MRP1 and Pgp were similar, 4.39 ± 1.93 and 5.83 ± 0.68 μM, respectively. The average of the three binding affinities for etoposide is 3.87 μM, which is similar to the Kd value of 3.75 ± 0.97 μM obtained in the absence of the blocking compounds. The results indicate that the binding affinity obtained for etoposide represents an average of its affinities for the BCRP, MRP1 and PgP transporters and that, if desired, the independent affinities can be obtained using this affinity chromatographic approach.
An alternative approach to the development of membrane affinity columns using the IAM support is the immobilization of the membranes within open tubular capillaries, the OT format [9]. The advantages of the OT format include the reduction in experimental time and the quantity of test compounds required for the study. Thus, this format appears to be more amenable to multiple compound screening. In order to create a screening method for the BCRP transporter, nuclear membranes and cellular membranes obtained from LN-229 cells were immobilized onto the surface of an open tubular capillary, generating NMAC(LN-229)-OT and CMAC(LN-229)-OT columns. In order to confirm that functional forms of the Pgp, MRP1 and BCRP transporters were immobilized, both columns were characterized with etoposide, biochanin A and verapamil (Table 3). The substrates were found to have similar affinities on both the NMAC(LN-229)-OT and CMAC(LN-229)-OT columns. For example, etoposide had a Kd of 1.26 ± 0.36 μM and 2.25 ± 0.49 μM, respectively.
Table 3.
Binding affinities (μM) of etoposide, biochanin A and verapamil determined on the NMAC(LN-229)-OT and CMAC(LN-229)-OT columns by frontal affinity chromatography using ammonium acetate [10mM, pH 7.4] as the mobile phase with a flow rate of 0.05 mL/min.
| Etoposide | Biochanin A | Verapamil | |
|---|---|---|---|
| NMAC(LN-229)-OT | 1.26 ± 0.36 | 1.75 ± 0.36 | 0.26 ± 0.12 |
| CMAC(LN-229)-OT | 2.25 ± 0.49 | 0.69 ± 0.17 | 0.56 ± 0.22 |
In order to determine whether there were differences between nuclear and cellular BCRPs, the screening was carried out in the presence of a saturating concentration of verapamil to eliminate the interaction of etoposide with the Pgp and MRP1 transporters. The binding affinity of verapamil was determined for both the NMAC(LN-229)-OT and CMAC(LN-229)-OT column, so as to determine the saturating concentration required for the BCRP screening study. The binding affinities obtained for verapamil were 0.26 ± 0.12 μM and 0.56 ± 0.22 μM, respectively. Based upon these results, the next set of experiments utilized 2 μM verapamil as the blocking compound and 0.75 μM etoposide as the marker ligand. Eight compounds, including etoposide, estrone-3-sulfate and biochanin A were screened on the NMAC(LN-229)-OT and CMAC(LN-229) OT columns (Figure 3). Previous studies of binding affinities of ligands to the nicotinic acetylcholine receptor (nAChR) using a CMAC(nAChR) column demonstrated that if a known displacer was used in the study a single displacement experiment could be used to rank the affinities of multiple test compounds relative to that of the known displacer [15]. In this study, the displacement observed with 5 μM etoposide was used as the known displacer and the shift in the breakthrough volume produced by this concentration, relative to the results obtained with 0.75 μM etoposide, was set as 100%, c.f. Figure 2A and 2B.
Figure 3.
Single frontal displacement studies of 8 compounds (4.25 μM), including etoposide, for the BCRP carried out on both CMAC(LN-229)-OT and NMAC(LN-229)-OT using ammonium acetate [10 mM, pH 7.4] in the presence of 2 μM verapamil and 0.75 μM etoposide as the mobile phase. The data was normalized to the change in breakthrough volume observed with etoposide and the relative changes from etoposide (0%) are reported.
The data from the single displacement studies demonstrated that, in the presence of 2 μM verapamil, all of the 8 test compounds displaced etoposide on both columns and that for 5 of the 7 compounds the reductions in the breakthrough volume ranged from ~70% to ~90% of the effect produced by 5 μM etoposide (Figure 3). AZT produced a greater relative displacement on the CMAC(LN-229)-OT column (115%), while it was rhodamine 123 that had the greatest relative displacement on the NMAC(LN-229)-OT column (108%) and biochanin A had the lowest effect on both columns, 39% and 24%, respectively. The weak displacement observed with biochanin A was of interest as the Kd obtained for biochanin A (1.75 and 0.69 μM for the NMAC and CMAC-OT columns, respectively) was similar to the Kd's obtained for etoposide (1.26 and 2.25 μM, respectively). The discrepancy between the screening results and binding data, suggests that biochanin A and etoposide are not binding to the same site on the BCRP protein. A comparison of the relative displacements produced by the test compounds on the CMAC(LN-229)-OT and NMAC(LN-229)-OT columns demonstrated that the difference between the effects were ≤15% for 4 of the 7 compounds, indicating that there were no major differences in the binding to the BCRP in both columns. It is important to note that the data from each column is normalized to the effect of 5μM etoposide and, therefore, should be independent of differences in the number of active sites, i.e. Bmax values, between the two columns. However, there were significant differences between the relative displacements of AZT (20%), quercitin (21%) and biochanin A (15%) on the two columns with the stronger effect observed on the CMAC(LN-229)-OT columns. The data suggest that these compounds display a higher affinity for the BCRP transporter found within the cellular membrane that of the BCRP found within the nuclear membrane. Thus, the results indicate that there are differences between BCRP contained within cellular and nuclear membranes, which could result from differences in post-translational modifications responsible for the translocation of the transporters to the different membranes or from conformational differences between the two proteins produced by elements within the membrane environment. These two possibilities are under investigation and the data will be reported elsewhere.
