Abstract
Nodal flow orients the left-right axis in some vertebrates, and is generated by clockwise-rotating cilia that are tilted posteriorly. In a recent paper, Song et al. (2010) add to mounting evidence that the planar cell polarity (PCP) pathway coordinates posterior positioning of cilia and subsequent determination of left-right asymmetry.
For centuries, anatomists have recognized that the human body's external bilateral symmetry masks internal asymmetry (Figure 1). To set up our left-right asymmetries, embryos first set up their anterior-posterior and dorsal-ventral axes which, as with any good Cartesian system, defines the third axis. Moreover, vertebrate embryos create a midline, which anchors the origin of the left-right axis. But, how does the embryo determine which side of this midline will be the left and which will be the right? In principle this choice could be made stochastically, but the fact that our heartbeats are routinely on our left reveals that it is not.
Figure 1. Nodal cilia function as the F molecule.
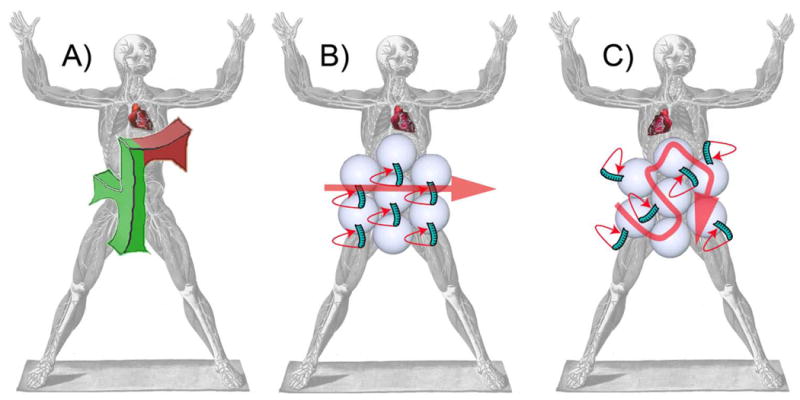
(A) Orientation of the theoretical F molecule along the anterior-posterior and dorsal-ventral axes (green) dictates the orientation of the left-right axis (red arm). Communication of the information to the rest of the embryo directs left-right asymmetric development, including heart looping. (B) PCP signals point the apical cilia of node cells posteriorly. This posterior orientation combined with the inherent clockwise rotation generates a leftward flow that specifies left in some vertebrate embryos. (C) Without Vangl function, cilia form and beat, but are not oriented posteriorly. Consequently, flow and left-right axis patterning are disrupted. Figure modified from Bartolommeo Eustachi Tabulae anatomicae XXV, 1783.
To explain how embryos might orient their left-right axis, Brown and Wolpert posited the existence of an “F molecule,” the stem and one arm of which would orient along the anterior-posterior and dorsal-ventral axes (Brown and Wolpert, 1990). The remaining arm, orthogonal to the other, would point to the left in a molecular equivalent of the right-hand rule (Fig. 1A). This molecular asymmetry would be communicated to F molecule-bearing cells, and from the cells to the embryo as a whole, to direct left-right specific developmental events.
Where could such an F molecule exist? Genetic evidence has long suggested that the specialized epithelial tissue called the embryonic node is integral to vertebrate left-right axis orientation. Node cells possess an apicobasal polarity that corresponds to the dorsal-ventral axis and acquire motile cilia on their apical surfaces. These cilia extend from the posterior aspect of the nodal cells and rotate in a clockwise direction, producing an asymmetric stroke that generates unidirectional flow towards the left (Nonaka et al., 2005; Okada et al., 2005) (Fig. 1B). Perhaps through the interpretation of mechanical flow forces or biased distribution of signals, this leftward flow induces left-specific gene expression, including Nodal and Lefty in the lateral plate mesoderm. Nodal subsequently induces expression of the homeobox gene Pitx2 which participates in the development of such organs as the heart, guts and lungs to give rise to asymmetric organ placement and morphology (Yoshioka et al., 1998). Thus, the cilium possesses the salient features of the F molecule. First, its apical position interprets the defined dorsal-ventral axis (one arm of the F). Second, its posterior position and tilt interprets the defined anterior-posterior axis (the stem of the F). And third, its clockwise rotation defines the orientation of the left-right axis (the remaining arm of the F). It is the resultant nodal flow that communicates this third axis to surrounding cells.
This analogy predicts that positioning the cilium posteriorly is critical to defining left. How might the embryo impart information about the anterior-posterior axis to nodal cells? Several recent reports, including a study from Song et al. in a recent issue of Nature, reveal that the PCP pathway performs this role (Song et al., 2010).
