Summary
Breast phyllodes tumors are fibroepithelial neoplasms with variable risk of aggressive local recurrence and distant metastasis, and the molecular pathogenesis is unclear. Here, we systematically study p16 and Rb expression in 34 phyllodes tumors in relation to proliferation. Tissue microarrays were constructed from 10 benign, 10 borderline, and 14 malignant phyllodes (5 cores/tumor) and from 10 fibroadenomas (2 cores/tumor). Tissue microarrays were labeled by immunohistochemistry for p16, Rb, and Ki-67 and by in situ hybridization for high-risk human papillomavirus. Cytoplasmic and nuclear p16 were scored by percentage labeling (0%-100%, diffuse >95%) and intensity. Nuclear Rb was scored by percentage labeling (0%-100%, diffuse >75%) and intensity. p16 and Rb labeling were repeated on whole sections of cases with Rb loss on the tissue microarray. Twenty-nine percent (4/14) malignant phyllodes showed diffuse strong p16 labeling with Rb loss in malignant cells (diffuse p16+/Rb−), whereas 21% (3/14) malignant phyllodes showed the reverse pattern of p16 loss with diffuse strong Rb (p16−/diffuse Rb+). Results were consistent between tissue microarrays and whole sections. No borderline phyllodes, benign phyllodes, or fibroadenoma showed diffuse p16+/Rb− or p16−/diffuse Rb+ phenotypes. No cases contained high-risk human papillomavirus. Average Ki-67 proliferation indices were 15% in malignant phyllodes, 1.7% in borderline phyllodes, 0.5% in benign phyllodes, and 0% in fibroadenoma. Ki-67 was highest in malignant phyllodes with diffuse p16+/Rb− labeling. In summary, 50% malignant phyllodes display evidence of Rb/p16 pathway alterations, likely reflecting p16 or Rb inactivation. These and other mechanisms may contribute to the increased proliferation in malignant phyllodes relative to other fibroepithelial neoplasms.
Keywords: p16, Rb, Phyllodes tumor, Breast, Fibroepithelial neoplasm
1. Introduction
Phyllodes tumors of the breast are fibroepithelial neoplasms [1,2] that carry variable risk for aggressive local recurrence and distant metastasis, with the rate of distant metastasis reaching more than 25% in malignant phyllodes (MP) tumors [1]. To date, the molecular pathogenesis of phyllodes tumors is unclear, but dysregulation of the cell cycle may play a role as evidenced by increased Ki-67 proliferation labeling in high-grade phyllodes tumors [3-7]. The cell cycle mediators Rb and p16 modulate the G1-S cell cycle checkpoint [8-10] and have been shown to be altered in invasive ductal carcinomas of the breast [11], particularly in basal-like breast carcinomas, which frequently demonstrate a diffuse p16+/Rb− phenotype by immunohistochemistry (IHC) [12]. However, the presence of p16 and Rb changes in phyllodes tumors has been inconsistent. Although several studies have shown increased p16 and Rb IHC protein labeling in high-grade phyllodes tumors [6,13], others have shown loss of chromosome 9p (encompassing the p16 locus 9p21) and/or chromosome 13 (encompassing the Rb locus 13q14) [14-16]. Importantly, few studies have correlated p16 and Rb expression in individual phyllodes tumors. Here, we systematically study the expression of p16 and Rb in a series of benign phyllodes (BP), borderline phyllodes (BLP) and MP tumors in relation to proliferation.
2. Materials and methods
2.1. Tissue microarray construction and case selection
This study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions. We evaluated a series of tissue microarrays (TMAs) constructed from archived paraffin tissue blocks of 34 fibroepithelial neoplasms of the breast, as previously described [17,18]. The fibroepithelial neoplasms included 10 BP, 10 BLP, 14 MP, and 10 fibroadenomas (FAs). The cases were selected from consecutive excisional specimens from the in-house surgical service and consultation service of our institution. Cases with minimal tumor on the excision specimen were excluded. The phyllodes tumors were subdivided into the categories of benign, borderline, and malignant based on tumor circumscription, the presence of stromal overgrowth, stromal cellularity, stromal pleomorphism, and stromal mitoses (Table 1), using established criteria [1,2]. All malignant phyllodes cases were high grade. All cases included in the TMA were selected as classic examples of each subtype; cases that were difficult to subclassify (ie, had features of >1 subtype) were purposely excluded. Each TMA consisted of 99 cores measuring 1.4 mm in diameter, including 9 cores of normal control organ tissues. Five cores were taken per phyllodes tumor and 2 cores per FA, to minimize sampling error. One core per phyllodes case included adjacent benign breast lobules as an internal control.