4. Conclusion
The results from this study demonstrate that cellular and nuclear membranes obtained from the LN-229 glioblastoma cell line can be immobilized in packed column or open tubular formats and resulting affinity columns used to pattern the ABC transporters contained within these membranes. The data from the current work indicate that the ABC transporters Pgp, MRP1 and BCRP are present in both membranes and global binding affinities could be determined using etoposide, which is a substrate for each of the three transporters. The selective characterization of each transporter was also carried out by saturation of the binding sites of the non-targeted transporters indicating that directed screening can be performed by placing the appropriate blocking compound(s) in the mobile phase. This approach was demonstrated using the affinity columns prepared in the open tubular format and by adding verapamil to the mobile phase, thereby blocking binding to the Pgp and MRP1 proteins. This technique was used to carry out a comparative screening study specifically targeted at the BCRP transporter and the results demonstrate that there are subtle binding differences between cellular and nuclear BCRP transporters. The data from this study also demonstrates that tumor cell lines can be used to screen for the presence and function of ABC transporters, to compare cell specific differences in these proteins as well as differences produced by inclusion in cellular or nuclear membranes, and to directly screen for new agents to treat tumor-specific MDR phenotypes.
Highlights
-
-
ABC columns
-
-
screening against BCRP
-
-
difference between cellular and nuclear BCRP
Table 1 A.
Binding affinities (μM) of Etoposide, biochanin A and FTC determined on the CMAC(LN-229)-IAM column by frontal affinity chromatography using ammonium acetate (10mM, pH 7.4) as the mobile phase with a flow rate of 0.4 mL min−1.
| Etoposide | Biochanin A | FTC | |
|---|---|---|---|
| CMAC(LN-229)-IAM | 1.91 ± 0.42 | 1.05 ± 0.55 | 1.36±0.37 |
Acknowledgement
This work was supported by the Intramural Research Program of the NIH. K.L.H. was supported by the European Union through the European Social Fund (DoRa program T6). Financial support was also provided by the Estonian Ministry of Education targeted financing no. SF0130010s12 and the European Regional Development Fund (Centre of Excellence “Mesosystems: Theory and Applications”, TK114) (K.-L. H., R. S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vasiliou V, Vasiliou K, Nebert DW. Hum Genomics. 2009;3:281. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Szyszko K, Pawłowski KM, Motyl T, Król M. Med Wet. 2011;9:453. [Google Scholar]
- [3].Król M, Pawłowski KM, Majchrzak K, Szyszko K, Motyl T. Pol J Vet Sci. 2010;9:399. [PubMed] [Google Scholar]
- [4].Honscha KU, Schirmer A, Reischauer A, Schoon HA, Einspanier A, Gäbel G. Reprod Domest Anim. 2009;44:218. doi: 10.1111/j.1439-0531.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- [5].Bhatia P, Bernier M, Sanghvi M, Moaddel R, Schwarting R, Ramamoorthy A, Wainer IW. Xenobiotica. 2012;42:748. doi: 10.3109/00498254.2012.662726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cooper MA. J.Mol. Recognit. 2004;17:286–315. doi: 10.1002/jmr.675. [DOI] [PubMed] [Google Scholar]
- [7].deJong LA, Uges DR, Franke JP, Bischoff R. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;829:1–25. doi: 10.1016/j.jchromb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- [8].Moaddel R, Wainer IW. JPBA. 2007;43:399–406. doi: 10.1016/j.jpba.2006.08.021. [DOI] [PubMed] [Google Scholar]
- [9].Moaddel R, Wainer IW. Nat Protocol. 2009;4:197. doi: 10.1038/nprot.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Habicht KL, Frazier C, Singh N, Shimmo R, Wainer IW, Moaddel R. J Pharm Biomed Anal. 2013;72:159. doi: 10.1016/j.jpba.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moaddel R, Calleri E, Massolini G, Frazier C, Wainer IW. Anal Biochem. 2007;364:216. doi: 10.1016/j.ab.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moaddel R, Bullock P, Wainer IW. J Chrom B Analyt Technol Biomed Life Sci. 2004;799:255. doi: 10.1016/j.jchromb.2003.10.054. [DOI] [PubMed] [Google Scholar]
- [13].Kimura T, Perry J, Anzai N, Pritchard JB, Moaddel R. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;859:267. doi: 10.1016/j.jchromb.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bhatia P, Moaddel R, Wainer IW. Talanta. 2010;81:1477. doi: 10.1016/j.talanta.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moaddel R, Jozwiak K, Yamaguchi R, Cobello C, Whittington K, Sarkar TK, Basak S, Wainer IW. J Chrom B. 2004;813:235. doi: 10.1016/j.jchromb.2004.09.042. [DOI] [PubMed] [Google Scholar]