Abstract
In the title compound, C19H17N5S, the dihedral angles between the purine ring system (r.m.s. deviation = 0.009 Å) and the S-bound and methylene-bound phenyl rings are 74.67 (8) and 71.28 (7)°, respectively. In the crystal, inversion dimers linked by pairs of N—H⋯N hydrogen bonds generate R 2 2(8) loops. C—H⋯N interactions link the dimers into (100) sheets.
Related literature
For background to the biological activity of thiopurine derivatives, see: Hadda et al. (2009 ▶); Nguyen et al. (2009 ▶). For further synthetic details, see: Banh et al. (2011 ▶); Salvatore et al. (2002 ▶, 2005 ▶).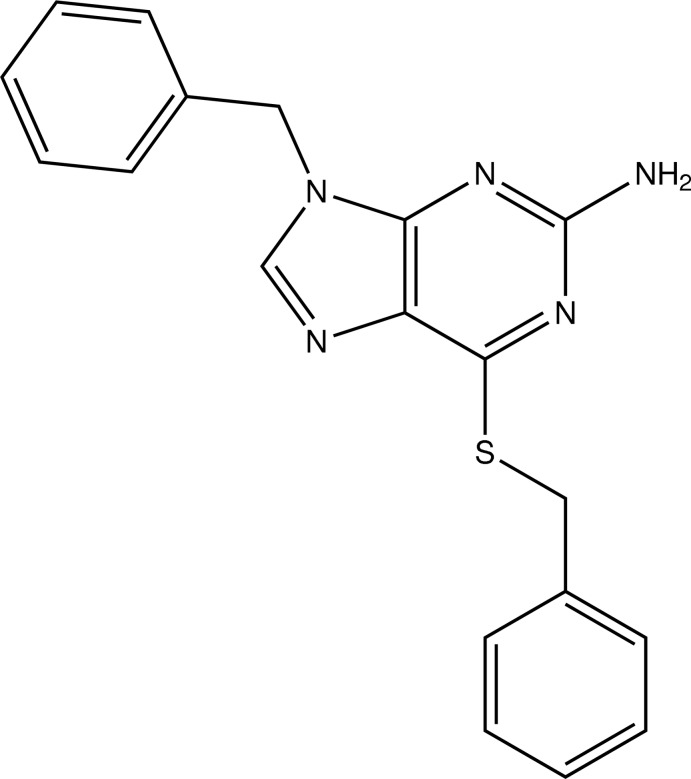
Experimental
Crystal data
C19H17N5S
M r = 347.44
Monoclinic,
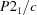
a = 16.7346 (7) Å
b = 5.5511 (3) Å
c = 20.4817 (10) Å
β = 121.325 (3)°
V = 1625.31 (14) Å3
Z = 4
Mo Kα radiation
μ = 0.21 mm−1
T = 100 K
0.69 × 0.19 × 0.14 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009) ▶ T min = 0.868, T max = 0.972
16728 measured reflections
4956 independent reflections
3416 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.137
S = 1.06
4956 reflections
234 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.33 e Å−3
Δρmin = −0.58 e Å−3
Data collection: APEX2 (Bruker, 2009) ▶; cell refinement: SAINT (Bruker, 2009) ▶; data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536814001986/hb7176sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814001986/hb7176Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814001986/hb7176Isup3.cml
CCDC reference: http://scripts.iucr.org/cgi-bin/cr.cgi?rm=csd&csdid=983914
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N5—H1N5⋯N3i | 0.91 (3) | 2.14 (3) | 3.040 (3) | 173 (2) |
| C7—H7B⋯N3ii | 0.99 | 2.57 | 3.548 (2) | 172 |
| C8—H8A⋯N2iii | 0.95 | 2.39 | 3.274 (2) | 155 |
Symmetry codes: (i) 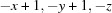 ; (ii)
; (ii) 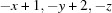 ; (iii)
; (iii) 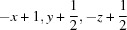 .
.
