Abstract
Ischemic preconditioning-induced neuroprotection is a well-known phenomenon. We hypothesize that this form of neuroprotection is transferable among the same type of cells. To test this hypothesis, human neuroblastoma SH-SY5Y cells were induced to become neuron-like cells. Primary rat cortical neuronal cultures were also used. These cells were subjected to various lengths of short oxygen-glucose deprivation (OGD, an in vitro simulation of ischemia) and then 1-h OGD. Some cells that were not exposed to a short episode of ischemia were incubated with culture medium from the cells that had 3- or 5-min OGD. Those cells were subjected to OGD for 1 h at 1 or 24 h after they were exposed to the medium. Cell injury was evaluated at 24 h after the 1-h OGD by lactate dehydrogenase release assay. In another experiment, cells subjected to a 3-min OGD or exposed to the medium from cells that had a 3-min OGD were harvested at 30 min after the OGD or the medium exposure for Western blotting of Akt, a prosurvival protein. Our study showed that a prior episode of ischemia lasting from 3 to 10 min significantly reduced the 1-h OGD-induced cell injury. Medium from cells subjected to a 3-min OGD also induced acute and delayed phases of neuroprotection in OGD-naïve human neuron-like cells and primary rat cortical neuronal cultures. Cells subjected to a 3-min OGD or incubated with the medium from cells exposed to a 3-min OGD had increased phosphorylated/activated Akt. The increased phosphorylated Akt and neuroprotection induced by medium transferring were inhibited by 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX), an adenosine A1 receptor inhibitor. The 3-min OGD-induced neuroprotection was inhibited by LY294002, an Akt activation inhibitor. These results suggest that ischemic preconditioning-induced neuroprotection is transferable among the cells. Small molecules, such as adenosine, may mediate this effect.
Keywords: adenosine A1 receptor, Akt, inter-cell preconditioning, neuroprotection
1. Introduction
Ischemic preconditioning is now a well-known phenomenon in which episodes of short ischemia induce robust protection against prolonged and detrimental ischemia in the same tissues or organs (Gidday, 2006; Murry, et al., 1986). Multiple endogenous mechanisms including activating prosurvival proteins, such as Akt, have been considered to mediate ischemic preconditioning-induced protection (Gidday, 2006). This form of protection has been found in various organs including brain. Ischemic preconditioning-induced neuroprotection has been actively studied to identify potential targets/interventions to improve neurological outcome after stroke (Gidday, 2006; Iadecola and Anrather, 2011).
The initial studies on ischemic preconditioning have been focused on applying short episodes of ischemia to the same tissues that are subjected to a subsequent prolonged ischemia. However, preconditioning effect can be induced by applying ischemia to a different tissue. For example, subjecting a limb to short episodes of ischemia can induce neuroprotection (remote ischemic preconditioning) (Zhao, et al., 2012). This finding significantly increases the applicability of using ischemic preconditioning for neuroprotection because application of ischemia to a limb is more practical and safer than inducing ischemia in the brain. However, it is not really clear how a preconditioning stimulus applied to an organ/tissue can induce protection in another organ/tissue. Signals transmitted through nervous system or molecules in the circulating blood have been proposed as the mediators for this phenomenon (Lim and Hausenloy, 2012; Zhao, et al., 2012). A third form of preconditioning has been found in the heart. The effluent from a preconditioned heart can induce protection in a naïve heart. This protection is called transferred inter-cardiac preconditioning (Dickson, et al., 1999). The mechanisms for this preconditioning are largely unknown.
In this study, we hypothesize that ischemic preconditioning-induced neuroprotection is transferable among the same type of cells and that this transferable preconditioning goes through mechanisms similar to those for ischemic preconditioning in the same tissues. To test our hypotheses, we induced human neuroblastoma SH-SY5Y cells to become neuron-like cells. Oxygen-glucose deprivation (OGD) was used to simulate ischemia in vitro. Culture medium transferring was used to determine whether the preconditioning is transferable.
2. Materials and Methods
2.1. SH-SY5Y cell culture
The human neuroblastoma SH-SY5Y cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). SH-SY5Y cells were cultured in a 1:1 mixture of Eagle’s minimum essential medium and F-12 supplemented with 10% fetal bovine serum. They were kept at 37°C in a humidified incubator gassed with 5% CO2 and 95% air and sub-cultured when they were 70 – 80% confluent. The cells were fed twice each week.