PCP genes were identified for their role in orienting cells within the plane of a sheet of cells, in a direction orthogonal to the apicobasal axis. Asymmetric cellular localization of core PCP proteins coordinates cellular morphogenesis and movements within the plane. Disruption of PCP in different tissues results in varied developmental defects, ranging from misoriented ommatidia to failure of convergent extension. Many core PCP pathway components are conserved from flies to vertebrates, with several genes, including Dishevelled (Dvl) and Van gogh (Vang) having undergone duplication.
Many mouse embryos lacking Vangl1 are viable and fertile, but a minority display hallmarks of defective PCP, including failure of convergent extension and neural tube closure. Notably, Vangl1 mutants also display laterality defects, such as bilateral Pitx2 expression (Antic et al. 2010). Mice harboring mutations in Vangl2 also exhibit open neural tubes, and two recent studies revealed roles for Vangl2 in left-right axis determination in the frog and fish. First, knockdown of Vangl2 in the Xenopus gastrocoel roof plate disrupted the posterior localization of cilia (Antic et al. 2010). Second, maternal-zygotic vangl2 (MZvangl2) zebrafish mutants exhibited aberrant lefty2 expression (Borovina et al. 2010). Although mutant cilia in the zebrafish equivalent of the node, Kupffer's vesicle, retained normal length and range of motion, many failed to tilt posteriorly, suggesting that Vangl2 regulates ciliary position.
Song et al. (2010) investigated the role of PCP in establishing nodal flow by examining mouse mutants lacking all Vangl function. Vangl1gt/gt Vangl2Δ/Δ mutants displayed PCP defects, as evidenced by misorientation of stereocilia and kinocilia in cochlear sensory hair cells. Excitingly, they also exhibited laterality anomalies, including defects in embryo turning, heart looping and proper lung lobe sidedness. Furthermore, the laterality markers Nodal, Lefty and Pitx2 were expressed bilaterally or on the right in many mutants, indicating that Vangl is required upstream of Nodal.
To elucidate the mechanism by which Vangl orients the left-right axis, Song et al. examined nodal cilia and flow (Song et al., 2010). In Vangl1gt/gt Vangl2Δ/Δ mutants, the cilia were morphologically similar and beat with similar frequencies as controls, suggesting that PCP is not required for ciliogenesis per se. Strikingly, however, leftward nodal flow and left-sided intracellular calcium release were disrupted. In the mutants, basal bodies, the foundations of cilia, were not appropriately positioned at the posterior, leading to randomized ciliary localization. Therefore, these data reveal that Vangl is necessary for posterior positioning of basal bodies, the posterior orientation of the cilia they give rise to, and thus the unidirectional leftward nodal flow that the cilia generate (Fig. 1C).
This study confirms the involvement of PCP in orienting the mouse left-right axis, previously suggested by analysis of Inversin, Bicaudal C, and Seahorse, proteins that interact with the core PCP component Dvl. An interesting question that arises from these findings is how PCP proteins position basal bodies, which relocate during development from a central to posterior location (Hashimoto et al. 2010). GFP-tagged Dvl2 and Dvl3 polarize to the posterior, and many node cells from compound Dvl mutants (Dvl1-/- Dvl2-/- Dvl3+/- and Dvl1-/- Dvl2+/- Dvl3-/-) display basal body positioning defects (Hashimoto et al. 2010). Furthermore, Vangl1 and another PCP protein, Prickle2, localizes anteriorly in node cells (Antic et al. 2010), suggesting that the creation of polarized fronts of opposing PCP components presages and directs the positioning of the basal body.
It is still unclear how information from the anterior-posterior axis polarizes PCP components in the node. A possibility raised by Hashimoto and Hamada is that PCP could be directed through a Wnt-independent mechanism involving the atypical cadherin Fat and its kinase Four-jointed, proteins required for PCP in Drosophila. Interestingly, a mammalian homolog of Fat localizes to cilia, and Fat and Four-jointed homologs genetically interact with Vangl2 in kidney cyst formation (Saburi et al., 2008). As ciliary defects can also result in kidney cysts, it will be exciting to learn whether Fat and Four-jointed cooperate with Vangl to regulate ciliary behavior in the node. Lastly, chick embryos orient the left-right axis through a cilium-independent mechanism (Gros et al., 2009), so perhaps Vangl/PCP-mediated cellular behavior represents the fundamental aspect of left-right axis determination that is evolutionarily conserved among vertebrates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Nat Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Brown NA, Wolpert L. Development. 1990;109:1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Science. 2009;324:941–944. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. Nat Cell Biol. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Nature. 2010;466:378–82. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, et al. Cell. 1998;94:299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]


