Abstract
In the title compound, C8H3N3O2 (systematic name: 4-nitrobenzene-1,2-dicarbonitrile), the nitro group is twisted out of the plane of the benzene ring to which it is attached [O—N—Cring—Cring torsion angle = 9.80 (13)°]. In the crystal packing, supramolecular layers with a zigzag topology in the ac plane are sustained by C—H⋯N interactions.
Related literature
For background to the synthesis of functional phthalocyanines, see: Chin et al. (2012 ▶). For a related structure, see: Lin et al. (2006 ▶). For the synthesis, see: Rasmussen et al. (1978 ▶).
Experimental
Crystal data
C8H3N3O2
M r = 173.13
Orthorhombic,

a = 12.8642 (3) Å
b = 9.2013 (2) Å
c = 13.2578 (3) Å
V = 1569.29 (6) Å3
Z = 8
Cu Kα radiation
μ = 0.94 mm−1
T = 100 K
0.35 × 0.30 × 0.25 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2013 ▶) T min = 0.626, T max = 1.000
7104 measured reflections
1638 independent reflections
1556 reflections with I > 2σ(I)
R int = 0.016
Refinement
R[F 2 > 2σ(F 2)] = 0.030
wR(F 2) = 0.088
S = 1.10
1638 reflections
130 parameters
All H-atom parameters refined
Δρmax = 0.21 e Å−3
Δρmin = −0.24 e Å−3
Data collection: CrysAlis PRO (Agilent, 2013 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶) and DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) general, I. DOI: 10.1107/S1600536814003468/wm5005sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814003468/wm5005Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814003468/wm5005Isup3.cml
CCDC reference: 987296
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯N3i | 0.962 (14) | 2.621 (14) | 3.3880 (13) | 136.9 (11) |
| C3—H3⋯N2ii | 0.950 (14) | 2.554 (14) | 3.3955 (13) | 147.8 (10) |
| C6—H6⋯N3iii | 0.943 (13) | 2.457 (13) | 3.3412 (13) | 156.1 (10) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii) 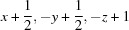 .
.
Acknowledgments
We gratefully acknowledge funding from the Brunei Research Council, and thank the Ministry of Higher Education (Malaysia) and the University of Malaya for funding structural studies through the High-Impact Research scheme (UM.C/HIR-MOHE/SC/03).
supplementary crystallographic information
1. Chemical context
As part of our on-going study of functional phthalocyanines, we have previously reported the synthesis and structure of 4-(prop-2-yn-1-yloxy)benzene-1,2-dicarbonitrile prepared from 4-nitrophthalonitrile (Chin et al., 2012). We now report the structure of the latter.
2. Structural commentary
In the title compound (Fig. 1), the nitro group is slightly twisted out of the plane of the benzene ring to which it is attached as seen in the value of the O1—N1—C1—C6 torsion angle of 9.80 (13)°. A similar small twist was found in the structure of the most closely related compound in the literature, i.e. 4-bromo-5-nitrophthalonitrile (Lin et al., 2006).
3. Supramolecular features
Supramolecular layers (Fig. 2) sustained by C—H···N interactions which form 22-membered {···NC4N···HC2H···NC3H···NC5H} synthons (Table 1) features in the crystal packing. The layers have a zigzag topolgy and extend parallel to the ac plane and stack along the b axis (Fig. 3).
4. Database survey
5. Synthesis and crystallization
The title compound was prepared by a literature procedure (Rasmussen et al., 1978). Thionyl chloride (4.3 ml, 0.56 mmol) was added drop wise with stiring over 5 minutes to 4-nitrophthalamide (2.83 g, 13.5 mmol) in dry DMF (10.4 ml, 0.20 mmol) at 263 to 258 K (salt-ice bath). After 4 h, the homogenous yellow solution was poured onto excess ice-water with vigorous stirring. The precipitate was vacuum filtered, washed with cold water and dried. Crystals for the X-ray study were grown from slow evaporation from its methanol solution. Yield = 1.92 g (68 %), M.pt: 408–413 K (lit. 413–415 K). IR (KBr) ν/cm-1: 2924, 2241, 1610, 1587, 1538, 1463, 1356, 1297, 1076, 931, 855, 802, 745, 718.
6. Refinement
All hydrogen atoms were refined freely.
Figures
Fig. 1.
The molecular structure of the title compound showing the atom-labelling scheme and displacement ellipsoids at the 70% probability level.
Fig. 2.
A view of the supramolecular layer in the title compound, sustained by C—H···N interactions shown as orange dashed lines.
Fig. 3.
A view of the unit-cell contents of the title compound in projection down the a axis. The C—H···N interactions are shown as orange dashed lines.