For experiments, SH-SY5Y cells were plated at a density of 5 × 103 cells/cm2 in 6-well plates. One day later, culture medium was changed into the Neurobasal medium (Invitrogen Life Technologies, GIBCO, Carlsbad, CA) supplemented with B27 supplement (Invitrogen Life Technologies) and L-glutamine (500 µM; Nacalai Tesque Inc., San Diego, CA). Retinoic acid (Sigma, St. Louis, MO) was added to the medium to make the final concentration at 10 µM for 3 days to induce SH-SY5Y cells to differentiate into a homogenous population of cells with neuronal morphology (Kume, et al., 2008; Lin, et al., 2011; Lin, et al., 2011). The cells were used in experiments the next day.
2.2. Primary rat cortical neuronal culture
These cells were purchased from Invitrogen Life Technologies and were isolated from 18-day old embryos of the Fischer 344 rats. They were plated at ~1.7 × 104 cells per well in 96-well plates and cultured in Neurobasal medium supplemented with 200 mM Glutamax-1 and B27. The cells were fed and maintained as we described before (Li and Zuo, 2011) and used in experiments on the fifth day after they were plated in our laboratory.
2.3. Experimental protocol
Two phases of neuroprotection, the acute and delayed phases, were determined. To investigate the acute phase, cells were exposed to OGD for 3, 5 or 10 min. One hour later, they were exposed to a 60-min OGD. To determine whether OGD preconditioning was transferable, cells were subjected to a 0-, 3- or 5-min OGD. Thirty minutes later, the culture medium was harvested and added to OGD-naïve cells whose culture medium was just removed. The OGD-naïve cells were subjected to the 60-min OGD 1 h later.
To determine the delayed phase of neuroprotection, the experimental protocol was the same as for studying the acute phase but the interval between the short episode of OGD or the medium transferring and the 60-min OGD was 24 h instead of 1 h.
2.4. Applications of drugs
The Akt activation inhibitor LY294002 (Sigma, St. Louis, MO) was administered to cells to make the final concentration of 10 µM immediately before a 3-min OGD or at 60 min before a 60-min OGD. The adenosine A1 receptor inhibitor 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX) was applied to the cells at the final concentration of 100 nM at 30 min after the 3-min OGD (just before the culture medium was harvested for transferring) or just before the 3-min OGD (for cells subjected to 3-min OGD and then 60-min OGD).
2.5. OGD
As we described before (Lin, et al., 2011), the culture medium was removed and the cells were washed with Dulbecco’s phosphate-buffered saline (Invitrogen Life Technologies). Neurobasal-A medium (Invitrogen Life technologies) that has no glucose, glutamine, or B27 was gassed in advance with 100% N2 for 10 min to eliminate oxygen in the medium. After 2 ml of this pre-gassed Neurobasal-A medium was added to each well, the culture plates were placed into the Billups-Rothenberg air-tight chamber. The chamber was closed and flushed through a channel with 100% N2 until O2 concentration in the gases of the chamber reached 2% as detected by a Datex™ infrared analyzer (Capnomac, Helsinki, Finland). The channel was tightly closed and cells were kept at 37°C for 3, 5, 10 or 60 min. At the end of incubation, oxygen concentrations in the gases from the chambers were confirmed to be lower than 2% and then the cells were exposed to room air. Glucose, glutamine and B27 were added to the cells and the cells were incubated at 37°C for the predetermined times.
2.6. Determination of lactate dehydrogenase (LDH) release
LDH activity in the culture medium and cells at 24 h after the 60-min OGD was determined by a colorimetric assay using LDH Cytotoxicity Detection Kit (Clontech Mountain View, CA). The culture medium was centrifuged at 100 g for 10 min and 100-µl cell-free supernatant was transferred to 96-well-plate. This supernatant was incubated with the same amount of the reaction mixture according to the manufacturer’s protocol. The absorbance of samples to light was measured at 490 nm with the reference wavelength of 655 nm in a spectrophotometer (Bio-Rad Laboratories, Hercules, CA). Background absorbance from the cell-free buffer solution was subtracted from all absorbance measurements. To measure the LDH activity in the cells, culture medium was removed and 1.0% Triton X-100 lysing solution was applied to the cells. After 20 min of incubation, 100 µl of cell lysates was incubated with the same amount of the reaction mixture and the absorbance of the samples was measured. The LDH release in percentage was calculated using the following equation: LDH level in the incubation medium × 100/(intracellular LDH level + LDH level in the culture medium).
2.6. Sample preparation for Western blotting
In the first experiment, cells were subjected to or were not subjected to a 3-min OGD. Cells were harvested at 30 min after the OGD. In the second experiment, culture medium from cells exposed to a 3-min OGD was harvested 30 min after the OGD and then added to OGD-naïve cells. The OGD-naïve cells were harvested 30 min later. Some cells received treatment with 100 nM DPCPX at 30 min after the 3-min OGD. The culture medium was then harvested to be added to OGD-naïve cells.
