Abstract
Sphingolipid-metabolizing enzymes are becoming targets for chemotherapeutic development with an increasing interest in the recent years. In this chapter we introduce the sphingolipid family of lipids, and the role of individual species in cell homeostasis. We also discuss their roles in several rare diseases and overall, in cancer transformation. We follow the biosynthesis pathway of the sphingolipid tree, focusing on the enzymes in order to understand how using small molecule inhibitors makes it possible to modulate cancer progression. Finally, we describe the most used and historically significant inhibitors employed in cancer research, their relationships to sphingolipid metabolism, and some promising results found in this field.
Keywords: Ceramide, Sphingosine, Glycosphingolipid, Cancer, Myriocin, Cycloserine, Fumonisin B1, 4-HRP, Scyphostatin, GW4869, Desipramine, NOE, B13, E-tb, MAPP, PDMP, Safingol, PF-543, FTY720, ABC294640
1 Sphingolipids
1.1 A Brief Introduction of Sphingolipids
Sphingolipids are a vast family of naturally occurring lipids containing a D-erythro-sphingoid (Shapiro and Flowers 1962) backbone (in sphingosine, (E,2S,3R)-2-aminooctadec-4-ene-1,3-diol). The sphingolipid group encompasses a growing variety of structures such as lysosphingolipids, ceramides, cerebrosides, sulfatides, and gangliosides. Sphingolipids were discovered studying the chemical composition of the brain. The father of neurochemistry, JLW Thudichum, described for the first time sphingomyelin and sphingosine in a study published in 1884. At that time, and for a long time thereafter, sphingolipids were considered as structural compounds in biological membranes (Stoffel 1970) of eukaryotes and in plasma lipoproteins. As an exception, sphingolipids have been also found in the Sphingomonas bacterial genus and other bacteria (Olsen and Jantzen 2001). Working as structural components, sphingomyelin and glycosphingolipids are the most abundant sphingolipids occurring in cells, normally accounting for 10–30 % of lipids in the cellular membranes whereas other sphingolipids such as ceramide or sphingosine are much less abundant. However, as it has been found for other lipids, the role of sphingolipids was found not to be only structural. In the middle 1980s sphingosine was found to inhibit protein kinase C, suggesting a bioactive role for sphingolipids as a second messenger (Hannun et al. 1986). After that, ceramide was shown to have regulatory roles in the cell. Following these findings, their phosphorylated forms, ceramide-1-phosphate (C1P) and sphingosine 1-phospate, were also described to have roles in apoptosis, proliferation, senescence, angiogenesis, and vesicular trafficking (Hannun and Obeid 2008). Interestingly, ceramide and sphingosine 1-phosphate, separated only by two bidirectional metabolic steps, have been described to exert opposites effects in the cell. Thus, ceramide has been reported to trigger apoptosis and cell arrest whereas S1P enhances cell survival and cell proliferation.
1.2 Biosynthesis of Sphingolipids
The de novo biosynthesis of sphingolipids in mammals begins by the condensation of serine with palmitoyl-coenzyme A to form 3-ketosphinganine, catalyzed by serine-palmitoyl transferase (SPT), a pyridoxal 5′-phosphate-dependent enzyme, and then reduced to sphinganine (Fig. 1). Less abundant, and biologically less studied, glycine or alanine can be incorporated instead of serine to form 1-desoxymethyl- or 1-deoxy- derivatives, respectively. N-acylation of the amino group of sphinganine with several possible coenzyme A-activated fatty acid (normally between C16/C16:1 and C24/C24:1, although shorter and longer backbone chains have been described, as well as a variety of backbone modifications Abe et al. 1996) leads to dihydroceramides, and this step is catalyzed by at least 6 known (dihydro)ceramide synthases (CerS1-6), each one with different fatty acid length preference. These enzymes are also responsible for N-acylation of sphingosine. Ceramides are formed by desaturation of dihydroceramides by dihydroceramide desaturase (DES) (Fabrias et al. 2012).
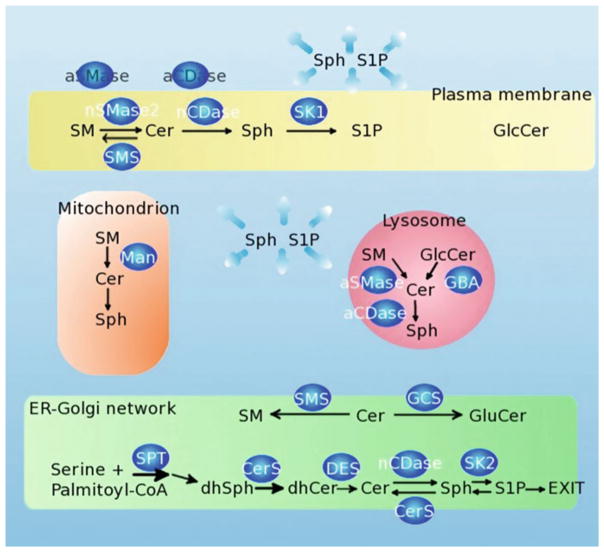
Fig. 1.
Sphingolipid pathway and subcellular localization of sphingolipid enzymes. The biosynthesis of sphingolipids begins in the endoplasmatic reticulum (ER)–Golgi network. Vesicular transport distributes sphingolipids to different compartments such as plasma membrane, and lysosome. Sphingolipids and a sphingomyelinase (Man) have been found in the mitochondrion. a/nSMase acid/neutral sphingonyelinase, a/nCDase acid/neutral ceramidase, nSMase neutral sphingomyelinase, SK1/2 sphingosine kinase 1/2, Man mitochondrial sphingomyelinase, GBA acid β-glucosidase, SMS sphingomyelinase synthase, DES dihydroceramide desaturase, GCS glucosylceramide synthase, SPT serine palmitoyl-CoA transferase
Classically, ceramide was treated as a single biological entity; however, ceramide is a family of structurally related molecules, and recently researchers have realized the biological diversity that may accompany this structural diversity. In mammals, it is possible to identify a growing number of ceramides, which are over 300 structures. Increasing the complexity, the same species of ceramide may be found in different subcellular compartments, in biological fluids, or in different metabolic contexts in the cell. This variability in structure and localization raised the concept of many ceramides (Hannun and Obeid 2011), with different possible functions.
Ceramides are also the central hub from which diverse chemical modifications such as N-acyl hydrolysis or esterification in C1 result in hundreds of diverse structures with different potential functions. Moreover, the catabolism of more complex sphingolipids leads to ceramide formation, which can be converted to other complex sphingolipids, or continue its catabolism. That positions ceramide as a central key regulatory step in the whole sphingolipid diversity and function.
One of the fates of ceramides is its hydrolysis by ceramidases (N-acylsphingosine amidohydrolase, EC 3.5.1.23, here abbreviated as CDase) to form sphingosine, which is considered a bioactive molecule by itself (as mentioned before it was discovered to inhibit PKC), and it is the main sphingoid base in mammals. There are different ceramidases in human, codified by five different genes. Depending on their pH range of activity, they have been named as acid, neutral, and alkaline. Acid CDase (aCDase) is codified by the gene ASAH1. The optimum activity of aCDase is pH 4.5, and it is found in the lysosome, secreted in the media, and it could also have a role in trafficking vesicles. Another CDase has its optimum activity at pH 7–9, and it has been dubbed neutral CDase (nCDase, codified by the gene ASAH2) which has been localized bound to the plasma membrane, and also secreted to the intestinal lumen, but also may be present in mitochondria (Novgorodov et al. 2011). Finally, three different CDases with activity at pH 8.5–9.5, named as alkaline CDases (alkCDase, codified by the genes ACER1 or ASAH3, ACER2, and ACER3), are localized in the endoplasmic reticulum and the Golgi complex (Mao et al. 2001).
The potential modifications of sphingosine are N-acylation (ceramides), N-methylation (N, N-dimethylsphingosine, DMS; N, N, N-trimethylsphingosine, TMS), and phosphorylation. Additionally, sphingosine can be reverted/recycled to ceramide by reverse activity of CDases or by CerS activity (the same enzyme seen working on the N-acylation of dihydrosphingosine in the biosynthesis of de novo dihydroceramide/ceramide). The N-acylation by CDases does not require fatty acid activation by coenzyme A, as it is necessary for N-acylations catalyzed by CerS (El Bawab et al. 2001; Mao et al. 2000). Phosphorylation of sphingosine to sphingosine 1-phosphate is carried out by one of the two sphingosine kinases (SK1, 2). DMS also has been reported to be phosphorylated to DMS-1-phosphate; however this compound has not been further studied (Yatomi et al. 1997).