Table 1.
Criteria for subclassification of breast phyllodes tumors
| Morphologic features | BP | BLP | MP |
|---|---|---|---|
| Circumscription | Well circumscribed | Intermediate | Infiltrative |
| Stromal pattern | Uniform | Heterogeneous | Overgrowth |
| Stromal cellularity | Moderate | Moderate | Marked |
| Stromal pleomorphism | Mild-moderate | Moderate | Marked |
| Stromal mitoses | Few (<5/10 HPF) | Intermediate (5-10/10 HPF) | Numerous (>10/10 HPF) |
2.2. IHC in situ hybridization and expression scoring
The TMAs were labeled by IHC for p16, Rb, and Ki-67 using methods previously described [12]. Briefly, the TMAs were labeled for p16 (E6H4 monoclonal, predilute, reference no. 705-4713) and Ki-67 (rabbit monoclonal, predilute, clone 30-9, catalog no. 790-4286; Ventana Medical Systems, Inc, Tucson, AZ) using the Benchmark XT automated slide stainer (Ventana Medical Systems, Inc). The TMAs were labeled for Rb with a manual method, using the G3-245 mouse monoclonal antibody (1:2000 dilution; BD Biosciences, Pharmingen, San Jose, CA) after 20-minute antigen retrieval in 10 mM citrate buffer. Rb labeling was visualized with the Dako LSAB kit (catalog no. K0690).
The IHC results were averaged over the 4 to 5 cores per tumor. Ki-67 proliferation indices were calculated as percent mitotic rate in 4 to 5 cores and averaged; if no mitoses were present in 1 high-power field, the proliferation index was considered 0% for calculation purposes. The presence of mitoses in the epithelial cells served as an internal control. Nuclear and cytoplasmic p16 labeling was scored by labeling intensity (weak [W], moderate [M], or strong [S]) and percentage labeling from 0% to 100%, with diffuse labeling defined as more than 95%. Nuclear Rb labeling was scored by labeling intensity (W, M, or S) and percentage labeling from 0% to 100%, with diffuse labeling defined as more than 75%. Cytoplasmic Rb labeling was considered nonspecific. Tumors were considered negative for Rb when the neoplastic stromal nuclei were negative and the benign stromal or epithelial cell nuclei were positive, serving as an internal control. The p16 and Rb IHC was repeated on whole sections from donor blocks of all cases with negative Rb labeling on the TMA. IHC scoring was reviewed and evaluated manually by 2 board-certified pathologists (A. C. M. and P. A.).
In addition, the TMAs were also labeled by in situ hybridization for high-risk human papillomavirus (HR-HPV) because p16 expression is also increased in human papillomavirus (HPV) infection [19]. The in situ hybridization was performed as previously described [20] using the HPV Inform III family 16 kit with iVIEW Blue+ v3 detection according to the manufacturer’s recommendations (Ventana Medical Systems, Inc).