Total protein lysates were prepared from the cells in the lysis buffer (Cell Signaling Technology, Danvers, MA) containing proteinase inhibitors (Proteinase Inhibitor Cocktail; Sigma, St Louis, MO) and phosphatase inhibitors (phosSTOP Phosphatase Inhibitor Cocktail Tablets; Roche, Nutley, NJ). The lysates were centrifuged at 13,000 g for 20 min at 4°C and the supernatant was kept for Western blotting. The protein concentrations in the supernatant were determined by a Bradford protein assay.
2.7. Western blot analysis
Proteins of 30 – 50 µg per lane were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrotransferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA). After being incubated with the Protein-free T20 Blocking Buffer (Thermo Scientific, Rockford, IL), the membranes were probed overnight at 4°C with various primary antibodies: rabbit polyclonal anti-Akt antibody (catalog number: 9272, Cell Signaling Technology), rabbit polyclonal anti-phospho-Ser473-Akt antibody (catalog number: 9271, Cell Signaling Technology) and rabbit polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (catalog number: G9545, Sigma, St Louis, MO). The membranes were incubated with the appropriate horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibodies for 1 h at room temperature. The protein bands were visualized with the enhanced chemiluminescence method using a Genomic and Proteomic Gel Documentation (Gel Doc) Systems from Syngene (Frederick, MD, USA). The densities of Akt and phospho-Akt protein bands were normalized to those of GAPDH to control for errors in protein sample loading and transferring during Western blotting. The results of various experimental conditions were normalized to the corresponding data of control cells in the same experiment.
2.8. Statistical analysis