Sphingosine 1-phosphate is a bioactive lipid, triggering biological responses such as cell migration, cell proliferation, and angiogenesis through five known GPCR receptors (S1P1-5) (Hannun and Obeid 2008; Maceyka et al. 2012; Pyne and Pyne 2011; Sanchez and Hla 2004). The biological effects of sphingosine 1-phosphate might be also mediated in a receptor-independent way, and intracellular targets have been described to mediate calcium homeostasis and cell growth (Spiegel and Milstien 2011). In blood, sphingosine 1-phosphate concentration is elevated (around 400 nM), mainly associated with lipoproteins and albumin. Stimulated platelets are a main source of blood sphingosine 1-phosphate. As seen before for other sphingolipids, the conversion to sphingosine 1-phosphate is reversible, and it can return to sphingosine by the action of phosphatases (SPP1 and SPP2). Importantly, sphingosine 1-phosphate can be irreversibly hydrolyzed by sphingosine 1-phosphate lyase (SPL) to hexadecenal and ethanolamine-phosphate, which are non-sphingolipid molecules, and it is the only exit point of the sphingolipid network. Interestingly, as with SPT (the entrance point enzyme to the sphingolipid structure), also SPL (the exit point) is a pyridoxal 5′-phosphate-dependent enzyme (Bourquin et al. 2011).
Returning to ceramide, its phosphorylation by ceramide kinase (CK) results in C1P, another bioactive sphingolipid involved in cell homeostasis, inflammation, and cell migration through interaction with phospholipase A1 and possibly also by acting on a G-protein-coupled receptor (Granado et al. 2009). Growing in complexity, two sphingomyelin synthases (SMS1, 2) add a phosphocholine group, from phosphatidylcholine, to ceramide resulting in formation of sphingomyelin and diacylglycerol. Sphingomyelin is one of the main structural lipid of biological membranes, and it has been described to form defined domains in the plasma membrane together with cholesterol, offering localization domains for certain proteins.
The hydrolysis of sphingomyelin by several sphingomyelinases (SMase) generates ceramide in different compartments, which can then trigger several and distinct cellular responses. As we saw for CDase, different genes also encode for SMases: an acid SMase (aSMAse, sphingomyelin phosphodiesterase 1, codified by the gene SMPD1) which can be lysosomal or secreted to the extracellular matrix (Jenkins et al. 2009). Three neutral SMase genes (nSMAse1, nSMase2, and nSMase3) have been identified and cloned. However, mammalian nSMase1, although with bacterial neutral SMase sequence homology, and in vitro SMase activity, acts primarily as a lyso-PAF phospholipase C in vivo, with no SMase detected in cells (Marchesini et al. 2003). On the other hand, nSMase2 shows SMase activity in vitro and in vivo, whereas nSMase3 has been shown to exhibit SMase activity in some studies but not others. As such, nSMase2 has been much more studied and found to be involved in a myriad of biological effects in cells (Wu et al. 2010). All of the cloned nSMase proteins require addition of magnesium in vitro assays, although only nSMase2 has been shown to really increase sphingomyelin hydrolysis in vivo when overexpressed in breast cancer MCF-7 cell line. Moreover, an additional SMase, a magnesium-independent nSMase activity has been described in cytosolic fractions. Nevertheless, this activity has not been associated to a gene yet (Okazaki et al. 1994).
Other modifications in the ceramide structure include condensation of hexosyl units. Commonly, in the endoplasmic reticulum, units of glucose or galactose are attached in β-linkage to ceramide forming the cerebrosides glucosyl-ceramide and galactosyl-ceramide by glucosylceramide transferase and galactosylceramide transferase, respectively. The addition of more units of sugars to the glucosylceramide structure including different numbers and combinations of galactose, glucose, N-acetylgalactosamine, neuraminic acid (sialic acid), fucose, etc. in a precise sequence, bond configuration, and with or without ramifications defines different subcategories of glycosphingolipids, such as globo-sphingolipids, gangliosides (rich in sialic acid, forming several series: GMs, GD, GTs, GQs, GPs), and lactoseries. Sulfation of galactoceramide by galactosylceramide sulfotransferase results in another subgroup of glycosphingolipids, named sulfatides, rich in axonal myelin sheath and associated with apolipoprotein E in cerebrospinal fluid. Glycosphingolipids in general have been identified as antigens defining for example some of the blood groups, and used to define tumor-associated antigens. Glycosphingolipids are also involved in cis and trans cell interactions, contact cell growth inhibition, cell adhesion, and signal transduction (Hakomori 2008).
1.3 Sphingolipids and Disease
The enormous variety of sphingolipid structures imparts on the family a tremendous range of biological functions, which are commonly discovered in disease conditions. The misregulation of one sphingolipid metabolic enzyme, a sphingolipid receptor, or any other sphingolipid-modulated protein can develop several severe and fatal diseases. Thus, there is a collection of lysosome storage diseases due to a mutation in different lysosomal sphingolipid catabolic enzymes resulting in accumulation of one or another sphingolipid in the lysosome and provoking cellular, tissue, and organ failure. The lack of aCDase activity in the lysosome results in Farber disease. There is an accumulation of ceramide in the lysosomes, linked to a deficient neurological and general organ development, normally with short life span. A deficiency of alpha-galactosidase A (GLA) causes Fabry’s disease, a multisystemic accumulation of globotriaosylceramide which results in severe complications in kidney, heart, and brain (Schaefer et al. 2009; Tarabuso 2011) commonly leading to early death. In Niemann–Pick disease, there is a lack of aSMase activity, resulting in storage of sphingomyelin in the endolysosomal compartment. The disease can present with different severities. Niemann–Pick type A (Ledesma et al. 2011) develops a severe neurological pathology with shorter life span. Type B is not as severe as type A but it still has a life expectancy during adulthood. Another lysosomal storage disorder is Gaucher’s disease, the most common lysosomal storage disorder. Gaucher’s disease presents accumulation of glucosylceramide due to lack of the lysosomal enzyme that hydrolyzes glucosylceramide to ceramide and glucose, glucocerebrosidase (acid β-glucosidase, GBA1 gene). Gaucher’s disease patients often show visceral disorders, and in some severe cases neurological abnormalities (Farfel-Becker et al. 2011). Moreover, accumulation of GM2 gangliosides comprises three different disorders (Tay–Sachs disease, Sandhoff disease, and the very rare GM2A deficiency) including GM2 gangliosidosis due to deficiency in beta-hexosaminidase activity. Accumulation of GM1 by beta-galactosidase activity deficiency results in GM1 gangliosidosis. Both types of gangliosidosis are fatal. Finally, accumulations of sulfatides are described in metachromatic leukodystrophy. These diseases illustrate how important is the regulation of the sphingolipid pathway, and how a malfunction of one of their enzymes seems not to have another exit than the accumulation of the lipid. Mutations in SPT underlie hereditary autonomic neuropathy which has been mechanistically linked to formation of deoxysphingolipids.
Other diseases have been also related to malfunction of the sphingolipid pathway, including Alzheimer’s disease, diabetes, atherosclerosis, cystic fibrosis, Wilson disease, and cancer. In this review, it is in cancer that we focus the drug development status involving targets in the sphingolipid pathway.
2 Sphingolipids and Cancer
Aberrations in bioactive sphingolipids, mainly ceramide, sphingosine 1-phosphate, and gangliosides, have been linked to several steps in cancer progression and response to cancer treatment, including resistance to chemotherapeutics.
In mammalian cells, accumulation of certain ceramide species in certain subcellular localizations might play a role in apoptosis (Birbes et al. 2002), cell cycle arrest, and inhibition of cell motility. Apoptosis is a natural response in tissue homeostasis, immune cell maturation, elimination of damaged or infected cells (for example exposed to UV-C light, or viral infection), or in the ontogenic process. Apoptosis can be triggered by a variety of stimuli such as cytokines (for example TNF) or DNA damage (activating p53 protein). In some cellular models the apoptotic mechanism has been shown to involve ceramide accumulation. Thus, several cancer cells and cancer models have been reported to have significant alterations in the enzymes involved in ceramide generation (aSMase, nSMAse, CerS) or degradation (aCDase Flowers et al. 2011, nCDase), often resulting in loss of ceramide, and could be one of the reasons of a cancer cell behavior, avoiding cell death.
In an opposite direction, changes in metabolism of sphingosine 1-phosphate have also been seen in cancer. S1P has been shown to induce cell proliferation, angiogenesis, cell invasion, and cell migration through five known G-protein-coupled receptors (S1P1-5). The enzymes responsible to catalyze sphingosine 1-phosphate have been reported to be overexpressed in several cancers, and thus, elevated levels of sphingosine 1-phosphate have been also reported in cancer cells and tissues. Especially relevant to cancer biology is the protein p53 which recently has been found to act upstream of SK1/sphingosine 1-phosphate pathway (Heffernan-Stroud et al. 2012).