3. Results
3.1. Clinicopathologic characteristics
The clinicopathologic characteristics of the patients with fibroepithelial neoplasms of the breast (n = 34) can be seen in Table 2. Briefly, the patients with MP (n = 14) had a mean age at diagnosis of 48.3 years (range, 30-67 years), with a mean tumor size of 7.6 cm (range, 2.5-20 cm). Of 6 patients with MP and available postsurgical follow-up data, 1 had a positive resection margin, and 5 had negative resection margins, and none developed local recurrences; however, 50% (3/6) developed metastatic disease (sites: 2 lung, 1 brain), and those 3 patients died due to their disease. The remaining 3 patients were alive with no evidence of disease at the time of last follow-up. Two patients with MP received adjuvant radiation therapy, and none received chemotherapy. The patients with BLP (n = 10) had a mean age of diagnosis of 45.9 years (range, 20-76 years), with a mean tumor size of 5.2 cm (range, 1-12 cm). Of the 9 patients with BLP and follow-up data, none developed local recurrence, metastasis, or death due to disease. One patient with BLP received adjuvant radiation therapy for a concomitant diagnosis of ductal carcinoma in situ, and no patient received chemotherapy. The patients with BP (n = 10) had a mean age of diagnosis of 38 years (range, 18-48 years), with a mean tumor size of 2.1 cm (range, 1-2.5 cm). Of the 9 patients with BP and follow-up data, none developed local recurrence, metastasis, or death due to disease. The patients in the control group with FA (n = 10) had a mean age of diagnosis of 26.2 years (range, 13-51 years), with a mean tumor size of 3.2 cm (range, 1-6.3 cm). None of the patients with FA developed local recurrence, metastasis, or death due to disease.
Table 2.
Clinical data and pathologic features of the breast fibroepithelial neoplasms
| MP | BLP | BP | FA | |
|---|---|---|---|---|
| n | 14 | 10 | 10 | 10 |
| Age (y), mean (range) | 48.3 (30-67) | 45.9 (20-76) | 38 (18-48) | 26.2 (13-51) |
| Race (n) | ||||
| White | 5 | 4 | 5 | 4 |
| Black | 3 | 3 | 4 | 4 |
| Other/NR | 6 | 3 | 1 | 2 |
| Resection (n) | ||||
| Lumpectomy | 8 | 10 | 10 | 10 |
| Mastectomy | 6 | 0 | 0 | 0 |
| Tumor size (cm) | 7.6 (2.5-20) | 5.2 (1-12) | 2.1 (1-2.5) | 3.2 (1-6.3) |
| Location | ||||
| Left | 8 | 5 | 3 | 7 |
| Right | 6 | 5 | 7 | 3 |
| Final margins | ||||
| Positive | 3 | 6 | 6 | 6 |
| Negative | 11 | 4 | 4 | 1 |
| Not reported | 0 | 0 | 0 | 3 |
| Subset of cases with available follow-up data |
||||
| Number | 6 | 9 | 9 | 9 |
| Mean follow-up (mo) | 39.3 (15–110) | 38.7 (7–112) | 47.9 (0.5-129) | 1 (0.3-6) |
| Neoadjuvant treatment (n) | 0 | 0 | 0 | 0 |
| Adjuvant treatment (n) | 2 a | 1 b | 0 | 0 |
| No evidence disease (n) | 3 (50%) | 9 (100%) | 9 (100%) | 9 (100%) |
| Local recurrence (n) | 0 | 0 | 0 | 0 |
| Metastasis (n) | 3 c | 0 | 0 | 0 |
| Died due to disease(n) | 3 | 0 | 0 | 0 |
Abbreviation: NR, not reported.
Radiation.
Radiation, for ductal carcinoma in situ.
Fifty percent of all MP cases with follow-up had metastases, which consisted of 2 to the lung and 1 to the brain. All metastases occurred within 3 years.
All 3 patients who had metastases died due to disease.