All data are expressed as means ± S.D. Results were analyzed by one-way analysis of variance followed by the Tukey test for post hoc analysis after confirmation of normal distribution of the data or by Kruskal-Wallis analysis of variance on ranks followed by the Tukey test when the data are not normally distributed. A P value < 0.05 was considered statistically significant. All statistical analyses were performed with the SigmaStat (Systat Software, Inc., Point Richmond, CA, USA).
3. Results
3.1. Transferable inter-cell OGD preconditioning induced acute and delayed phases of neuroprotection
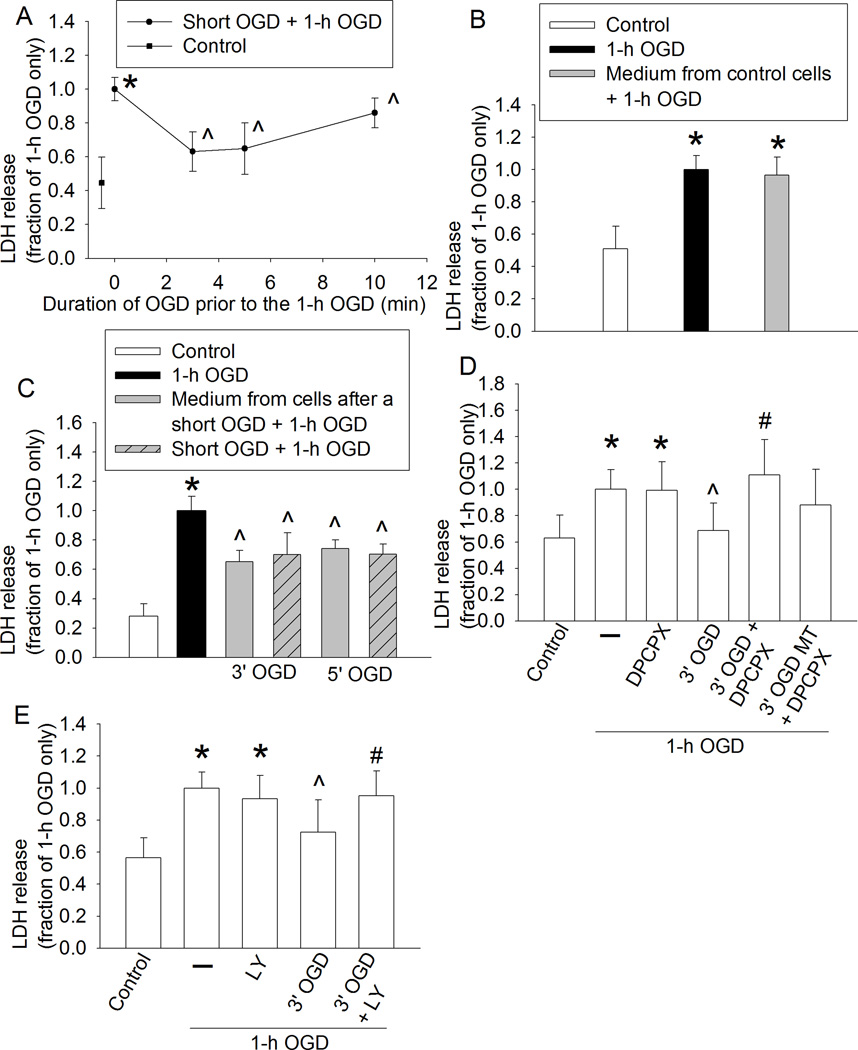
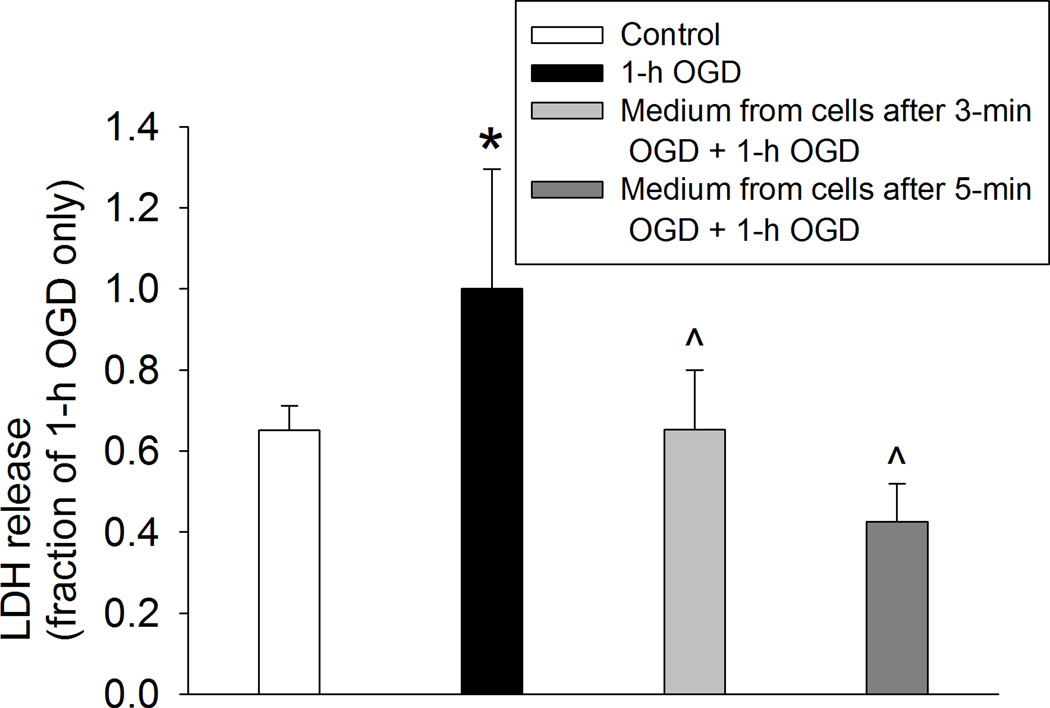
We showed that an OGD of 3, 5 or 10 min at 1 h before the 60-min OGD significantly reduced LDH release in human neuron-like cells (Fig. 1A). These results suggest that preconditioning with a short OGD induces protection in human neuron-like cells. Although cell culture medium from control cells did not affect the LDH release after a 60-min OGD (Fig. 1B), cells incubated for 1 h with culture medium from cells subjected to a 3- or 5-min OGD had a reduced LDH release after the 60-min OGD. The reduction of LDH release was at the level similar to those cells that had a 3- or 5-min OGD prior to the 60-min OGD (Fig. 1C). These results suggest that the OGD preconditioning is transferable to induce an acute phase of neuroprotection. Consistent with the results from human neuron-like cells, 60-min OGD also significantly increased LDH release in the primary rat cortical neuronal cultures and this increase was attenuated by culture medium from the rat cortical neuronal cultures subjected to a 3- or 5-min OGD (Fig. 2).
Fig. 1. Oxygen-glucose deprivation (OGD) preconditioning-induced acute phase of neuroprotection in human neuron-like cells.