Aberrant glycosylation has become a marker of the majority of cancers (Durrant et al. 2012). One of the recognized functions for glycosphingolipids is cell adhesion and cell motility. Changing the pattern of glycosphingolipid by expressing aberrant glycosphingolipids, and/or truncating the glycosphingolipids biosynthetic pathway, or overexpression of the glycosphingolipids, can dramatically promote or inhibit the spreading of cancer cells.
Because of these multiple contributions of sphingolipid in regulating cancer cells, their key enzymes have become targets in drug development.
3 Chemotherapeutical Drugs
Despite the observation that many sphingolipid enzymes have been implicated directly in cancer pathobiology, and even though drug inhibitors of virtually all of them have been developed, it is mainly glucosyl ceramide synthase, acid ceramidase, and sphingosine kinase that have garnered most attention as targets in recent research (Table 1).
Table 1.
Commonly used sphingolipid enzyme inhibitors
| Enzyme targeted | Inhibitor | Structure |
|---|---|---|
| Serine-palmitoyl transferase | Myriocin |
|
| L-cycloserine |
|
|
| (Dihydro)ceramide desaturase | 4-HPR |
|
| Ceramide synthase | Fumonisin B1 |
|
| Neutral sphingomyelinase2 | Scyphostatin |
|
| GW4869 |
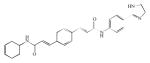
|
|
| Acid sphingomyelinase/acid ceramidase | Desipramine |
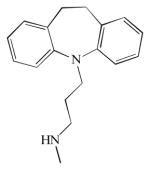
|
| Acid ceramidase | NOE |
|
| B13 |
|
|
| E-tb |
|
|
| Neutral ceramidase/alkaline ceramidase | D-e-MAPP |
|
| Glucosylceramide synthase | D-threo-PDMP |
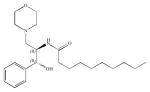
|
| Sphingosine kinase | Safingol |
|
| DMS |
|
|
| PF-543 |
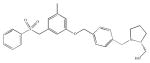
|
|
| SK1-I |
|
|
| SKI-II |
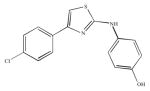
|
|
| (s)-FTY720 vinylphosphonate |
|
|
| ABC294640 |
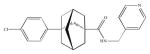
|
In this chapter we review the main inhibitors of sphingolipid enzymes, and we mainly focus on those with more chemotherapeutic possibilities.
3.1 Serine-Palmitoyl Transferase
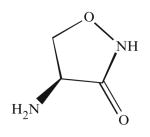
One of the first SPT inhibitors was beta-chloroalanine which was shown to inhibit SPT in vitro using rat liver microsomes as a source of SPT and in vivo, using Chinese hamster ovary (CHO) cells (Medlock and Merrill 1988). Nonetheless, this compound also inhibits other pyridoxal phosphate-dependent enzymes and transaminases. From the fungus Isaria sinclairii, (ISP-1) was isolated as an immunosuppressant compound along with the antibiotics myriocin and thermozymocidin, but once the chemical structures were resolved, it was appreciated that the three of them were identical (Miyake et al. 1995). The structural similarity of myriocin with sphingosine led to the discovery of its inhibitory effect on SPT. The biological effects of myriocin as inhibitor of cell growth were reverted by addition of sphingosine and sphingosine 1-phosphate. Other sphingosine analogues, such as DMS, had no effect on SPT (Miyake et al. 1995). Myriocin has been shown to reduce melanoma cell proliferation by decreasing sphingolipid levels and increasing p53 and p21 expression. In vivo, injection of myriocin reduced tumor growth in murine melanoma with a similar decrease in sphingolipids and increase in p53 and p21 showed in cells (Lee et al. 2012). L-cycloserine, another SPT inhibitor, has been shown to block taxol-induced ceramide, reducing apoptosis in breast cancer MCF-7 and MDA-MB-231 cell lines (Charles et al. 2001).
Recently, geranyl linalool, phytol, and farnesol have been also described as novel SPT inhibitors that reduce fumonisin B1-induced sphinganine accumulation and thus inhibit the first step of sphingolipid de novo synthesis (Shin et al. 2012).
3.2 Ceramide Synthases
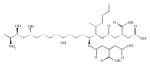
Fumonisins were the first specific inhibitors described for the sphingolipid pathway. They are natural mycotoxins, with 15 different fumonisins, whereas FB1 is the most toxic. Fumonisins inhibit N-acylation of sphingoid bases by CerS, blocking the de novo synthesis of ceramide, and its recycling from sphingosine, causing an accumulation of free dihydrosphingosine and sphingosine resulting in a reduction of the total amount of complex sphingolipids and a total disruption of sphingolipid metabolism and eventually cellular metabolism failure.
3.3 Dihydroceramide Desaturase
The first DES inhibitor came from rational design. Based on the inhibitory mechanism of cyclopropene fatty acids on fatty acyl desaturase, the cyclopropene analogue of ceramide, N-[(1R, 2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1 cyclopropenyl)ethyl] octanamide, or briefly, GT11, was developed and found to inhibit DES (Triola et al. 2001). GT11 functioned as a competitive inhibitor with a Ki = 6 μM with N-octanoyl-sphinganine (Triola et al. 2003). In vivo studies showed GT11 to inhibit DES in different cultured cell lines. However, at higher concentrations (>5 μM), GT11 caused the accumulation of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate in primary cerebellar neurons due to inhibition of sphingosine 1-phosphate lyase (Triola et al. 2004). From GT11 structure, another ceramide analogue, 5-thiadihydro- ceramide (XM462), containing a sulfur atom instead of the cyclopropene ring of GT11, also inhibited DES but with less potency than GT11, but was a more stable compound (Munoz-Olaya et al. 2008). From the work on GT11 and XM462, a new library of compounds was designed showing in vitro and in vivo inhibition of DES (Camacho et al. 2012).
Interestingly, although not designed and initially not used to inhibit DES, the drugs celecoxib and methylcelecoxib, which are COX-2 inhibitors, were shown to cause the accumulation of dihydroceramide species and dihydrosphingosine by inhibiting DES in HCT-116 cells, having no effect on reducing ceramide species (Schiffmann et al. 2009).
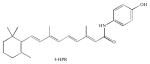
The most explored DES inhibitor is the synthetic retinoid N-(4-hydroxyphenyl) retinamide (fenretinide or 4-HPR) which has been shown to inhibit cell growth and induce cell death in several cancer cell lines. Moreover, 4-HPR has been approved for phase I and II in clinical trials. For example, 4-HPR is currently in clinical trials in pediatric patients with recurrent neuroblastoma (Villablanca et al. 2011), in premenopausal breast cancer risk (Macis et al. 2012), and in recurrent prostate cancer (Moore et al. 2010). 4-HPR was shown to cause accumulation of dihydroceramide and to act as an inhibitor of DES in cells (Wang et al. 2008). Mechanistically, the direct inhibition of DES by 4-HPR was demonstrated in in vitro studies using microsomes as a source of DES (Rahmaniyan et al. 2011). The cytotoxicity of 4-HPR has been related to accumulation of reactive oxygen species (ROS) and to inhibition of DES, although a recent study suggests that 4-HPR induction of cell death may occur independent of dihydroceramide and ROS accumulation (Apraiz et al. 2012).
3.4 Sphingomyelinases
In a screening amongst 10,000 microbial extract to find an nSMase2-specific inhibitor, a mycelial extract of Trichopeziza mollissima showed a micromolar-range inhibition for nSMase2 activity from rat liver microsomes. The active compound was scyphostatin, a water-insoluble and unstable compound when dried that can be stored at 20 °C in methanol (Nara et al. 1999a, b). The characterization of this inhibitor showed a reversible inhibitor, where the Km and Vmax were modified, and its specificity for neutral versus acid SMase was shown to be around 50-fold higher for nSMase, with IC50 for aSMase around IC50 = 49.3 μM, versus 1 μM for nSMase (Nara et al. 1999a, b). Of note, nSMase3 was inhibited by scyphostatin (Krut et al. 2006). Interesting synthetic analogues of scyphostatin have been developed looking for more potent nSMase inhibitors. Amongst them, kotylostatin, modified in its acyl chain, resulted in it being an irreversible inhibitor (Wascholowski et al. 2006). Not many biological studies have been carried out with scyphostatin or their analogues. Moreover, another natural and structural related inhibitor, manumycin A, is a reversible nSMase inhibitor with an affinity to nSMase comparable to the natural substrate, sphingomyelin. Some synthetic manumycin A analogues were shown to be irreversible inhibitors. Manumycin A has been shown to have antitumor activity. However, manumycin A has been shown to have Ras farnesyltransferase- and interleukin-1-converting enzyme inhibitory activities (Arenz et al. 2001). Manumycin A is currently used in cancer research, but its activity is attributed to Ras farnesyltransferase inhibition.