Acknowledgments
MH and HAW acknowledge the Malaysian Ministry of Science, Technology and Innovations (MOSTI) for funding the synthetic chemistry work under 304/PFARMASI/650600/I121. MH thanks Universiti Sains Malaysia for the award of a postgraduate fellowship
supplementary crystallographic information
1. Introduction
Thiopurine and its analogues possess a broad pharmacological activity for example as a cytotoxic agent (Nguyen et al., 2009) and in the treatment of lupus nephritis (Hadda et al., 2009). As part of our studies in this area, we report the synthesis and structure of the title compound.
2. Experimental
The method to synthesize the title compound was modified from a few papers (Banh et al., 2011; Salvatore et al., 2002; Salvatore et al., 2005). 2-amino-9H-purine-6-thiol (0.598 mmol) was mixed with cesium carbonate (0.598 mmol) in 3.5 ml of dimethylformamide and then stirred vigorously for 15 minutes. Another mixture containing benzyl bromide (1.315 mmol), tetrabutylammonium iodide (0.598 mmol) in 3.5 ml of DMF was added to the first mixture and the stirring was continued at room temperature for six hours. The reaction progress was monitored by TLC using n-hexane:ethyl acetate (0.5:3.5) as a solvent. After the product being formed, the reaction mixture was diluted with 70 mL of water and then extracted using 3 × 70 ml of ethyl acetate. The organic phase was collected, washed with 3× 70 ml of water and then dried over anhydrous magnesium sulfate. This organic phase was then evaporated in vacuo and the crude product was re-crystallized from a hot methanol to afford the title compound as colourless blocks.
2.1. Refinement
N bound H atoms were located from difference Fourier maps and freely refined. The remaining H atoms were positioned geometrically and refined using a riding model with with C–H = 0.95–0.99 Å and Uiso(H) = 1.2Ueq(C).
3. Results and discussion
The purin ring is almost planar with the maximum deviation of 0.014 (2)Å at atom C11. It makes a dihedral angle of 71.28 and 74.67 (8)° with the two benzene rings, C1—C6 and C14—C19, respectively and these two benzene rings make a dihedral angle of 76.04)10)° with each other (Fig. 1).
In the crytsal structure, two dimers involving N5—H1N5···N3i and C7—H7B···N3ii are observed. These two dimers formed stacked molecules down the b-axis. Intermolecular interactions of C8—H8A···N2iii further expand the molecules into infinite layers parallel to the bc-plane (Fig. 2).
Figures
Fig. 1.
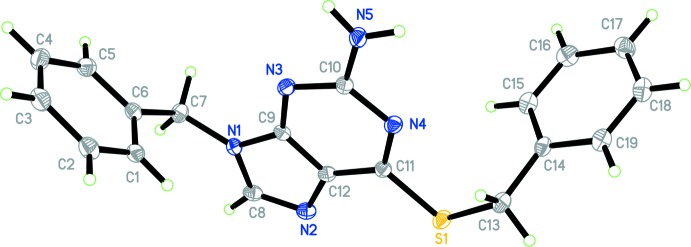
The asymmetric unit of the title compound, showing 50% probability displacement ellipsoids.
Fig. 2.
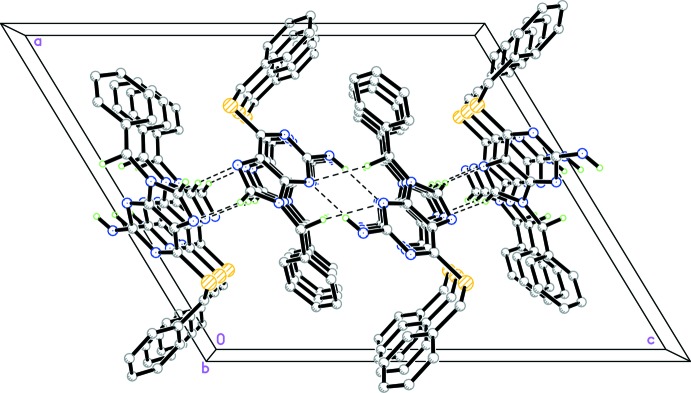
The crystal packing of (I). Dashed lines indicate hydrogen bonds. H atoms not involved in the hydrogen bond interactions have been omitted for clarity.