A: The differentiated human SH-SY5Y cells were subjected to various lengths of OGD. One hour later, they were subjected to a 60-min OGD. Cell injury was assessed by lactate dehydrogenase (LDH) release at 24 h after the 60-min OGD. B: Cells were subjected to the 60-min OGD only. Some cells were incubated with the culture medium from control cells for 1 h before the 60-min OGD. C: Cells were subjected to a 3- or 5-min OGD and then 1-h reperfusion or incubated for 1 h with the culture medium from cells exposed to a 3- or 5-min OGD before the 60-min OGD. D: 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX) was added to the medium immediately before the 3-min OGD or 60 min before the 60-min OGD in the OGD plus DPCPX only group. E: LY294002 was added to the medium immediately before the 3-min OGD or 60 min before the 60-min OGD in the OGD plus LY294002 only group. Results are means ± S.D. (n = 18 – 24). * P < 0.05 compared with the corresponding control cells, ^ P < 0.05 compared with cells subjected to the 60-min OGD only. # P < 0.05 compared with 3-min OGD and then 60-min OGD. Statistical analysis of all data was performed by one-way analysis of variance except for the data presented in panel A that was analyzed by Kruskal-Wallis analysis of variance on ranks. MT: medium transferring.
Fig. 2. Oxygen-glucose deprivation (OGD) preconditioning-induced acute phase of neuroprotection in primary rat cortical neuronal culture.
Cells were incubated for 1 h with or without the culture medium from cells exposed to a 3- or 5-min OGD before the 60-min OGD. Cell injury was assessed by lactate dehydrogenase (LDH) release at 24 h after the 60-min OGD. Results are means ± S.D. (n = 6). * P < 0.05 compared with control cells, ^ P < 0.05 compared with cells subjected to the 60-min OGD only. Statistical analysis was performed by one-way analysis of variance.
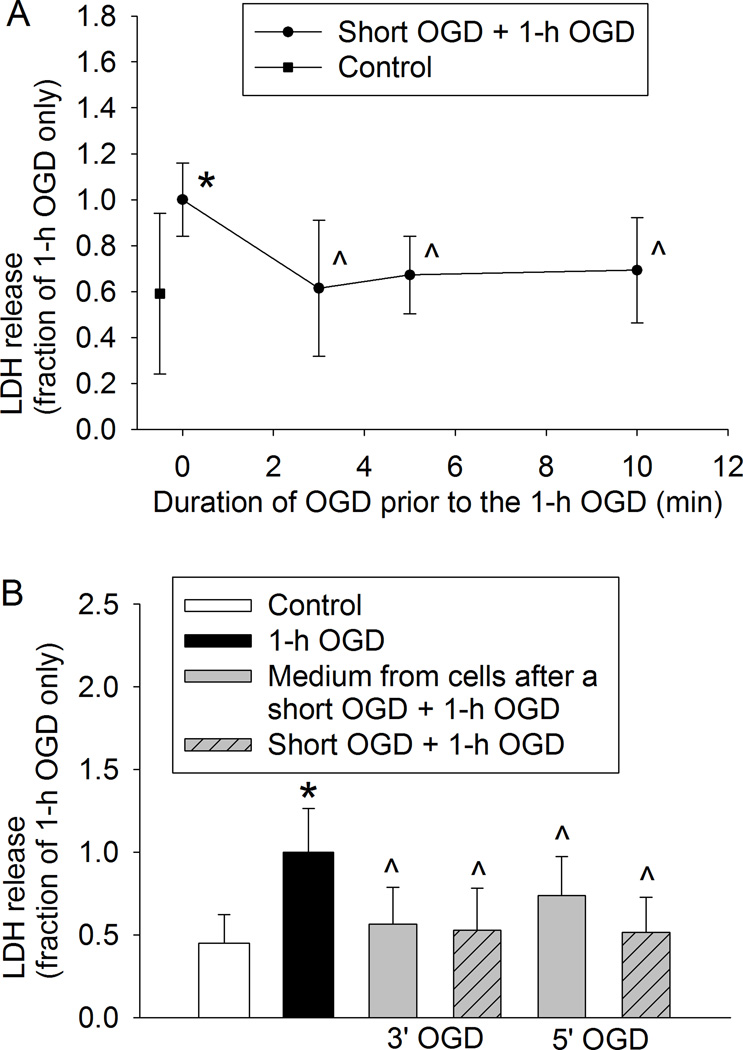
Similar to the results of acute phase of preconditioning-induced neuroprotection, an OGD of 3, 5 or 10 min at 24 h before the 60-min OGD significantly reduced LDH release (Fig. 3A). Cells exposed to the culture medium from cells that were subjected to a 3- or 5-min OGD had a decreased LDH release after the 60 min OGD applied at 24 h after the medium exposure (Fig. 3B). These results suggest that a short OGD induced a delayed phase of neuroprotection and that this preconditioning effect is transferable.
Fig. 3. Oxygen-glucose deprivation (OGD) preconditioning-induced delayed phase of neuroprotection in human neuron-like cells.