Probably, one of the most used nSMase2 inhibitors in research is GW4869. It is a potent, cell-permeable, noncompetitive, and specific nSMase inhibitor with an in vitro IC50 of 1 μM for nSMase, and it does not inhibit aSMase up to 150 μM. The inhibitor was shown to work in vivo as well by blocking the hydrolysis of sphingomyelin by nSMase induced by TNF (Luberto et al. 2002). GW4869 has been shown to reduce cellular ceramide levels and increase hyaluronic acid secretion (Qin et al. 2012), reducing secretion of miRNA (Kosaka et al. 2010) and counteracting the retinoic acid-induced growth inhibition (Somenzi et al. 2007).
Screening guanidinium derivatives, undecylidene-aminoguanidine (C11AG) was shown to inhibit nSMase and block HSV-1 replication by 50 % using 2.5 μM. It also blocked LPS-stimulated sphingomyelin hydrolysis (Amtmann et al. 2003) and enhanced cell death in Jurkat T-cell lymphoma cells.
There is not much literature on SMase3 in cancer progression, although it has been shown to be deregulated in several primary tumors (Corcoran et al. 2008), and it could play important roles in cellular homeostasis.
Similarly for nSMase, there are no selective inhibitors for this enzyme. However, aSMase was found to participate in evodiamine, a cytotoxic alkaloid, and induction of apoptosis in human gastric cancer SGC-7901 cells (Huang et al. 2011a, b), and it mediates apoptosis by ceramide production and radiosensibilization by the combination of the synergistic compounds sorafenib and vorinostat in cancer treatment (Park et al. 2010). The difluoromethylene sphingomyelin analogue SMA-7 is an SMase inhibitor that reduces levels of aSMase to basal in LPS-induced colon cancer cells, blocking the release of pro-inflammatory cytokines (Sakata et al. 2007). However, SMA-7 also inhibits nSMAse with a similar IC50 = 3.3 μM (Yokomatsu et al. 2001). The tricyclic group of compounds, such as desipramine, have been shown to indirectly inhibit aSMase by inducing its degradation.
3.5 Ceramidases
Ceramidases, and more exactly aCDase, has been a common target to drug development since they have a dual role in cancer progression. The first role is decreasing ceramide levels. Ceramide has been shown to drive cellular apoptosis, often in response of cytokines, or chemotherapeutic drugs. Thus activation of CDase results in decrease of ceramide levels, and failure of apoptosis. The second role is to participate in generation of sphingosine 1-phoshate, a pro-proliferative agonist. In that way, CDase activity hydrolyzes ceramide to form sphingosine, which in the presence of sphingosine kinase activity is converted to sphingosine 1-phosphate. This dual role makes CDase a strong player in cancer progression, and an attractive target for anticancer drug development. This opposite effect of ceramide and sphingosine 1-phosphate is not just limited to cell proliferation; for example, in our group we have recently described that the pro-migratory family of proteins ezrin, radixin, and moesin, which are overexpressed in several cancer cells, are also oppositely regulated by ceramide and sphingosine 1-phosphate (Canals et al. 2010).
Lyosomal aCDase and aSMase are inhibited by some amphiphilic tricyclic agents such as desipramine, and by other amphipathic amines such as chlorpromazine and chloroquine. These agents downregulate aCDase and aSMase protein levels, although they do not affect the RNA message levels. These compounds are not specific for SMases or CDases, affecting some, but not all, lysosomal enzymes (Canals et al. 2011). Looking for more specific inhibitors, and based on the sphingoid-base structure, synthetic chemistry has brought some families of CDase inhibitors. The first sphingolipid analogue-based CDase inhibitor was N-oleoylethanolamine (NOE) (Sugita et al. 1975). NOE has been broadly used as an aCDase inhibitor, increasing cellular ceramide, and inducing apoptosis in several cell lines, such as the mouse L929 fibroblast (Strelow et al. 2000), human glioma U87-MG cells, primary placenta trophoblast (Payne et al. 1999), and bone marrow-derived dendritic cells. However, NOE was found to also inhibit ceramide glycosylation and later, its aCDase inhibitor potency was found to be weak in vitro and in vivo (Grijalvo et al. 2006). Currently, NOE is not used as an aCDase inhibitor but as an endocannabinoid-related molecule. However, NOE has served as a scaffold to design other aCDase inhibitors.
Another attempt for CDase inhibitor was a ceramide analogue, (1S, 2R)-D-erythro-2-(N-Myristoylamino)-1-phenyl-1-propanol or D-e-MAPP, which was shown to accumulate intracellular ceramide in human promyelocytic HL-60 leukemia cells causing cell cycle arrest. It was shown to inhibit in vitro neutral and alkaline CDase at micromolar concentrations, but it had no effect on aCDase (Bielawska et al. 1996).
In a study of D-e-MAPP analogues, (1R, 2R)-2-(N-tetradecanoylamino)-1-(4-nitrophenyl)-1,3-propanediol (or B13) was shown to be a potent in vitro aCDase inhibitor (Bielawska et al. 2008). B13 also accumulated cellular ceramide in vivo, although the mechanism in vivo has been argued, since the neutral nature of the compound makes it difficult to be accumulated in the lysosome. To solve this problem, B13 was modified to enter in the lysosome, resulting in series of lysomotrophic molecules such as LCL204 (also known as AD 2646). Thus, LCL204 inhibits aCDase in vitro as well as in vivo, increasing ceramide levels in a myriad of cultured cells, and counteracting the resistance to apoptosis caused by overexpression of aCDase found in some cancers. LCL04 was found to reduce aCDase protein levels, and destroy the lysosomes and thus affect other lysosomal proteins, including aSMase (Bai et al. 2009).
3.6 Glucosylceramide Synthase
The ceramide analogue, D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol or D-threo-PDMP or just D-PDMP, is a competitive inhibitor of GSC. Interestingly, the L-PDMP isoform was found not to have an inhibitory effect on cells (Inokuchi et al. 1990). D-PDMP treatment of human leukemia HL-60 cells resulted in reduction of basal levels of glucosylceramide, lactosylceramide, and GM3. The reduction of glucosylceramide species and derivatives has been also observed in a myriad of cancer cells and it has been suggested as a chemotherapeutic agent, alone or to sensitize cells to other chemotherapeutic drugs. For example, Lewis lung carcinoma cells lost its lung-colonizing capacity after micromolar PDMP treatment. D-PDMP has been used in combination with imatinib to kill K562 leukemic cells (Baran et al. 2011), in combination with nanoliposomal C6-ceramide (Jiang et al. 2011), and with GNF-2- Bcr-Abl inhibitor to induce apoptosis in human chronic myeloid leukemia cell line (Huang et al. 2011a, b). Moreover, D-PDMP was seen to inhibit galactosylceramide synthase as well. The L-isomer of PDMP was not only found to have no inhibitory effect on GSC but also had the opposite effect of increasing the levels of glucosyl- and lactosylceramides and their respective metabolites (Chatterjee et al. 1996). However, L-PDMP was found to inhibit glycosylceramide glycanases, which are abnormally expressed in some tumor cancers such as colon cancer, neuroblastoma, and some breast cancer cell lines (Basu et al. 1999).
3.7 Sphingosine Kinases
There are two enzymes with sphingosine kinase activity to generate sphingosine 1-phosphate, SK1 (with three N-terminal variants Tonelli et al. 2010) and SK2. The participation of SK1 in cancer progression has been reported in many studies, and SK1 has been repeatedly considered as an oncogene. For example, overexpression of SK1 in NIH3T3 fibroblast cell line resulted in increase in cell proliferation, loss of cell growth inhibition by cell contact, increase in colony formation, and increase in the number of established tumor in mice injected with SK1-transfected cells. Microarray databases, like oncomine (http://www.oncomine.org/), show statistically significant up-regulation of SK1 in a large number of cancers.
The N-methyl natural derivative of sphingosine, N,N-dimethylsphingosine (DMS), was found to inhibit SK activity in human platelets (Yatomi et al. 1996) and it has been reported to block chemotaxis towards growth factors, as well as inducing apoptosis in U937 human leukemic monocytes. As with other sphingosine analogues, DMS also inhibits PKC (Khan et al. 1990). Moreover, the quaternary ammonium derivative of DMS, TMS, which is a more stronger PKC inhibitor, has also been found to inhibit in vitro cancer cell growth, and in vivo growth of human tumor cells in nude mice, in a similar way than DMS (Endo et al. 1991). The similar results between DMS and TMS make it difficult to distinguish between SK and PKC effects using these compounds.