Crystal data
| C19H17N5S | F(000) = 728 |
| Mr = 347.44 | Dx = 1.420 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3425 reflections |
| a = 16.7346 (7) Å | θ = 2.3–30.0° |
| b = 5.5511 (3) Å | µ = 0.21 mm−1 |
| c = 20.4817 (10) Å | T = 100 K |
| β = 121.325 (3)° | Block, colourless |
| V = 1625.31 (14) Å3 | 0.69 × 0.19 × 0.14 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII CCD diffractometer | 4956 independent reflections |
| Radiation source: fine-focus sealed tube | 3416 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.048 |
| φ and ω scans | θmax = 30.6°, θmin = 2.0° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −23→23 |
| Tmin = 0.868, Tmax = 0.972 | k = −7→7 |
| 16728 measured reflections | l = −25→29 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.050 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.137 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0602P)2 + 0.4349P] where P = (Fo2 + 2Fc2)/3 |
| 4956 reflections | (Δ/σ)max < 0.001 |
| 234 parameters | Δρmax = 0.33 e Å−3 |
| 0 restraints | Δρmin = −0.58 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.24764 (3) | 0.72454 (8) | 0.12637 (3) | 0.01824 (13) | |
| N1 | 0.52964 (10) | 1.0733 (3) | 0.14394 (8) | 0.0147 (3) | |
| N2 | 0.42255 (10) | 1.0996 (3) | 0.17932 (8) | 0.0169 (3) | |
| N3 | 0.47003 (10) | 0.7208 (3) | 0.06126 (8) | 0.0148 (3) | |
| N4 | 0.32973 (10) | 0.5624 (3) | 0.05291 (8) | 0.0151 (3) | |
| N5 | 0.39014 (11) | 0.3896 (3) | −0.01293 (9) | 0.0183 (3) | |
| C1 | 0.70179 (12) | 0.7950 (3) | 0.20967 (10) | 0.0170 (4) | |
| H1A | 0.6619 | 0.7724 | 0.2293 | 0.020* | |
| C2 | 0.77591 (13) | 0.6376 (3) | 0.23027 (10) | 0.0200 (4) | |
| H2A | 0.7865 | 0.5080 | 0.2642 | 0.024* | |
| C3 | 0.83454 (13) | 0.6682 (4) | 0.20170 (10) | 0.0211 (4) | |
| H3A | 0.8838 | 0.5570 | 0.2147 | 0.025* | |
| C4 | 0.82082 (13) | 0.8621 (3) | 0.15408 (10) | 0.0210 (4) | |
| H4A | 0.8617 | 0.8863 | 0.1355 | 0.025* | |
| C5 | 0.74731 (12) | 1.0206 (3) | 0.13374 (10) | 0.0190 (4) | |
| H5A | 0.7385 | 1.1538 | 0.1016 | 0.023* | |
| C6 | 0.68604 (12) | 0.9858 (3) | 0.16023 (9) | 0.0154 (3) | |
| C7 | 0.60378 (12) | 1.1554 (3) | 0.13203 (10) | 0.0176 (4) | |
| H7A | 0.6271 | 1.3124 | 0.1581 | 0.021* | |
| H7B | 0.5767 | 1.1826 | 0.0767 | 0.021* | |