3.2. p16 and Rb immunohistochemistry
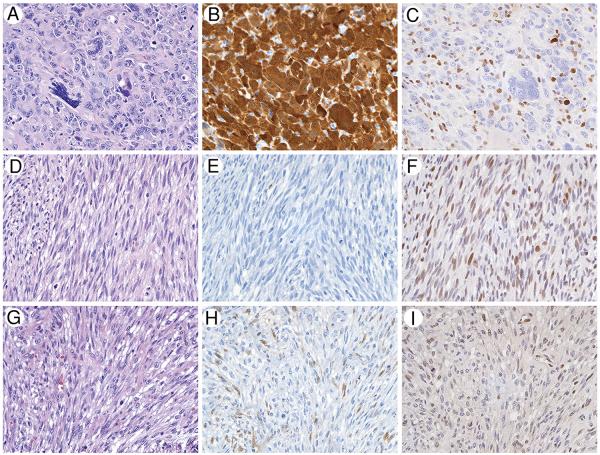
The patterns of p16 and Rb alterations by IHC are listed in Table 3. Among the MP cases, 29% (4/14) demonstrated diffuse strong p16 labeling with Rb loss in cytologically malignant stromal cells (“diffuse p16+/Rb−” phenotype) (Fig. A-C), whereas 21% (3/14) demonstrated the reverse pattern of p16 loss with diffuse strong Rb labeling (“p16−/diffuse Rb+” phenotype) (Fig. 1D-F). Abundant admixed benign vessels and nonatypical stromal cells (representing entrapped native stroma) exhibited intact Rb labeling in the cases with Rb loss in the malignant stromal cells, serving as an intact internal positive control. These results were consistent between TMA and whole sections. The remaining 50% of MP (7/14) demonstrated weak p16 labeling and weak Rb labeling (“low p16+/low Rb+” phenotype). The pattern of p16 and Rb immunolabeling did not correlate with patient survival in the 6 patients with MP and clinical follow-up. Of the 3 patients with MP who died due to disease, 1 displayed the p16−/diffuse Rb+ phenotype, and 2 displayed the low p16+/low Rb+ phenotype. Of the 3 patients with MP who had no evidence of disease at last follow-up, 1 displayed the p16−/diffuse Rb+ phenotype, 1 displayed the diffuse p16+/Rb1 phenotype, and 1 displayed the low p16+/low Rb+ phenotype.
Table 3.
Patterns of alteration in the p16/Rb pathway in breast fibroepithelial neoplasms
| p16/Rb expression pattern |
MP | BLP | BP | FA |
|---|---|---|---|---|
| Diffuse p16+/Rb− | 4 (29%) | 0 | 0 | 0 |
| p16−/diffuse Rb+ | 3 (21%) | 0 | 0 | 0 |
| Low p16+/low Rb+ | 7 (50%) | 10 (100%) | 7 (70%) | 10 (100%) |
| p16−/low Rb+ | 0 | 0 | 3 (30%) | 0 |
| Total (n) | 14 | 10 | 10 | 10 |
Fig.
Half of malignant phyllodes show p16 and Rb alterations by IHC, compared with no cases of BLP or BP. C, Twenty-nine percent of MP (A, hematoxylin and eosin) show strong, diffuse cytoplasmic, and nuclear p16 positivity (B), with loss of nuclear Rb labeling (C) in the pleomorphic malignant cells. Positive internal control labeling of nuclear Rb is present the admixed benign stromal cells and inflammatory cells. Twenty-one percent of MP (D, hematoxylin and eosin) show loss of p16 labeling (E), with strong and diffuse nuclear Rb labeling (F). All BLP (G, hematoxylin and eosin) show weak, patchy p16 labeling (H) in lesional stromal cells, with weak patchy-diffuse Rb labeling (I). (×160).
No case of BLP, BP, or FA showed the “diffuse p16+/Rb−” or “p16−/diffuse Rb+” phenotype seen in 50% of the MP (P = .02, Fisher exact test). Instead, 100% BLP, 70% BP, and 100% FA demonstrated the “low p16+/low Rb+” phenotype (Fig. G-H). The remaining 30% of BP demonstrated p16 loss with weak Rb labeling (“p16−/low Rb” phenotype).
3.3. Ki-67 proliferation indices
The mean Ki-67 proliferation indices in the fibroepithelial neoplasms were 15% in MP, 1.7% in BLP, 0.5% in BP, and 0% in FA (Table 4). In the MP, the cases with diffuse p16+/Rb− expression had higher mean Ki-67 (19%) than the cases with p16−/diffuse Rb+ expression (8%); however, this difference was not statistically significant in this relatively small cohort (P = .16, t test).
Table 4.