A: The differentiated human SH-SY5Y cells were subjected to various lengths of OGD. Twenty-four hour later, they were subjected to a 60-min OGD. Cell injury was assessed by lactate dehydrogenase (LDH) release at 24 h after the 60-min OGD. B: Cells were subjected to a 3- or 5-min OGD and then 24-h reperfusion or incubated for 24 h with the culture medium from cells exposed to a 3- or 5-min OGD before the 60-min OGD. Results are means ± S.D. (n = 24 – 30). * P < 0.05 compared with the corresponding control cells, ^ P < 0.05 compared with cells subjected to 60-min OGD only. Statistical analysis of the data presented in panel A was performed by one-way analysis of variance and the data presented in panel B was analyzed by Kruskal-Wallis analysis of variance on ranks.
3.2. Transferable inter-cell OGD preconditioning-induced neuroprotection might be mediated by adenosine A1 receptor
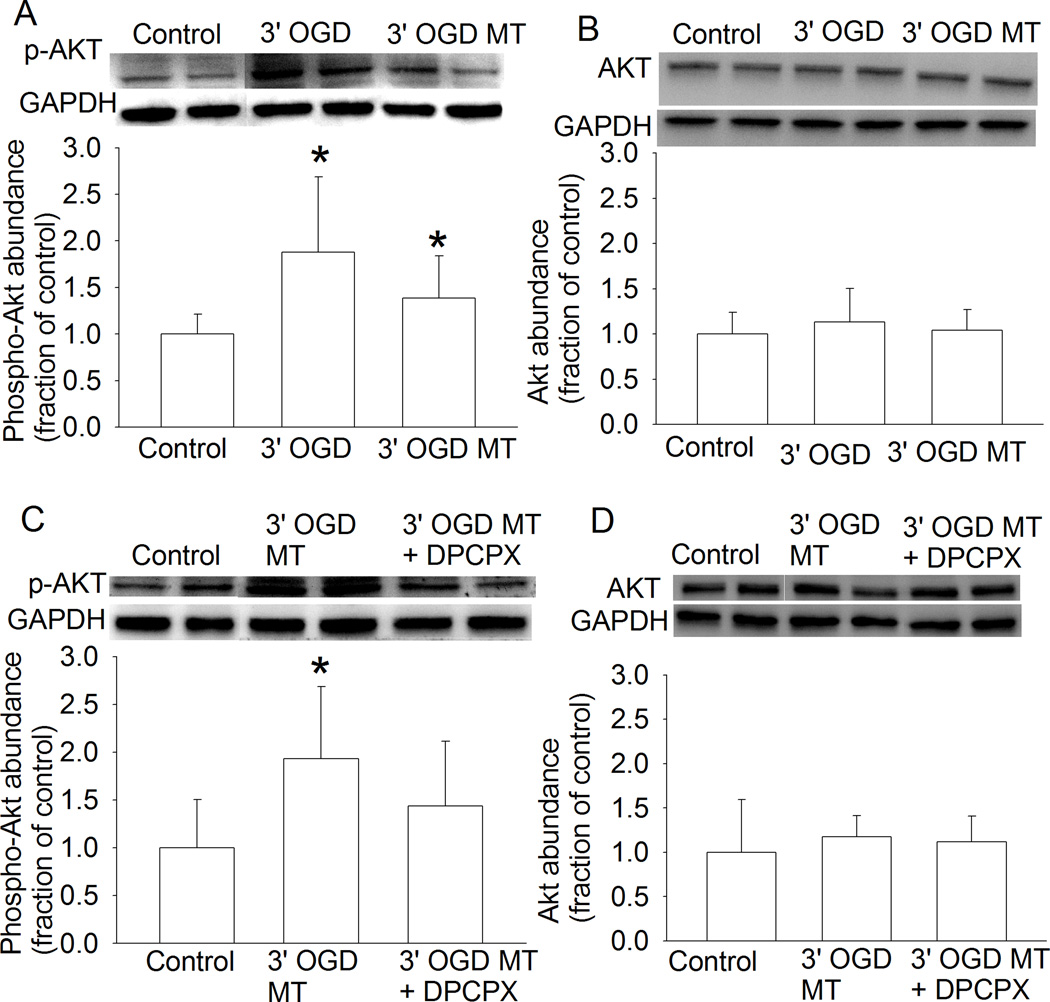
Since adenosine A1 receptors have been shown to play a role in remote preconditioning-induced neuroprotection (Hu, et al., 2012), we tested the role of these receptors in the OGD preconditioning-induced protection. Although DPCPX, a specific adenosine A1 receptor inhibitor, did not affect the 60-min OGD-induced LDH release, DPCPX significantly attenuated the reduction of LDH release caused by the 3-min OGD or the medium of cells subjected to the 3-min OGD (Fig. 1D). Since activation of adenosine A1 receptors can stimulate Akt (Park, et al., 2010) and Akt is a prosurvival protein (Li and Zuo, 2011), we determined the role of Akt in the OGD preconditioning-induced protection. LY294002, an Akt activation inhibitor, abolished the 3-min OGD induced reduction of LDH release after the 60-min OGD (Fig. 1E). Also, 3-min OGD or the medium from cells subjected to a 3-min OGD increased the phosphorylated/activated Akt (Fig. 4A). The increased expression of phospho-Akt by the medium of cells subjected to a 3-min OGD was attenuated by DPCPX (Fig. 3C). Neither 3-min OGD nor the medium from cells subjected to a 3-min OGD affected Akt expression (Figs. 4B and 4D). These results suggest a role of adenosine A1 receptors and Akt in the OGD preconditioning-induced neuroprotection in these human neuron-like cells.