| C8 | 0.49754 (13) | 1.1938 (3) | 0.18504 (10) | 0.0165 (4) | |
| H8A | 0.5274 | 1.3330 | 0.2149 | 0.020* | |
| C9 | 0.46824 (11) | 0.8869 (3) | 0.10795 (9) | 0.0133 (3) | |
| C10 | 0.39805 (12) | 0.5647 (3) | 0.03571 (9) | 0.0144 (3) | |
| C11 | 0.33280 (12) | 0.7292 (3) | 0.10083 (9) | 0.0138 (3) | |
| C12 | 0.40253 (12) | 0.9046 (3) | 0.13023 (9) | 0.0141 (3) | |
| C13 | 0.19169 (13) | 0.4383 (3) | 0.08654 (10) | 0.0186 (4) | |
| H13A | 0.2409 | 0.3215 | 0.0950 | 0.022* | |
| H13B | 0.1636 | 0.3806 | 0.1160 | 0.022* | |
| C14 | 0.11669 (12) | 0.4322 (3) | 0.00268 (10) | 0.0166 (4) | |
| C15 | 0.10259 (13) | 0.6128 (3) | −0.04927 (10) | 0.0198 (4) | |
| H15A | 0.1421 | 0.7504 | −0.0327 | 0.024* | |
| C16 | 0.03108 (13) | 0.5934 (3) | −0.12532 (11) | 0.0221 (4) | |
| H16A | 0.0219 | 0.7190 | −0.1601 | 0.027* | |
| C17 | −0.02712 (13) | 0.3932 (3) | −0.15127 (11) | 0.0217 (4) | |
| H17A | −0.0761 | 0.3812 | −0.2033 | 0.026* | |
| C18 | −0.01235 (13) | 0.2113 (3) | −0.09980 (11) | 0.0217 (4) | |
| H18A | −0.0512 | 0.0726 | −0.1168 | 0.026* | |
| C19 | 0.05850 (13) | 0.2296 (3) | −0.02383 (11) | 0.0196 (4) | |
| H19A | 0.0677 | 0.1032 | 0.0107 | 0.023* | |
| H1N5 | 0.4310 (15) | 0.370 (4) | −0.0289 (12) | 0.030 (6)* | |
| H2N5 | 0.3390 (15) | 0.304 (4) | −0.0358 (12) | 0.024 (6)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0183 (2) | 0.0203 (2) | 0.0209 (2) | −0.00120 (18) | 0.0136 (2) | −0.00239 (18) |
| N1 | 0.0136 (7) | 0.0158 (7) | 0.0139 (7) | −0.0025 (6) | 0.0066 (6) | −0.0020 (6) |
| N2 | 0.0205 (8) | 0.0153 (7) | 0.0157 (7) | 0.0009 (6) | 0.0100 (6) | −0.0001 (6) |
| N3 | 0.0160 (7) | 0.0152 (7) | 0.0150 (7) | −0.0017 (6) | 0.0095 (6) | −0.0013 (6) |
| N4 | 0.0148 (7) | 0.0163 (7) | 0.0158 (7) | 0.0013 (6) | 0.0090 (6) | −0.0001 (6) |
| N5 | 0.0161 (8) | 0.0203 (8) | 0.0209 (8) | −0.0053 (7) | 0.0113 (7) | −0.0083 (6) |
| C1 | 0.0161 (9) | 0.0189 (9) | 0.0148 (8) | −0.0039 (7) | 0.0072 (7) | −0.0020 (7) |
| C2 | 0.0188 (9) | 0.0186 (9) | 0.0177 (9) | −0.0028 (7) | 0.0061 (7) | −0.0005 (7) |
| C3 | 0.0174 (9) | 0.0218 (9) | 0.0200 (9) | 0.0004 (7) | 0.0067 (8) | −0.0035 (7) |
| C4 | 0.0183 (9) | 0.0238 (10) | 0.0231 (9) | −0.0039 (8) | 0.0121 (8) | −0.0046 (8) |
| C5 | 0.0203 (9) | 0.0188 (9) | 0.0176 (8) | −0.0052 (7) | 0.0097 (8) | −0.0025 (7) |