Ki-67 proliferation indices in breast fibroepithelial neoplasms
| Ki-67 proliferation index (mean) |
||||
|---|---|---|---|---|
| MP | BLP | BP | FA | |
| All cases | 15 % | 1.7% | 0.5% | 0% |
| Diffuse p16+/Rb− | 19% | N/A | N/A | N/A |
| p16−/diffuse Rb+ | 8% | N/A | N/A | N/A |
Abbreviation: N/A, not applicable.
3.4. HPV in situ hybridization
Because diffuse p16 expression is also associated with HR-HPV infection in the oropharynx and cervix [19], the TMAs were also labeled by in situ hybridization for HR-HPV. No case contained evidence of HR-HPV DNA integration by in situ hybridization.
4. Discussion
Fibroepithelial lesions of the breast are neoplastic proliferations of the mammary stroma [1,2,21,22], accompanied by epithelial components that may or may not also display clonal alterations [23,24]. Breast fibroepithelial lesions range from benign to malignant, including FA and phyllodes tumors, which have variable grades (BP, BLP, and MP). Although the morphologic distinction between FA and MP is not difficult, the criteria for separating cellular FA from BP are less straightforward [21]. However, the distinction is critical because phyllodes tumors carry variably increased risk for aggressive local recurrence and metastases. The rate of local recurrence varies from less than 20% in BP to greater than 25% in MP [22], with the presence of positive margins at the time of surgical resection for phyllodes tumor strongly associated with the risk of local recurrence [25-27]. Overall, approximately 2% of phyllodes tumors metastasize [2]. The reported rate of distant metastases ranges from less than 5% in BP and BLP to 25% in MP [1], although the reports of metastases in the BP likely represent undersampling of more aggressive lesions. However, histologic features have failed to accurately predict which MP will behave most aggressively.
The exact pathogenesis of fibroepithelial lesions of the breast remains unclear. Although FA was initially believed to be hyperplastic rather than neoplastic [28], subsequent studies revealed monoclonality in FA as well as shared chromosomal aberrations between FA and phyllodes tumors in the same patient [29-31]. Furthermore, the molecular changes underlying the progression of phyllodes tumors are unclear. MP tumors are known to have increased Ki-67 proliferation indices as compared with BLP, and BLP as compared with BP [3-7], lending support to the hypothesis that cell cycle regulation plays a key role in the progression of phyllodes tumors.
The proteins Rb (retinoblastoma protein) and p16 are key regulators of the cell cycle and proliferation [8-10]. Rb functions to repress the E2F transcription factors, thereby acting to put a brake on entry into the S-phase of the cell cycle. Cyclin-dependent kinases act to hyperphosphorylate Rb, causing dissociation of Rb from the E2F transcription factors and allowing progression through the cell cycle. The protein p16 is an inhibitor of cyclin-dependent kinase D1 and thus inhibits the hyperphosphorylation of Rb and entry into the cell cycle.
Loss of Rb in human cancers including breast cancer typically results in compensatory up-regulation of p16, and vice versa [11,32,33]. Invasive basal-like ductal breast carcinomas often show a p16+/Rb− phenotype [12], but the results in phyllodes tumors have been inconsistent. Studies by Kuijper et al [6] and Karim et al [13] showed an increase in both p16 and Rb protein expression by IHC with increasing grade of phyllodes tumor and reported no inverse relationship between p16 and Rb. In contrast, Esposito et al [7] found no significant association between p16 expression by IHC and grade of phyllodes tumor but did not investigate Rb expression.
The molecular changes undermining the pathogenesis of phyllodes tumors have also been investigated by comparative genomic hybridization studies, which show that phyllodes tumors but not FAs display chromosomal instability [16]. Furthermore, BP and BLP/MP segregate based on genomic alterations [14,15,34], with higher grade phyllodes tumor consistently showing gain of 1q [14,15,35]. Despite the IHC evidence of increased p16 and Rb expression in high-grade phyllodes tumors [6,13], genomic studies have actually demonstrated loss of chromosomal material encompassing the p16 locus (9p21) and/or Rb locus (13q14) in high-grade phyllodes tumors [14-16]. Jones et al [15] also demonstrated loss of p16 expression by IHC in phyllodes tumors showing −9p, but no decrease in Rb expression by mRNA in tumors with −13.