Crystal data
| C8H3N3O2 | F(000) = 704 |
| Mr = 173.13 | Dx = 1.466 Mg m−3 |
| Orthorhombic, Pbca | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 4240 reflections |
| a = 12.8642 (3) Å | θ = 3.3–76.1° |
| b = 9.2013 (2) Å | µ = 0.94 mm−1 |
| c = 13.2578 (3) Å | T = 100 K |
| V = 1569.29 (6) Å3 | Prism, colourless |
| Z = 8 | 0.35 × 0.30 × 0.25 mm |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 1638 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 1556 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.016 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 76.3°, θmin = 6.7° |
| ω scan | h = −8→16 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2013) | k = −10→11 |
| Tmin = 0.626, Tmax = 1.000 | l = −16→16 |
| 7104 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.030 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.088 | All H-atom parameters refined |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0522P)2 + 0.3777P] where P = (Fo2 + 2Fc2)/3 |
| 1638 reflections | (Δ/σ)max < 0.001 |
| 130 parameters | Δρmax = 0.21 e Å−3 |
| 0 restraints | Δρmin = −0.24 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.55783 (6) | 0.56540 (8) | 0.23140 (6) | 0.0224 (2) | |
| O2 | 0.41285 (6) | 0.62811 (9) | 0.16017 (6) | 0.0284 (2) | |
| N1 | 0.46305 (7) | 0.56035 (9) | 0.22296 (6) | 0.0185 (2) | |
| N2 | 0.49465 (7) | 0.20831 (10) | 0.59515 (7) | 0.0245 (2) | |
| N3 | 0.20184 (7) | 0.10850 (10) | 0.54001 (7) | 0.0214 (2) | |
| C1 | 0.40488 (7) | 0.46610 (10) | 0.29308 (7) | 0.0159 (2) | |
| C2 | 0.30038 (8) | 0.44112 (11) | 0.27448 (7) | 0.0185 (2) | |
| C3 | 0.24639 (7) | 0.34933 (11) | 0.33907 (7) | 0.0183 (2) | |
| C4 | 0.29752 (7) | 0.28688 (10) | 0.42094 (7) | 0.0158 (2) | |
| C5 | 0.40307 (7) | 0.31687 (10) | 0.43884 (7) | 0.0152 (2) | |
| C6 | 0.45807 (7) | 0.40675 (10) | 0.37365 (7) | 0.0156 (2) | |
| C7 | 0.45469 (7) | 0.25464 (10) | 0.52497 (7) | 0.0176 (2) | |
| C8 | 0.24319 (7) | 0.18890 (10) | 0.48760 (7) | 0.0173 (2) | |
| H2 | 0.2670 (11) | 0.4827 (16) | 0.2163 (10) | 0.027 (3)* | |
| H3 | 0.1745 (11) | 0.3317 (13) | 0.3282 (9) | 0.020 (3)* | |
| H6 | 0.5294 (10) | 0.4254 (13) | 0.3839 (9) | 0.017 (3)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0156 (4) | 0.0246 (4) | 0.0271 (4) | −0.0022 (3) | 0.0032 (3) | 0.0031 (3) |
| O2 | 0.0248 (4) | 0.0300 (4) | 0.0304 (4) | −0.0019 (3) | −0.0029 (3) | 0.0151 (3) |
| N1 | 0.0177 (4) | 0.0174 (4) | 0.0204 (4) | −0.0004 (3) | 0.0013 (3) | 0.0015 (3) |
| N2 | 0.0198 (4) | 0.0303 (5) | 0.0235 (5) | 0.0001 (3) | −0.0016 (3) | 0.0064 (4) |
| N3 | 0.0183 (4) | 0.0241 (4) | 0.0217 (4) | −0.0026 (3) | 0.0008 (3) | 0.0014 (3) |
| C1 | 0.0166 (5) | 0.0145 (4) | 0.0167 (5) | 0.0002 (3) | 0.0030 (3) | −0.0003 (3) |
| C2 | 0.0176 (5) | 0.0204 (5) | 0.0174 (5) | 0.0014 (3) | −0.0017 (3) | 0.0002 (4) |
| C3 | 0.0132 (5) | 0.0219 (5) | 0.0199 (5) | −0.0009 (3) | −0.0010 (3) | −0.0005 (4) |
| C4 | 0.0155 (4) | 0.0152 (4) | 0.0166 (4) | −0.0002 (3) | 0.0021 (3) | −0.0022 (3) |
| C5 | 0.0152 (4) | 0.0144 (4) | 0.0159 (4) | 0.0021 (3) | −0.0001 (3) | −0.0020 (3) |
| C6 | 0.0125 (4) | 0.0153 (4) | 0.0190 (5) | 0.0005 (3) | 0.0006 (3) | −0.0021 (4) |