| C6 | 0.0151 (9) | 0.0153 (8) | 0.0140 (8) | −0.0039 (7) | 0.0062 (7) | −0.0047 (6) |
| C7 | 0.0180 (9) | 0.0156 (8) | 0.0214 (9) | −0.0016 (7) | 0.0117 (8) | 0.0005 (7) |
| C8 | 0.0214 (9) | 0.0140 (8) | 0.0140 (8) | −0.0004 (7) | 0.0092 (7) | −0.0013 (6) |
| C9 | 0.0140 (8) | 0.0134 (8) | 0.0107 (7) | 0.0003 (6) | 0.0051 (6) | 0.0004 (6) |
| C10 | 0.0148 (8) | 0.0141 (8) | 0.0142 (8) | 0.0004 (7) | 0.0074 (7) | 0.0006 (6) |
| C11 | 0.0134 (8) | 0.0149 (8) | 0.0129 (8) | 0.0017 (6) | 0.0066 (7) | 0.0026 (6) |
| C12 | 0.0165 (8) | 0.0131 (8) | 0.0139 (8) | 0.0007 (7) | 0.0088 (7) | 0.0002 (6) |
| C13 | 0.0196 (9) | 0.0171 (9) | 0.0222 (9) | −0.0022 (7) | 0.0129 (8) | 0.0012 (7) |
| C14 | 0.0133 (8) | 0.0187 (9) | 0.0196 (8) | −0.0001 (7) | 0.0099 (7) | −0.0001 (7) |
| C15 | 0.0189 (9) | 0.0178 (9) | 0.0241 (9) | 0.0003 (7) | 0.0122 (8) | 0.0008 (7) |
| C16 | 0.0227 (10) | 0.0212 (9) | 0.0225 (9) | 0.0048 (8) | 0.0118 (8) | 0.0056 (8) |
| C17 | 0.0168 (9) | 0.0252 (10) | 0.0194 (9) | 0.0033 (8) | 0.0068 (7) | 0.0007 (8) |
| C18 | 0.0180 (9) | 0.0203 (9) | 0.0268 (10) | −0.0015 (7) | 0.0117 (8) | −0.0013 (8) |
| C19 | 0.0184 (9) | 0.0187 (9) | 0.0235 (9) | 0.0003 (7) | 0.0122 (8) | 0.0017 (7) |
Geometric parameters (Å, º)
| S1—C11 | 1.7551 (17) | C5—C6 | 1.400 (2) |
| S1—C13 | 1.8091 (18) | C5—H5A | 0.9500 |
| N1—C9 | 1.372 (2) | C6—C7 | 1.513 (2) |
| N1—C8 | 1.384 (2) | C7—H7A | 0.9900 |
| N1—C7 | 1.456 (2) | C7—H7B | 0.9900 |
| N2—C8 | 1.307 (2) | C8—H8A | 0.9500 |
| N2—C12 | 1.395 (2) | C9—C12 | 1.396 (2) |
| N3—C9 | 1.340 (2) | C11—C12 | 1.393 (2) |
| N3—C10 | 1.349 (2) | C13—C14 | 1.512 (2) |
| N4—C11 | 1.331 (2) | C13—H13A | 0.9900 |
| N4—C10 | 1.360 (2) | C13—H13B | 0.9900 |
| N5—C10 | 1.348 (2) | C14—C15 | 1.390 (2) |
| N5—H1N5 | 0.90 (2) | C14—C19 | 1.399 (2) |
| N5—H2N5 | 0.87 (2) | C15—C16 | 1.390 (3) |
| C1—C2 | 1.392 (2) | C15—H15A | 0.9500 |
| C1—C6 | 1.392 (2) | C16—C17 | 1.388 (3) |
| C1—H1A | 0.9500 | C16—H16A | 0.9500 |
| C2—C3 | 1.390 (3) | C17—C18 | 1.386 (3) |
| C2—H2A | 0.9500 | C17—H17A | 0.9500 |
| C3—C4 | 1.389 (3) | C18—C19 | 1.385 (3) |
| C3—H3A | 0.9500 | C18—H18A | 0.9500 |
| C4—C5 | 1.388 (3) | C19—H19A | 0.9500 |
| C4—H4A | 0.9500 | ||
| C11—S1—C13 | 100.87 (8) | N3—C9—N1 | 127.88 (15) |