Here, we demonstrate for the first time an inverse relationship of p16 and Rb expression in phyllodes tumors. Most notably, these alterations in the p16/Rb pathway were specifically seen in half of MP, but not in BLP, BP, or FA. Twenty-nine percent of MP displayed strong diffuse p16 labeling with Rb loss in malignant cells (diffuse p16+/Rb−), suggesting inactivation of Rb with compensatory increase in p16 expression, a pattern previously reported in invasive ductal carcinoma [11,12]. A separate subset of MP, 21% of cases, displayed the reverse pattern of p16 loss with diffuse strong Rb expression in malignant cells (p16−/diffuse Rb+). This pattern likely reflects inactivation of p16. A caveat to this is that it is unclear whether the Rb antibody is able to recognize the hyperphosphorylated form of Rb [9], in which case the diffuse, strong Rb labeling could potentially reflect the accumulation of inactive Rb. Nevertheless, these data suggest that the G1-S cell cycle checkpoint is inactivated in half of MP.
In addition, our results support previous reports of increased Ki-67 proliferation indices in MP and BLP compared with BP [3-7]. Furthermore, the subset of MP with diffuse p16 labeling and Rb loss (diffuse p16+/Rb−) had the highest average Ki-67 proliferation index. Although not statistically significant, this finding is evidence that the cells are actively proceeding through the cell cycle and proliferating, supporting the concept that Rb is lost in these cells.
Our finding that alterations in the p16/Rb pathway are limited to MP illustrates several points. First, as detailed above, it suggests that these and other mechanisms contribute to the increased proliferation in MP relative to the other fibroepithelial neoplasms. Second, it suggests that immunolabeling for p16, Rb, and Ki-67 may be useful in differentiating between MP and BLP on a limited sample such as a core needle biopsy, where the distinction can be difficult [22]. Third, it shows that the diffuse p16+/Rb− phenotype in breast is not specific to basal-like invasive ductal carcinoma, as it may also be seen in MP. Finally, recent studies have suggested that triple negative breast carcinomas with loss of Rb respond better to chemotherapy [36-38]. Our findings suggest that the MP with the diffuse p16+/Rb− phenotype might be a subset of phyllodes tumors, which could be responsive to chemotherapy.
We recognize that our case series is relatively small with partial follow-up, and larger studies are clearly needed. In addition, the study is limited by the TMA methodology used, and phyllodes tumors are known to have considerable intratumoral heterogeneity even on genomic studies [15]. However, we attempted to address this issue by taking multiple large cores per tumor, which were selected from numerous areas of the tumor both adjacent to and far from the lesional epithelium.
In summary, malignant breast phyllodes tumors are fibroepithelial neoplasms with variable risk of aggressive local recurrence and distant metastasis. Here, we demonstrate alterations in the p16/Rb protein modulators of the G1-S cell cycle checkpoint in a significant subset of MP but not BLP, BP or FA. Our results suggest that alterations in the p16/Rb pathway contribute to the increased proliferation seen in MP. Additional large studies are needed to further clarify the role of the cell cycle in the pathogenesis of phyllodes tumors.