Fig. 4. Oxygen-glucose deprivation (OGD) preconditioning-increased phosphorylation of Akt in human neuron-like cells.
A and B: The differentiated human SH-SY5Y cells were subjected to a 3-min OGD or were incubated with culture medium from cells subjected to a 3-min OGD. Thirty minutes later, they were harvested for Western blotting of phospho-Akt (p-Akt) and Akt. C and D: 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX) was added to the medium 30 min after the 3-min OGD. The medium was collected 30 min after the OGD and was incubated with OGD-naïve cells for 30 min before the cells were harvested for Western blotting of p-Akt and Akt. Results are means ± S.D. (n = 12 – 18). * P < 0.05 compared with the corresponding control cells. Statistical analysis of all data was performed by one-way analysis of variance. MT: medium transferring.
4. Discussion
Our results showed that culture medium from cells subjected to a short episode of OGD induced a protective effect in OGD-naïve human neuron-like cells and primary rat cortical neuron cultures. These results clearly suggest that the preconditioning effect was transferable. Transferred inter-cardiac ischemic preconditioning has been shown (Dickson, et al., 2001; Dickson, et al., 1999) but similar phenomenon has not been demonstrated in other tissues/organs. Our study suggests the existence of these effects in the neural tissues. Similar to the autologous preconditioning effect in which a preconditioning stimulus is applied to the same tissues/organs that are subjected to a subsequent detrimental insult (Gidday, 2006; Iadecola and Anrather, 2011), our results provide initial evidence that the transferred inter-tissue/organ preconditioning can induce acute and delayed phases of protection.
The transferred inter-neural tissue preconditioning may have significant clinical implication. Brain and spinal cord are soaked in cerebral spinal fluid. This set-up will obviously help transfer the preconditioning effects from one brain or spinal cord region to other regions. Transient ischemic attack is a clinical phenomenon and may improve the ischemic tolerance of the whole central nervous system if our findings in this study are confirmed in human neural tissues. This effect may be part of endogenous system developed to protect tissues/organs at risk.
Very little is known about the mechanisms for the transferred inter-cardiac ischemic preconditioning. We reasoned that small molecules, such as adenosine, may be released into the extracellular fluid to make the preconditioning effects transferable. Our results support this theory because DPCPX, a specific adenosine A1 receptor inhibitor, attenuated the transferred inter-cell OGD preconditioning. Consistent with this theory, activation of adenosine A1 receptors is known to induce neuroprotection (Cunha, 2005). Also, limb remote ischemic preconditioning-induced neuroprotection may be mediated by adenosine A1 receptor (Hu, et al., 2012). ATP and adenosine are known to be released from tissues including neural tissues under stress, such as during hypoxia and ischemia (Dale and Frenguelli, 2009; Milusheva, et al., 1990) The released ATP may be a major source to form adenosine extracellularly by ecto-ATPase in neural tissues (Melani, et al., 2012).
Our results also suggest that Akt activation may be an event downstream of adenosine A1 receptors because the increase of phospho-Akt induced by the culture medium from cells subjected to a 3-min was attenuated by DPCPX and previous findings that activation of adenosine A1 receptor can activate Akt (Park, et al., 2010) and that Akt is a prosurvival protein (Li and Zuo, 2011). The adenosine A1 receptor-Akt signaling pathway is also important for the preconditioning effects of a short episode of OGD on the same cells because these autologous preconditioning effects were inhibited by DPCPX and LY294002 and the short episode of OGD increased the phosphorylation/activation of Akt.