| C9—N1—C8 | 105.78 (14) | N3—C9—C12 | 126.53 (15) |
| C9—N1—C7 | 127.68 (14) | N1—C9—C12 | 105.59 (14) |
| C8—N1—C7 | 125.79 (14) | N5—C10—N3 | 118.33 (15) |
| C8—N2—C12 | 103.57 (14) | N5—C10—N4 | 114.32 (15) |
| C9—N3—C10 | 111.85 (14) | N3—C10—N4 | 127.34 (15) |
| C11—N4—C10 | 117.98 (14) | N4—C11—C12 | 120.50 (15) |
| C10—N5—H1N5 | 123.6 (14) | N4—C11—S1 | 118.92 (13) |
| C10—N5—H2N5 | 118.7 (14) | C12—C11—S1 | 120.58 (13) |
| H1N5—N5—H2N5 | 117.1 (19) | C11—C12—N2 | 133.42 (15) |
| C2—C1—C6 | 120.00 (16) | C11—C12—C9 | 115.76 (15) |
| C2—C1—H1A | 120.0 | N2—C12—C9 | 110.81 (15) |
| C6—C1—H1A | 120.0 | C14—C13—S1 | 117.49 (13) |
| C3—C2—C1 | 120.58 (17) | C14—C13—H13A | 107.9 |
| C3—C2—H2A | 119.7 | S1—C13—H13A | 107.9 |
| C1—C2—H2A | 119.7 | C14—C13—H13B | 107.9 |
| C4—C3—C2 | 119.68 (18) | S1—C13—H13B | 107.9 |
| C4—C3—H3A | 120.2 | H13A—C13—H13B | 107.2 |
| C2—C3—H3A | 120.2 | C15—C14—C19 | 118.40 (17) |
| C5—C4—C3 | 119.92 (17) | C15—C14—C13 | 124.32 (16) |
| C5—C4—H4A | 120.0 | C19—C14—C13 | 117.28 (16) |
| C3—C4—H4A | 120.0 | C16—C15—C14 | 120.45 (17) |
| C4—C5—C6 | 120.63 (17) | C16—C15—H15A | 119.8 |
| C4—C5—H5A | 119.7 | C14—C15—H15A | 119.8 |
| C6—C5—H5A | 119.7 | C17—C16—C15 | 120.97 (17) |
| C1—C6—C5 | 119.13 (16) | C17—C16—H16A | 119.5 |
| C1—C6—C7 | 122.79 (15) | C15—C16—H16A | 119.5 |
| C5—C6—C7 | 118.07 (15) | C18—C17—C16 | 118.67 (17) |
| N1—C7—C6 | 115.28 (14) | C18—C17—H17A | 120.7 |
| N1—C7—H7A | 108.5 | C16—C17—H17A | 120.7 |
| C6—C7—H7A | 108.5 | C19—C18—C17 | 120.73 (18) |
| N1—C7—H7B | 108.5 | C19—C18—H18A | 119.6 |
| C6—C7—H7B | 108.5 | C17—C18—H18A | 119.6 |
| H7A—C7—H7B | 107.5 | C18—C19—C14 | 120.75 (17) |
| N2—C8—N1 | 114.24 (15) | C18—C19—H19A | 119.6 |
| N2—C8—H8A | 122.9 | C14—C19—H19A | 119.6 |
| N1—C8—H8A | 122.9 | ||
| C6—C1—C2—C3 | −0.2 (3) | C10—N4—C11—C12 | 2.0 (2) |
| C1—C2—C3—C4 | 2.1 (3) | C10—N4—C11—S1 | −178.00 (12) |
| C2—C3—C4—C5 | −1.6 (3) | C13—S1—C11—N4 | 9.65 (15) |
| C3—C4—C5—C6 | −0.6 (3) | C13—S1—C11—C12 | −170.34 (14) |
| C2—C1—C6—C5 | −2.0 (2) | N4—C11—C12—N2 | 178.87 (17) |
| C2—C1—C6—C7 | 176.53 (16) | S1—C11—C12—N2 | −1.1 (3) |
| C4—C5—C6—C1 | 2.4 (2) | N4—C11—C12—C9 | −1.9 (2) |
| C4—C5—C6—C7 | −176.18 (16) | S1—C11—C12—C9 | 178.12 (12) |
| C9—N1—C7—C6 | −69.9 (2) | C8—N2—C12—C11 | 179.67 (19) |