The synthetic sphingosine analogue L-threo-dihydrosphingosine (safingol) was first described as an inhibitor of PKC, and as with other sphingosine isomers and analogues was also found to inhibit SK activity in partially purified rat brain SK and from human platelet SK (Buehrer and Bell 1992). Moreover, safingol has been related to ceramide generation and induction of apoptosis (Noda et al. 2009), and in a sphingolipid-independent way inducing autophagy in human HCT-116 colon carcinoma cell line (Coward et al. 2009). Other protein kinases have been reported to be directly or indirectly inhibited by safingol such as the PI3k/Akt/mTOR pathway, and the ERK MAPK signaling upon bradykinin and PDGF stimulation (Tolan et al. 1996). Safingol has been shown to inhibit cancer cell growth (Schwartz et al. 1993) and sensitization of cancer cells to chemotherapeutic drugs, and it has been successfully used in animal models and successfully passed a phase I clinical trial in combination with doxorubicin (Schwartz et al. 1997). Safingol alone and in combination with cisplatin is ready to start clinical phase II trials (Dickson et al. 2011). From the safingol structure, an analogue library was constructed finding L-threo-N-(4-heptylbenzoyl)dihydrosphingosine derivative having a dramatic apoptotic effect in lung cancer A549 cells (Villorbina et al. 2007).
It is important to note that some of the effects of sphingoid analogues that were initially attributed to PKC inhibition could also be due to SK inhibition, or vice versa, or combination of the two enzymes.
Recently, another competitive inhibitor, PF-543, has been described to inhibit SK1 with a Ki of 3.6 nM, reducing the level of sphingosine 1-phosphate, and increasing the level of sphingosine in head and neck 1,483 carcinoma cell line. Surprisingly PF-543 did not affect the growth rate and total ceramide levels, but it affected the rate of newly synthesized ceramide (Schnute et al. 2012).
Other SK1 inhibitors are FTY720 (fingolimod) and (S)-FTY720 vinylphosphonate which were shown to inhibit SK1 activity (with a sphingosine competition inhibition of Kic = 2 μM and uncompetitive inhibition of Kuc = 17 μM, respectively). The mechanism involves SK1 proteosomal degradation in smooth muscle, breast cancer MCF-7, and LNCaP prostate cancer cell lines. Thus, whereas FTY720 phosphate results in cell proliferation and migration, the FTY720 parent compound induces apoptosis. Moreover, the two compounds also showed opposite effects on ERK1/2, Akt, FAK, and caspase-3 (Tonelli et al. 2010). Of note, (s)-FTY720 vinylphosphonate acts as an antagonist of S1P receptors, increasing the potential therapeutical effect of the drug to induce cancer cell death. Interestingly, (R)-FTY720 methyl ester has been shown to inhibit selectively SK2 (Ki ~16 μM with sphingosine), implicating SK2 in cytoskeleton rearrangements in breast cancer MCF-7 cells (Lim et al. 2011).
Inhibition of SK using SKI-II (2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole) (IC50 = 0.5 μM, for human recombinant SK1) typically employed a concentration of 10 μM, and this resulted in decreased multidrug-resistant breast cancer proliferation and viability (Antoon et al. 2012), blocked the activation of ezrin protein by S1P required for migration in cervical cancer HeLa cells (Canals et al. 2010), and resulted in SK1 degradation in MDA-MB-453 breast cancer cells (Ohotski et al. 2012). Indeed, this inhibitor has been suggested of not inhibiting directly SK1, but enhancing its lysosomal degradation (Ren et al. 2010). Recently, it has been used in combination with bortezomib to induce caspase-dependent apoptosis in leukemic cells (Li et al. 2011).
Specific for SK1, (2R,3S,4E)-N-methyl-5-(4-pentylphenyl)-2-aminopent-4-ene-1,3-diol (BML-258 or SK1-I) is a water-soluble ATP-competitive inhibitor with no activity towards SK2 and PKC. This inhibitor showed antiproliferative effects on human leukemia U937 cells inducing caspase- and BCL-2-dependent apoptosis. SK1-I showed an anti-proliferative effect and inhibition of migration and invasion in glioblastoma U373 and LN229 cells towards serum, EGF, and lysophosphatidic acid. Moreover, SK1-I inhibited the growth of acute myeloid leukemia xenograft tumors in mice and reduced tumor burden, serum S1P levels, metastasis, hemangiogenesis, and lymphangiogenesis in mouse metastasic breast cancer model (Nagahashi et al. 2012; Paugh et al. 2008).
Selective inhibitors for SK2 have been identified, and these include ABC294640 [3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide], a competitive inhibitor with respective sphingosine, Ki ~10 μM. In cell culture, ABC294640 inhibited cell proliferation, cell migration in several cancer cell lines (French et al. 2010), induced autophagy and apoptosis in PC-3 prostate and breast cancer MDA-MB-231 cancer cells, and reduced tumor incidence in an azoxymethane/dextran sulfate sodium mouse model (Beljanski et al. 2011; Chumanevich et al. 2010).
It is not the goal of this chapter to describe small molecules that inhibit sphingosine 1-phosphate receptors. However, it is important to point out that another set of inhibitors are available for these receptors, and they are also important targets in the current drug discovery research (Huwiler and Pfeilschifter 2008).
3.8 Antibodies as Chemotherapeutics
This chapter would not be complete if we do not mention that antibodies raised against sphingosine 1-phosphate and antibodies which recognize aberrant glycolipids expressed in tumors have become strong activators of the complement system, or directly induce cell death (Durrant et al. 2012). Amongst all the glycolipids, glycosphingolipids are the most up-regulated in tumor cells, and thus, appear to be the most important targets for cancer drug development. In this context, sphingomab, a murine monoclonal antibody that binds sphingosine 1-phosphate (the human version is known as sonepcizumab), has shown reduction in cancer progression in a murine model and in human cancers (Milstien and Spiegel 2006; Visentin et al. 2006). Sphingomab has already completed phase I clinical trials for cancer treatment and is being considered for phase II studies (Sabbadini 2011).
3.9 Chemotherapeutics that Modify Sphingolipid Pathway
Although the majority of therapeutics used in cancer are not directly targeted to sphingolipid enzymes, their action mechanism might require the participation of sphingolipids. For example resveratrol, which is an apoptotic compound used to induce cancer cells to die, may involve ceramide generation, since when breast cancer MDA-MB-231 cell lines are pretreated with myriocin, the cells do not accumulate ceramide in response to resveratrol and cells are rescued from the induction of apoptosis. Moreover, resveratrol has been reported to inhibit sphingosine kinase 1 in breast cancer MCF-7 cells (Lim et al. 2012). Other chemotherapeutic apoptotic agents such as fenretinide (Maurer et al. 1999), doxorubicin (Saad et al. 2007), ara-C (Grazide et al. 2002), etoposide (Perry et al. 2000), and Δ9-tetrahydrocannabinol have been shown to induce cell death by accumulation of ceramide (Galve-Roperh et al. 2000).
4 Perspectives
The identification of a role of sphingolipids in tumor behavior, development, and prognosis suggests a point of regulation in cancer progression, and new targets to block cancer growth or metastasis. While a few sphingolipid analogues have been already incorporated in clinical trials, other sphingolipid-regulating enzymes have poor- or low-efficiency inhibitors; other inhibitors are specific and potent in vitro but poor when used in vivo, or there is still a lack in their metabolism by the cell, or in blood. However, this is an active and exponentially growing field in cancer research, and we expect that in the near future chemotherapies involving inhibitors of sphingolipid-metabolizing enzymes, and other sphingolipid-regulated proteins, will be part of clinical trials and cancer patient treatments.
Acknowledgments
We gratefully acknowledge Dr. Mike Airola and María Hernández-Corbacho for the careful reading of this manuscript. This work was supported by NIH grants CA87584 and CA97132.
Abbreviations
- D-e-MAPP
(1S, 2R)-D-erythro-2-(N-Myristoylamino)-1-phenyl-1-propanol
- CDase (aCDase nCDase, alkCDase)
Ceramidase (acid, neutral, and alkaline ceramidase)
- CerS
Ceramide synthase
- DES
Dihydroceramide desaturase
- GSC
Glucosylceramide synthase
- DMS
N, N-dimethylsphingosine
- TMS
N, N, N-Trimethylsphingosine
- NOE
N-oleoylethanolamine
- PKC
Protein kinase C
- SPT
Serine-palmitoyl transferase
- SMase (aSMase nSMase)
Sphingomyelinase (acid, neutral sphingomyelinase)
- SMS
Sphingomyelin synthase
- SPP
Sphingosine 1-phosphate phosphatase
- SK
Sphingosine kinase
Contributor Information
Daniel Canals, Department of Medicine, University of Stony Brook, Stony Brook, New York 11794.