| C7 | 0.0135 (4) | 0.0185 (5) | 0.0208 (5) | −0.0015 (3) | 0.0019 (3) | 0.0000 (4) |
| C8 | 0.0139 (4) | 0.0196 (5) | 0.0184 (4) | 0.0002 (3) | −0.0014 (3) | −0.0023 (4) |
Geometric parameters (Å, º)
| O1—N1 | 1.2252 (12) | C2—H2 | 0.962 (14) |
| O2—N1 | 1.2243 (11) | C3—C4 | 1.3932 (13) |
| N1—C1 | 1.4752 (12) | C3—H3 | 0.949 (13) |
| N2—C7 | 1.1453 (13) | C4—C5 | 1.4057 (13) |
| N3—C8 | 1.1459 (13) | C4—C8 | 1.4431 (13) |
| C1—C6 | 1.3811 (14) | C5—C6 | 1.3898 (13) |
| C1—C2 | 1.3859 (14) | C5—C7 | 1.4397 (13) |
| C2—C3 | 1.3889 (14) | C6—H6 | 0.943 (13) |
| O2—N1—O1 | 124.59 (8) | C3—C4—C5 | 120.44 (9) |
| O2—N1—C1 | 117.40 (8) | C3—C4—C8 | 120.41 (8) |
| O1—N1—C1 | 118.01 (8) | C5—C4—C8 | 119.15 (8) |
| C6—C1—C2 | 123.54 (9) | C6—C5—C4 | 120.23 (9) |
| C6—C1—N1 | 117.94 (8) | C6—C5—C7 | 119.68 (8) |
| C2—C1—N1 | 118.52 (9) | C4—C5—C7 | 120.09 (8) |
| C1—C2—C3 | 118.44 (9) | C1—C6—C5 | 117.66 (9) |
| C1—C2—H2 | 120.7 (8) | C1—C6—H6 | 121.4 (8) |
| C3—C2—H2 | 120.8 (8) | C5—C6—H6 | 120.9 (8) |
| C2—C3—C4 | 119.67 (9) | N2—C7—C5 | 178.04 (11) |
| C2—C3—H3 | 119.8 (8) | N3—C8—C4 | 178.30 (10) |
| C4—C3—H3 | 120.5 (8) | ||
| O2—N1—C1—C6 | −170.25 (9) | C3—C4—C5—C7 | 178.52 (9) |
| O1—N1—C1—C6 | 9.80 (13) | C8—C4—C5—C7 | −2.37 (13) |
| O2—N1—C1—C2 | 10.12 (13) | C2—C1—C6—C5 | 0.25 (14) |
| O1—N1—C1—C2 | −169.82 (9) | N1—C1—C6—C5 | −179.36 (8) |
| C6—C1—C2—C3 | −1.26 (15) | C4—C5—C6—C1 | 1.09 (14) |
| N1—C1—C2—C3 | 178.34 (8) | C7—C5—C6—C1 | −178.85 (8) |
| C1—C2—C3—C4 | 0.91 (15) | C6—C5—C7—N2 | 96 (3) |
| C2—C3—C4—C5 | 0.38 (14) | C4—C5—C7—N2 | −84 (3) |
| C2—C3—C4—C8 | −178.71 (9) | C3—C4—C8—N3 | 122 (3) |
| C3—C4—C5—C6 | −1.41 (14) | C5—C4—C8—N3 | −57 (4) |
| C8—C4—C5—C6 | 177.69 (8) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···N3i | 0.962 (14) | 2.621 (14) | 3.3880 (13) | 136.9 (11) |
| C3—H3···N2ii | 0.950 (14) | 2.554 (14) | 3.3955 (13) | 147.8 (10) |
| C6—H6···N3iii | 0.943 (13) | 2.457 (13) | 3.3412 (13) | 156.1 (10) |
Symmetry codes: (i) x, −y+1/2, z−1/2; (ii) x−1/2, −y+1/2, −z+1; (iii) x+1/2, −y+1/2, −z+1.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: WM5005).
References
- Agilent (2013). CrysAlis PRO Agilent Technologies Inc., Santa Clara, CA, USA.
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Chin, Y. J., Tan, A. L., Wimmer, F. L., Mirza, A. H., Young, D. J., Ng, S. W. & Tiekink, E. R. T. (2012). Acta Cryst. E68, o2293–o2294. [DOI] [PMC free article] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Lin, M.-J., Wang, J.-D., Chen, N.-S. & Huang, J.-L. (2006). J. Coord. Chem. 59, 607–615.
- Rasmussen, C. R., Gardocki, J. F., Plampin, J. N., Twardzik, B. L., Reynolds, B. E., Molinari, A. J., Schwartz, N., Bennetts, W. W., Price, B. E. & Marakowski, J. (1978). J. Med. Chem. 21, 1044–1054. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) general, I. DOI: 10.1107/S1600536814003468/wm5005sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814003468/wm5005Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814003468/wm5005Isup3.cml
CCDC reference: 987296
Additional supporting information: crystallographic information; 3D view; checkCIF report