| C8—N1—C7—C6 | 121.46 (18) | C8—N2—C12—C9 | 0.37 (18) |
| C1—C6—C7—N1 | −14.7 (2) | N3—C9—C12—C11 | 0.5 (3) |
| C5—C6—C7—N1 | 163.78 (15) | N1—C9—C12—C11 | −179.30 (14) |
| C12—N2—C8—N1 | −0.76 (19) | N3—C9—C12—N2 | 179.94 (15) |
| C9—N1—C8—N2 | 0.87 (19) | N1—C9—C12—N2 | 0.13 (18) |
| C7—N1—C8—N2 | 171.58 (15) | C11—S1—C13—C14 | −83.26 (14) |
| C10—N3—C9—N1 | −179.61 (16) | S1—C13—C14—C15 | 15.4 (2) |
| C10—N3—C9—C12 | 0.6 (2) | S1—C13—C14—C19 | −165.09 (13) |
| C8—N1—C9—N3 | 179.63 (17) | C19—C14—C15—C16 | 1.2 (3) |
| C7—N1—C9—N3 | 9.2 (3) | C13—C14—C15—C16 | −179.25 (16) |
| C8—N1—C9—C12 | −0.55 (17) | C14—C15—C16—C17 | −0.6 (3) |
| C7—N1—C9—C12 | −171.03 (16) | C15—C16—C17—C18 | −0.3 (3) |
| C9—N3—C10—N5 | 178.94 (15) | C16—C17—C18—C19 | 0.6 (3) |
| C9—N3—C10—N4 | −0.5 (2) | C17—C18—C19—C14 | 0.0 (3) |
| C11—N4—C10—N5 | 179.75 (15) | C15—C14—C19—C18 | −0.9 (3) |
| C11—N4—C10—N3 | −0.8 (3) | C13—C14—C19—C18 | 179.50 (16) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N5—H1N5···N3i | 0.91 (3) | 2.14 (3) | 3.040 (3) | 173 (2) |
| C7—H7B···N3ii | 0.99 | 2.57 | 3.548 (2) | 172 |
| C8—H8A···N2iii | 0.95 | 2.39 | 3.274 (2) | 155 |
Symmetry codes: (i) −x+1, −y+1, −z; (ii) −x+1, −y+2, −z; (iii) −x+1, y+1/2, −z+1/2.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: HB7176).
References
- Banh, T. N., Kode, N. R. & Phadtare, S. (2011). Lett. Drug. Des. Discov. 8, 709–716.
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Hadda, V., Pandey, B. D., Gupta, R. & Goel, A. (2009). JPGM, 55, 139–140. [DOI] [PubMed]
- Nguyen, T., Vacek, P. M., O’Neill, P., Colletti, R. B. & Finette, B. A. (2009). Cancer Res. 69, 7004–7012. [DOI] [PMC free article] [PubMed]
- Salvatore, R. N., Nagle, A. S. & Jung, K. W. (2002). J. Org. Chem. 67, 674–683. [DOI] [PubMed]
- Salvatore, R. N., Smith, R. A., Nischwitz, A. K. & Gavin, T. (2005). Tetrahedron Lett. 46, 8931–8935.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536814001986/hb7176sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814001986/hb7176Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814001986/hb7176Isup3.cml
CCDC reference: http://scripts.iucr.org/cgi-bin/cr.cgi?rm=csd&csdid=983914
Additional supporting information: crystallographic information; 3D view; checkCIF report