Yusuf A. Hannun, Email: yusuf.hannun@stonybrookmedicine.edu, Health Science Center, Stony Brook University, 100 Nicolls Road, L-4, 178, Stony Brook, NY 11794, USA
References
- Abe A, Shayman JA, Radin NS. A novel enzyme that catalyzes the esterification of N-acetylsphingosine. Metabolism of C2-ceramides. J Biol Chem. 1996;271:14383–14389. doi: 10.1074/jbc.271.24.14383. [DOI] [PubMed] [Google Scholar]
- Amtmann E, Baader W, Zoller M. Neutral sphingomyelinase inhibitor C11AG prevents lipopolysaccharide-induced macrophage activation. Drugs Exp Clin Res. 2003;29:5–13. [PubMed] [Google Scholar]
- Antoon JW, White MD, Burow ME, Beckman BS. Dual inhibition of sphingosine kinase isoforms ablates TNF-induced drug resistance. Oncol Rep. 2012;27:1779–1786. doi: 10.3892/or.2012.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apraiz A, Idkowiak-Baldys J, Nieto-Rementeria N, Boyano MD, Hannun YA, Asumendi A. Dihydroceramide accumulation and reactive oxygen species are distinct and nonessential events in 4-HPR-mediated leukemia cell death. Biochem Cell Biol. 2012;90:209–223. doi: 10.1139/o2012-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenz C, Thutewohl M, Block O, Waldmann H, Altenbach HJ, Giannis A. Manumycin A and its analogues are irreversible inhibitors of neutral sphingomyelinase. Chembiochem. 2001;2:141–143. doi: 10.1002/1439-7633(20010202)2:2<141::AID-CBIC141>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bai A, Szulc ZM, Bielawski J, Mayroo N, Liu X, Norris J, Hannun YA, Bielawska A. Synthesis and bioevaluation of omega-N-amino analogs of B13. Bioorg Med Chem. 2009;17:1840–1848. doi: 10.1016/j.bmc.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran Y, Bielawski J, Gunduz U, Ogretmen B. Targeting glucosylceramide synthase sensitizes imatinib-resistant chronic myeloid leukemia cells via endogenous ceramide accumulation. J Cancer Res Clin Oncol. 2011;137:1535–1544. doi: 10.1007/s00432-011-1016-y. [DOI] [PubMed] [Google Scholar]
- Basu M, Kelly P, O’Donnell P, Miguel M, Bradley M, Sonnino S, Banerjee S, Basu S. Ceramide glycanase activities in human cancer cells. Biosci Rep. 1999;19:449–460. doi: 10.1023/a:1020220524180. [DOI] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, Zhuang Y, Smith CD. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest New Drugs. 2011;29:1132–1142. doi: 10.1007/s10637-010-9452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawska A, Bielawski J, Szulc ZM, Mayroo N, Liu X, Bai A, Elojeimy S, Rembiesa B, Pierce J, Norris JS, Hannun YA. Novel analogs of D-e-MAPP and B13. Part 2: signature effects on bioactive sphingolipids. Bioorg Med Chem. 2008;16:1032–1045. doi: 10.1016/j.bmc.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawska A, Greenberg MS, Perry D, Jayadev S, Shayman JA, McKay C, Hannun YA. (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J Biol Chem. 1996;271:12646–12654. doi: 10.1074/jbc.271.21.12646. [DOI] [PubMed] [Google Scholar]
- Birbes H, Bawab SE, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- Bourquin F, Capitani G, Grutter MG. PLP-dependent enzymes as entry and exit gates of sphingolipid metabolism. Protein Sci. 2011;20:1492–1508. doi: 10.1002/pro.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrer BM, Bell RM. Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. J Biol Chem. 1992;267:3154–3159. [PubMed] [Google Scholar]
- Camacho L, Simbari F, Garrido M, Abad JL, Casas J, Delgado A, Fabrias G. 3-Deoxy-3,4-dehydro analogs of XM462. Preparation and activity on sphingolipid metabolism and cell fate. Bioorg Med Chem. 2012;20:3173–3179. doi: 10.1016/j.bmc.2012.03.073. [DOI] [PubMed] [Google Scholar]
- Canals D, Jenkins RW, Roddy P, Hernandez-Corbacho MJ, Obeid LM, Hannun YA. Differential effects of ceramide and sphingosine 1-phosphate on ERM phosphorylation: probing sphingolipid signaling at the outer plasma membrane. J Biol Chem. 2010;285:32476–32485. doi: 10.1074/jbc.M110.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals D, Perry DM, Jenkins RW, Hannun YA. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br J Pharmacol. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AG, Han TY, Liu YY, Hansen N, Giuliano AE, Cabot MC. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Cleveland T, Shi WY, Inokuchi J, Radin NS. Studies of the action of ceramide-like substances (D- and L-PDMP) on sphingolipid glycosyltransferases and purified lactosylceramide synthase. Glycoconj J. 1996;13:481–486. doi: 10.1007/BF00731481. [DOI] [PubMed] [Google Scholar]
- Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, Hofseth LJ. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CA, He Q, Ponnusamy S, Ogretmen B, Huang Y, Sheikh MS. Neutral sphingomyelinase-3 is a DNA damage and nongenotoxic stress-regulated gene that is deregulated in human malignancies. Mol Cancer Res. 2008;6:795–807. doi: 10.1158/1541-7786.MCR-07-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, Wang E, Merrill AH, Jr, Schwartz GK. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5:184–193. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Carvajal RD, Merrill AH, Jr, Gonen M, Cane LM, Schwartz GK. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin Cancer Res. 2011;17:2484–2492. doi: 10.1158/1078-0432.CCR-10-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant LG, Noble P, Spendlove I. Immunology in the clinic review series; focus on cancer: glycolipids as targets for tumour immunotherapy. Clin Exp Immunol. 2012;167:206–215. doi: 10.1111/j.1365-2249.2011.04516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bawab S, Birbes H, Roddy P, Szulc ZM, Bielawska A, Hannun YA. Biochemical characterization of the reverse activity of rat brain ceramidase. A CoA-independent and fumonisin B1-insensitive ceramide synthase. J Biol Chem. 2001;276:16758–16766. doi: 10.1074/jbc.M009331200. [DOI] [PubMed] [Google Scholar]
- Endo K, Igarashi Y, Nisar M, Zhou QH, Hakomori S. Cell membrane signaling as target in cancer therapy: inhibitory effect of N, N-dimethyl and N, N, N-trimethyl sphingosine derivatives on in vitro and in vivo growth of human tumor cells in nude mice. Cancer Res. 1991;51:1613–1618. [PubMed] [Google Scholar]
- Fabrias G, Munoz-Olaya J, Cingolani F, Signorelli P, Casas J, Gagliostro V, Ghidoni R. Dihydroceramide desaturase and dihydrosphingolipids: debutant players in the sphingolipid arena. Prog Lipid Res. 2012;51:82–94. doi: 10.1016/j.plipres.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Farfel-Becker T, Vitner EB, Futerman AH. Animal models for Gaucher disease research. Dis Model Mech. 2011;4:746–752. doi: 10.1242/dmm.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers M, Fabrias G, Delgado A, Casas J, Abad JL, Cabot MC. C6-Ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Res Treat. 2011;133:447–458. doi: 10.1007/s10549-011-1768-8. [DOI] [PubMed] [Google Scholar]
- French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh I, Sanchez C, Cortes ML, Gomez del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Granado MH, Gangoiti P, Ouro A, Arana L, Gonzalez M, Trueba M, Gomez-Munoz A. Ceramide 1-phosphate (C1P) promotes cell migration Involvement of a specific C1P receptor. Cell Signal. 2009;21:405–412. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Grazide S, Maestre N, Veldman RJ, Bezombes C, Maddens S, Levade T, Laurent G, Jaffrezou JP. Ara-C- and daunorubicin-induced recruitment of Lyn in sphingomyelinase-enriched membrane rafts. FASEB J. 2002;16:1685–1687. doi: 10.1096/fj.01-0794fje. [DOI] [PubMed] [Google Scholar]
- Grijalvo S, Bedia C, Triola G, Casas J, Llebaria A, Teixido J, Rabal O, Levade T, Delgado A, Fabrias G. Design, synthesis and activity as acid ceramidase inhibitors of 2-oxooctanoyl and N-oleoylethanolamine analogues. Chem Phys Lipids. 2006;144:69–84. doi: 10.1016/j.chemphyslip.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Hakomori SI. Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochim Biophys Acta. 2008;1780:325–346. doi: 10.1016/j.bbagen.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Loomis CR, Merrill AH, Jr, Bell RM. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261:12604–12609. [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan-Stroud LA, Helke KL, Jenkins RW, De Costa AM, Hannun YA, Obeid LM. Defining a role for sphingosine kinase 1 in p53-dependent tumors. Oncogene. 2012;31:1166–1175. doi: 10.1038/onc.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang Y, Liu X, Li Z, Xu W, He S, Huang Y, Zhang H. Acid sphingomyelinase contributes to evodiamine-induced apoptosis in human gastric cancer SGC-7901 cells. DNA Cell Biol. 2011a;30:407–412. doi: 10.1089/dna.2010.1122. [DOI] [PubMed] [Google Scholar]