Acknowledgments
Funding source: Joseph C. Eggleston Fund in Surgical Pathology, The Johns Hopkins Hospital.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Rosen PP. Fibroepithelial neoplasms. In: Rosen PP, editor. Rosen’s Breast Pathology. 3rd Lippincott Williams & Wilkins; Philadelphia, PA: 2009. pp. 187–229. [Google Scholar]
- 2.Tan PH, Tse G, Lee A, et al. Fibroepithelial tumours. In: Lakhani SR, Ellis IO, Schnitt SK, et al., editors. World Health Organization Classification of Tumours of the Breast. IARC Press; Lyon, France: 2012. pp. 142–7. [Google Scholar]
- 3.Kleer CG, Giordano TJ, Braun T, Oberman HA. Pathologic, immunohistochemical, and molecular features of benign and malignant phyllodes tumors of the breast. Mod Pathol. 2001;14:185–90. doi: 10.1038/modpathol.3880282. [DOI] [PubMed] [Google Scholar]
- 4.Niezabitowski A, Lackowska B, Rys J, et al. Prognostic evaluation of proliferative activity and DNA content in the phyllodes tumor of the breast: immunohistochemical and flow cytometric study of 118 cases. Breast Cancer Res Treat. 2001;65:77–85. doi: 10.1023/a:1006457304526. [DOI] [PubMed] [Google Scholar]
- 5.Shpitz B, Bomstein Y, Sternberg A, et al. Immunoreactivity of p53, Ki-67, and c-erbB-2 in phyllodes tumors of the breast in correlation with clinical and morphologic features. J Surg Oncol. 2002;79:86–92. doi: 10.1002/jso.10049. [DOI] [PubMed] [Google Scholar]
- 6.Kuijper A, de Vos RA, Lagendijk JH, van der Wall E, van Diest PJ. Progressive deregulation of the cell cycle with higher tumor grade in the stroma of breast phyllodes tumors. Am J Clin Pathol. 2005;123:690–8. [PubMed] [Google Scholar]
- 7.Esposito NN, Mohan D, Brufsky A, et al. Phyllodes tumor: a clinicopathologic and immunohistochemical study of 30 cases. Arch Pathol Lab Med. 2006;130:1516–21. doi: 10.5858/2006-130-1516-PTACAI. [DOI] [PubMed] [Google Scholar]
- 8.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 9.Karim RZ, Scolyer RA, Tse GM, et al. Pathogenic mechanisms in the initiation and progression of mammary phyllodes tumours. Pathology. 2009;41:105–17. doi: 10.1080/00313020802579342. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011;50:5566–82. doi: 10.1021/bi200642e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dublin EA, Patel NK, Gillett CE, et al. Retinoblastoma and p16 proteins in mammary carcinoma: their relationship to cyclin D1 and histopathological parameters. Int J Cancer. 1998;79:71–5. doi: 10.1002/(sici)1097-0215(19980220)79:1<71::aid-ijc14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Subhawong AP, Subhawong T, Nassar H, et al. Most basal-like breast carcinomas demonstrate the same Rb−/p16+ immunophenotype as the HPV-related poorly differentiated squamous cell carcinomas which they resemble morphologically. Am J Surg Pathol. 2009;33:163–75. doi: 10.1097/PAS.0b013e31817f9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karim RZ, Gerega SK, Yang YH, et al. p16 and pRb immunohistochemical expression increases with increasing tumour grade in mammary phyllodes tumours. Histopathology. 2010;56:868–75. doi: 10.1111/j.1365-2559.2010.03562.x. [DOI] [PubMed] [Google Scholar]
- 14.Laé M, Vincent-Salomon A, Savignoni A, et al. Phyllodes tumors of the breast segregate in two groups according to genetic criteria. Mod Pathol. 2007;20:435–44. doi: 10.1038/modpathol.3800756. [DOI] [PubMed] [Google Scholar]
- 15.Jones AM, Mitter R, Springall R, et al. A comprehensive genetic profile of phyllodes tumours of the breast detects important mutations, intra-tumoral genetic heterogeneity and new genetic changes on recurrence. J Pathol. 2008;214:533–44. doi: 10.1002/path.2320. [DOI] [PubMed] [Google Scholar]