Our study has limitations. We used human neuron-like cell cultures and primary rat cortical neuronal cultures. It is not appropriate to extrapolate our findings directly to human neural tissues under in vivo conditions. However, fresh human neural tissues are difficult to obtain and performing an experiment to test the hypotheses as proposed in our study in human under in vivo conditions is not possible. Another limitation is that we determined the role of adenosine A1 receptors in the transferred inter-cell OGD preconditioning effects but did not identify the specific ligand(s) for this effect. In addition to adenosine, other small molecules, such as adenosine 5'-monophosphate, are agonists for adenosine A1 receptors (Rittiner, et al., 2012). Future studies are needed to determine the agonist(s) involved in this transferred inter-cell preconditioning effect induced by OGD.
In summary, we have shown that a short episode of OGD induces acute and delayed phases of protection in human neuron-like cells. This preconditioning-induced protection is transferable between human neuron-like cells or primary rat cortical neuronal cultures and may be mediated by adenosine A1 receptors and Akt.
Highlights.
Oxygen-glucose deprivation (OGD) preconditioning induces neuroprotection
Oxygen-glucose deprivation preconditioning-induced neuroprotection is transferable among the same cells
The transferred inter-cell OGD preconditioning may be mediated by adenosine A1 receptors and Akt
Acknowledgments
Grant support: This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia.
Abbreviations
- DPCPX
8-cyclopentyl-1, 3-dipropylxanthine
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- LDH
lactate dehydrogenase
- OGD
oxygen-glucose deprivation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study was performed in and should be attributed to the Department of Anesthesiology, University of Virginia, USA.
Conflict of interest: No.
References
- Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signaling. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EW, Blehar DJ, Carraway RE, Heard SO, Steinberg G, Przyklenk K. Naloxone blocks transferred preconditioning in isolated rabbit hearts. Journal of Molecular and Cellular Cardiology. 2001;33:1751–1756. doi: 10.1006/jmcc.2001.1436. [DOI] [PubMed] [Google Scholar]
- Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, Przyklenk K. Rabbit heart can be "preconditioned" via transfer of coronary effluent. American Journal of Physiology. 1999;277:H2451–H2457. doi: 10.1152/ajpheart.1999.277.6.H2451. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, Zhang J, Xiong L. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Research. 2012;1459:81–90. doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nature Neuroscience. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T, Kawato Y, Osakada F, Izumi Y, Katsuki H, Nakagawa T, Kaneko S, Niidome T, Takada-Takatori Y, Akaike A. Dibutyryl cyclic AMP induces differentiation of human neuroblastoma SH-SY5Y cells into a noradrenergic phenotype. Neuroscience Letters. 2008;443:199–203. doi: 10.1016/j.neulet.2008.07.079. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neuroscience. 2011;199:44–50. doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Hausenloy DJ. Remote ischemic conditioning: from bench to bedside. Front Physiol. 2012;3:27. doi: 10.3389/fphys.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Feng C, Cao M, Zuo Z. Volatile anesthetics may not induce significant toxicity to human neuron-like cells. Anesthesia and Analgesia. 2011 doi: 10.1213/ANE.0b013e3181fdf69d. In press. [DOI] [PubMed] [Google Scholar]
- Lin D, Li G, Zuo Z. Volatile anesthetic post-treatment induces protection via inhibition of glycogen synthase kinase 3beta in human neuron-like cells. Neuroscience. 2011;179:73–79. doi: 10.1016/j.neuroscience.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani A, Corti F, Stephan H, Muller CE, Donati C, Bruni P, Vannucchi MG, Pedata F. Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Experimental Neurology. 2012;233:193–204. doi: 10.1016/j.expneurol.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Milusheva E, Sperlagh B, Kiss B, Szporny L, Pasztor E, Papasova M, Vizi ES. Inhibitory effect of hypoxic condition on acetylcholine release is partly due to the effect of adenosine released from the tissue. Brain Research Bulletin. 1990;24:369–373. doi: 10.1016/0361-9230(90)90091-d. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Park SW, Chen SW, Kim M, Brown KM, D'Agati VD, Lee HT. Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. Journal of Pharmacology and Experimental Therapeutics. 2010;333:736–747. doi: 10.1124/jpet.110.166884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. AMP is an adenosine A1 receptor agonist. Journal of Biological Chemistry. 2012;287:5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ren C, Chen X, Shen J. From rapid to delayed and remote postconditioning: the evolving concept of ischemic postconditioning in brain ischemia. Curr Drug Targets. 2012;13:173–187. doi: 10.2174/138945012799201621. [DOI] [PMC free article] [PubMed] [Google Scholar]