- Huang WC, Tsai CC, Chen CL, Chen TY, Chen YP, Lin YS, Lu PJ, Lin CM, Wang SH, Tsao CW, Wang CY, Cheng YL, Hsieh CY, Tseng PC, Lin CF. Glucosylceramide synthase inhibitor PDMP sensitizes chronic myeloid leukemia T315I mutant to Bcr-Abl inhibitor and cooperatively induces glycogen synthase kinase-3-regulated apoptosis. FASEB J. 2011b;25:3661–3673. doi: 10.1096/fj.10-180190. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J. New players on the center stage: sphingosine 1-phosphate and its receptors as drug targets. Biochem Pharmacol. 2008;75:1893–1900. doi: 10.1016/j.bcp.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Inokuchi J, Jimbo M, Momosaki K, Shimeno H, Nagamatsu A, Radin NS. Inhibition of experimental metastasis of murine Lewis lung carcinoma by an inhibitor of glucosylceramide synthase and its possible mechanism of action. Cancer Res. 1990;50:6731–6737. [PubMed] [Google Scholar]
- Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21:836–846. doi: 10.1016/j.cellsig.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, DiVittore NA, Kaiser JM, Shanmugavelandy SS, Fritz JL, Heakal Y, Tagaram HR, Cheng H, Cabot MC, Staveley-O’Carroll KF, Tran MA, Fox TE, Barth BM, Kester M. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol Ther. 2011;12:574–585. doi: 10.4161/cbt.12.7.15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WA, Dobrowsky R, el Touny S, Hannun YA. Protein kinase C and platelet inhibition by D-erythro-sphingosine: comparison with N, N-dimethylsphingosine and commercial preparation. Biochem Biophys Res Commun. 1990;172:683–691. doi: 10.1016/0006-291x(90)90728-6. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Kronke M. Novel tumor necrosis factor-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J Biol Chem. 2006;281:13784–13793. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Prinetti A, Sonnino S, Schuchman EH. Brain pathology in Niemann Pick disease type A: insights from the acid sphingomyelinase knockout mice. J Neurochem. 2011;116:779–788. doi: 10.1111/j.1471-4159.2010.07034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Choi KM, Lee S, Sin DM, Lim Y, Lee YM, Hong JT, Yun YP, Yoo HS. Myriocin, a serine palmitoyltransferase inhibitor, suppresses tumor growth in a murine melanoma model by inhibiting de novo sphingolipid synthesis. Cancer Biol Ther. 2012;13:92–100. doi: 10.4161/cbt.13.2.18870. [DOI] [PubMed] [Google Scholar]
- Li QF, Yan J, Zhang K, Yang YF, Xiao FJ, Wu CT, Wang H, Wang LS. Bortezomib and sphingosine kinase inhibitor interact synergistically to induces apoptosis in BCR/ABl + cells sensitive and resistant to STI571 through down-regulation Mcl-1. Biochem Biophys Res Commun. 2011;405:31–36. doi: 10.1016/j.bbrc.2010.12.111. [DOI] [PubMed] [Google Scholar]
- Lim KG, Gray AI, Pyne S, Pyne NJ. Resveratrol dimers are novel sphingosine kinase 1 inhibitors and affect sphingosine kinase 1 expression and cancer cell growth and survival. Br J Pharmacol. 2012;166:1605–1616. doi: 10.1111/j.1476-5381.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KG, Sun C, Bittman R, Pyne NJ, Pyne S. (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cell Signal. 2011;23:1590–1595. doi: 10.1016/j.cellsig.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, Hazen-Martin DJ, Obeid LM, Hannun YA, Smith GK. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macis D, Gandini S, Guerrieri-Gonzaga A, Johansson H, Magni P, Ruscica M, Lazzeroni M, Serrano D, Cazzaniga M, Mora S, Feroce I, Pizzamiglio M, Sandri MT, Gulisano M, Bonanni B, Decensi A. Prognostic effect of circulating adiponectin in a randomized 2 × 2 trial of low-dose tamoxifen and fenretinide in premenopausal women at risk for breast cancer. J Clin Oncol. 2012;30:151–157. doi: 10.1200/JCO.2011.35.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Xu R, Bielawska A, Obeid LM. Cloning of an alkaline ceramidase from Saccharomyces cerevisiae. An enzyme with reverse (CoA-independent) ceramide synthase activity. J Biol Chem. 2000;275:6876–6884. doi: 10.1074/jbc.275.10.6876. [DOI] [PubMed] [Google Scholar]
- Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. J Biol Chem. 2001;276:26577–26588. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- Medlock KA, Merrill AH., Jr Inhibition of serine palmitoyltransferase in vitro and long-chain base biosynthesis in intact Chinese hamster ovary cells by beta-chloroalanine. Biochemistry. 1988;27:7079–7084. doi: 10.1021/bi00418a061. [DOI] [PubMed] [Google Scholar]
- Milstien S, Spiegel S. Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell. 2006;9:148–150. doi: 10.1016/j.ccr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun. 1995;211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- Moore MM, Stockler M, Lim R, Mok TS, Millward M, Boyer MJ. A phase II study of fenretinide in patients with hormone refractory prostate cancer: a trial of the Cancer Therapeutics Research Group. Cancer Chemother Pharmacol. 2010;66:845–850. doi: 10.1007/s00280-009-1228-x. [DOI] [PubMed] [Google Scholar]
- Munoz-Olaya JM, Matabosch X, Bedia C, Egido-Gabas M, Casas J, Llebaria A, Delgado A, Fabrias G. Synthesis and biological activity of a novel inhibitor of dihydroceramide desaturase. Chem Med Chem. 2008;3:946–953. doi: 10.1002/cmdc.200700325. [DOI] [PubMed] [Google Scholar]
- Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, Takabe K. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara F, Tanaka M, Hosoya T, Suzuki-Konagai K, Ogita T. Scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima: taxonomy of the producing organism, fermentation, isolation, and physico-chemical properties. J Antibiot (Tokyo) 1999a;52:525–530. doi: 10.7164/antibiotics.52.525. [DOI] [PubMed] [Google Scholar]
- Nara F, Tanaka M, Masuda-Inoue S, Yamasato Y, Doi-Yoshioka H, Suzuki-Konagai K, Kumakura S, Ogita T. Biological activities of scyphostatin, a neutral sphingomyelinase inhibitor from a discomycete, Trichopeziza mollissima. J Antibiot (Tokyo) 1999b;52:531–535. doi: 10.7164/antibiotics.52.531. [DOI] [PubMed] [Google Scholar]
- Noda T, Iwai S, Hamada M, Fujita Y, Yura Y. Induction of apoptosis of detached oral squamous cell carcinoma cells by safingol. Possible role of Bim, focal adhesion kinase and endonuclease G. Apoptosis. 2009;14:287–297. doi: 10.1007/s10495-009-0319-9. [DOI] [PubMed] [Google Scholar]
- Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J Biol Chem. 2011;286:25352–25362. doi: 10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohotski J, Long JS, Orange C, Elsberger B, Mallon E, Doughty J, Pyne S, Pyne NJ, Edwards J. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2012;106:1453–1459. doi: 10.1038/bjc.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Bielawska A, Domae N, Bell RM, Hannun YA. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha, 25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1994;269:4070–4077. [PubMed] [Google Scholar]
- Olsen I, Jantzen E. Sphingolipids in Bacteria and Fungi. Anaerobe. 2001;7:103–112. [Google Scholar]
- Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Haussinger D, Reinehr R, Larner A, Spiegel S, Fisher PB, Voelkel-Johnson C, Ogretmen B, Grant S, Dent P. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70:6313–6324. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh BS, Paugh SW, Bryan L, Kapitonov D, Wilczynska KM, Gopalan SM, Rokita H, Milstien S, Spiegel S, Kordula T. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 2008;22:455–465. doi: 10.1096/fj.07-8276com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne SG, Brindley DN, Guilbert LJ. Epidermal growth factor inhibits ceramide-induced apoptosis and lowers ceramide levels in primary placental trophoblasts. J Cell Physiol. 1999;180:263–270. doi: 10.1002/(SICI)1097-4652(199908)180:2<263::AID-JCP14>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Selectivity and specificity of sphingosine 1-phosphate receptor ligands: “off-targets” or complex pharmacology? Front Pharmacol. 2011;2:26. doi: 10.3389/fphar.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Berdyshev E, Poirer C, Schwartz NB, Dawson G. Neutral sphingomyelinase 2 deficiency increases hyaluronan synthesis by up-regulation of Hyaluronan synthase 2 through decreased ceramide production and activation of akt. J Biol Chem. 2012;287:13620–13632. doi: 10.1074/jbc.M111.304857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmaniyan M, Curley RW, Jr, Obeid LM, Hannun YA, Kraveka JM. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J Biol Chem. 2011;286:24754–24764. doi: 10.1074/jbc.M111.250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Xin C, Pfeilschifter J, Huwiler A. A novel mode of action of the putative sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (SKI II): induction of lysosomal sphingosine kinase 1 degradation. Cell Physiol Biochem. 2010;26:97–104. doi: 10.1159/000315110. [DOI] [PubMed] [Google Scholar]
- Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, Turner LS, Cheng J, Bielawska A, Bielawski J, Keane TE, Obeid LM, Hannun YA, Norris JS, Liu X. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol Ther. 2007;6:1455–1460. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol. 2011;162:1225–1238. doi: 10.1111/j.1476-5381.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata A, Ochiai T, Shimeno H, Hikishima S, Yokomatsu T, Shibuya S, Toda A, Eyanagi R, Soeda S. Acid sphingomyelinase inhibition suppresses lipopolysaccharide-mediated release of inflammatory cytokines from macrophages and protects against disease pathology in dextran sulphate sodium-induced colitis in mice. Immunology. 2007;122:54–64. doi: 10.1111/j.1365-2567.2007.02612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Schaefer RM, Tylki-Szymanska A, Hilz MJ. Enzyme replacement therapy for Fabry disease: a systematic review of available evidence. Drugs. 2009;69:2179–2205. doi: 10.2165/11318300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schiffmann S, Sandner J, Schmidt R, Birod K, Wobst I, Schmidt H, Angioni C, Geisslinger G, Grosch S. The selective COX-2 inhibitor celecoxib modulates sphingolipid synthesis. J Lipid Res. 2009;50:32–40. doi: 10.1194/jlr.M800122-JLR200. [DOI] [PubMed] [Google Scholar]
- Schnute ME, McReynolds MD, Kasten T, Yates M, Jerome G, Rains JW, Hall T, Chrencik J, Kraus M, Cronin CN, Saabye M, Highkin MK, Broadus R, Ogawa S, Cukyne K, Zawadzke LE, Peterkin V, Iyanar K, Scholten JA, Wendling J, Fujiwara H, Nemirovskiy O, Wittwer AJ, Nagiec MM. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem J. 2012;444:79–88. doi: 10.1042/BJ20111929. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Jiang J, Kelsen D, Albino AP. Protein kinase C: a novel target for inhibiting gastric cancer cell invasion. J Natl Cancer Inst. 1993;85:402–407. doi: 10.1093/jnci/85.5.402. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Ward D, Saltz L, Casper ES, Spiess T, Mullen E, Woodworth J, Venuti R, Zervos P, Storniolo AM, Kelsen DP. A pilot clinical/pharmacological study of the protein kinase C-specific inhibitor safingol alone and in combination with doxorubicin. Clin Cancer Res. 1997;3:537–543. [PubMed] [Google Scholar]
- Shapiro D, Flowers HM. Studies on sphingolipids VII. Synthesis and configuration of natural sphingomyelins. J Am Chem Soc. 1962;84:1047–1050. [Google Scholar]
- Shin KO, Park MY, Seo CH, Lee YI, Kim HS, Yoo HS, Hong JT, Jung JK, Lee YM. Terpene alcohols inhibit de novo sphingolipid biosynthesis. Planta Med. 2012;78:434–439. doi: 10.1055/s-0031-1298155. [DOI] [PubMed] [Google Scholar]
- Somenzi G, Sala G, Rossetti S, Ren M, Ghidoni R, Sacchi N. Disruption of retinoic acid receptor alpha reveals the growth promoter face of retinoic acid. PLoS One. 2007;2:e836. doi: 10.1371/journal.pone.0000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W. Studies on the biosynthesis and degradation of sphingosine bases. Chem Phys Lipids. 1970;5:139–158. doi: 10.1016/0009-3084(70)90014-9. [DOI] [PubMed] [Google Scholar]
- Strelow A, Bernardo K, Adam-Klages S, Linke T, Sandhoff K, Kronke M, Adam D. Overexpression of acid ceramidase protects from tumor necrosis factor-induced cell death. J Exp Med. 2000;192:601–612. doi: 10.1084/jem.192.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M, Willians M, Dulaney JT, Moser HW. Ceramidase and ceramide synthesis in human kidney and cerebellum. Description of a new alkaline ceramidase. Biochim Biophys Acta. 1975;398:125–131. doi: 10.1016/0005-2760(75)90176-9. [DOI] [PubMed] [Google Scholar]
- Tarabuso AL. Fabry disease. Skinmed. 2011;9:173–177. [PubMed] [Google Scholar]
- Tolan D, Conway AM, Steele L, Pyne S, Pyne NJ. The identification of DL-threo dihydrosphingosine and sphingosine as novel inhibitors of extracellular signal-regulated kinase signalling in airway smooth muscle. Br J Pharmacol. 1996;119:185–186. doi: 10.1111/j.1476-5381.1996.tb15967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triola G, Fabrias G, Casas J, Llebaria A. Synthesis of cyclopropene analogues of ceramide and their effect on dihydroceramide desaturase. J Org Chem. 2003;68:9924–9932. doi: 10.1021/jo030141u. [DOI] [PubMed] [Google Scholar]
- Triola G, Fabrias G, Dragusin M, Niederhausen L, Broere R, Llebaria A, van Echten-Deckert G. Specificity of the dihydroceramide desaturase inhibitor N-[(1R,2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1-cyclopropenyl)ethyl]octanami de (GT11) in primary cultured cerebellar neurons. Mol Pharmacol. 2004;66:1671–1678. doi: 10.1124/mol.104.003681. [DOI] [PubMed] [Google Scholar]
- Triola G, Fabrias G, Llebaria A. Synthesis of a cyclopropene analogue of ceramide, a potent inhibitor of dihydroceramide desaturase This work was supported by the Direccion General de Ensenanza Superior e Investigacion Cientifica (grant PB97-1171) and the Departament d’Universitats, Recerca i Societat de la Informacio, Generalitat de Catalunya (grant 1999-SGR 00187 and a Predoctoral fellowship to G.T.). We thank Dr. J. Casas, Dr. A. Delgado, and Dr. J. Joglar for their help in different aspects of this work. Angew Chem Int Ed Engl. 2001;40:1960–1962. [PubMed] [Google Scholar]
- Villablanca JG, London WB, Naranjo A, McGrady P, Ames MM, Reid JM, McGovern RM, Buhrow SA, Jackson H, Stranzinger E, Kitchen BJ, Sondel PM, Parisi MT, Shulkin B, Yanik GA, Cohn SL, Reynolds CP. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the Children’s Oncology Group. Clin Cancer Res. 2011;17:6858–6866. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villorbina G, Canals D, Carde L, Grijalvo S, Pascual R, Rabal O, Teixido J, Fabrias G, Llebaria A, Casas J, Delgado A. Solid-phase synthesis of a combinatorial library of dihydroceramide analogues and its activity in human alveolar epithelial cells. Bioorg Med Chem. 2007;15:50–62. doi: 10.1016/j.bmc.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Wang H, Maurer BJ, Liu YY, Wang E, Allegood JC, Kelly S, Symolon H, Liu Y, Merrill AH, Jr, Gouaze-Andersson V, Yu JY, Giuliano AE, Cabot MC. N-(4-Hydroxyphenyl) retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther. 2008;7:2967–2976. doi: 10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- Wascholowski V, Giannis A, Pitsinos EN. Influence of the scyphostatin side chain on the mode of inhibition of neutral sphingomyelinase. ChemMedChem. 2006;1:718–721. doi: 10.1002/cmdc.200600099. [DOI] [PubMed] [Google Scholar]
- Wu BX, Clarke CJ, Hannun YA. Mammalian neutral sphingomyelinases: regulation and roles in cell signaling responses. Neuromolecular Med. 2010;12:320–330. doi: 10.1007/s12017-010-8120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N, N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35:626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Yamamura S, Ruan F, Kume S, Ozaki Y, Igarashi Y. N, N-dimethylsphingosine 1-phosphate activates human platelets. FEBS Lett. 1997;417:341–344. doi: 10.1016/s0014-5793(97)01321-5. [DOI] [PubMed] [Google Scholar]
- Yokomatsu T, Takechi H, Akiyama T, Shibuya S, Kominato T, Soeda S, Shimeno H. Synthesis and evaluation of a difluoromethylene analogue of sphingomyelin as an inhibitor of sphingomyelinase. Bioorg Med Chem Lett. 2001;11:1277–1280. doi: 10.1016/s0960-894x(01)00179-2. [DOI] [PubMed] [Google Scholar]