- 16.Kuijper A, Snijders AM, Berns EM, et al. Genomic profiling by array comparative genomic hybridization reveals novel DNA copy number changes in breast phyllodes tumours. Cell Oncol. 2009;31:31–9. doi: 10.3233/CLO-2009-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cimino-Mathews A, Subhawong AP, Elwood H, et al. Neural crest transcription factor Sox10 is preferentially expressed in triple negative and metaplastic breast carcinomas. HUM PATHOL. 2013;44:1341–9. doi: 10.1016/j.humpath.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimino-Mathews A, Subhawong AP, Illei PB, et al. GATA-3 expression in breast carcinoma: utility in triple negative sarcomatoid and metastatic carcinomas. HUM PATHOL. 2013;44:1341–9. doi: 10.1016/j.humpath.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4a) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–84. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 20.Cimino-Mathews A, Sharma R, Illei PB. Detection of human papillomavirus in small cell carcinomas of the anus and rectum. Am J Surg Pathol. 2012;36:1087–92. doi: 10.1097/PAS.0b013e3182549b6d. [DOI] [PubMed] [Google Scholar]
- 21.Lee AH. Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast. Histopathology. 2008;52:45–57. doi: 10.1111/j.1365-2559.2007.02893.x. [DOI] [PubMed] [Google Scholar]
- 22.Giri D. Recurrent challenges in the evaluation of fibroepithelial lesions. Arch Pathol Lab Med. 2009;133:713–21. doi: 10.5858/133.5.713. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer EJ, Hanby AM, Ellis P, et al. Molecular analysis of phyllodes tumors reveals distinct changes in the epithelial and stromal components. Am J Pathol. 2000;156:1093–8. doi: 10.1016/S0002-9440(10)64977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy RP, Zhang S, Bostwick DG, et al. Molecular genetic evidence for different clonal origins of epithelial and stromal components of phyllodes tumor of the prostate. Am J Pathol. 2004;165:1395–400. doi: 10.1016/S0002-9440(10)63397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffat CJ, Pinder SE, Dixon AR, et al. Phyllodes tumours of the breast: a clinicopathological review of thirty-two cases. Histopathology. 1995;27:205–18. doi: 10.1111/j.1365-2559.1995.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 26.Tan PH, Jayabaskar T, Chuah KL, et al. Phyllodes tumors of the breast: the role of pathologic parameters. Am J Clin Pathol. 2005;123:529–40. doi: 10.1309/U6DV-BFM8-1MLJ-C1FN. [DOI] [PubMed] [Google Scholar]
- 27.Ben Hassouna J, Damak T, Gamoudi A, et al. Phyllodes tumors of the breast: a case series of 106 patients. Am J Surg. 2006;192:141–7. doi: 10.1016/j.amjsurg.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi S, Motomura K, Inaji H, Imaoka S, Koyama H. Clonal analysis of fibroadenoma and phyllodes tumor of the breast. Cancer Res. 1993;53:4071–4. [PubMed] [Google Scholar]
- 29.Noguchi S, Yokouchi H, Aihara T, et al. Progression of fibroadenoma to phyllodes tumor demonstrated by clonal analysis. Cancer. 1995;76:1779–85. doi: 10.1002/1097-0142(19951115)76:10<1779::aid-cncr2820761015>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Kuijper A, Buerger H, Simon R, et al. Analysis of the progression of fibroepithelial tumours of the breast by PCR-based clonality assay. J Pathol. 2002;197:575–81. doi: 10.1002/path.1161. [DOI] [PubMed] [Google Scholar]
- 31.Hodges KB, Abdul-Karim FW, Wang M, et al. Evidence for transformation of fibroadenoma of the breast to malignant phyllodes tumor. Appl Immunohistochem Mol Morphol. 2009;17:345–50. doi: 10.1097/PAI.0b013e318194d992. [DOI] [PubMed] [Google Scholar]
- 32.Parry D, Bates S, Mann DJ, Peters G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 1995;14:503–11. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muirhead DM, Hoffman HT, Robinson RA. Correlation of clinicopathological features with immunohistochemical expression of cell cycle regulatory proteins p16 and retinoblastoma: distinct association with keratinisation and differentiation in oral cavity squamous cell carcinoma. J Clin Pathol. 2006;59:711–5. doi: 10.1136/jcp.2005.030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv S, Niu Y, Wei L, et al. Chromosomal aberrations and genetic relations in benign, borderline and malignant phyllodes tumors of the breast: a comparative genomic hybridization study. Breast Cancer Res Treat. 2008;112:411–8. doi: 10.1007/s10549-007-9876-1. [DOI] [PubMed] [Google Scholar]
- 35.Jee KJ, Gong G, Ahn SH, Park JM, Knuutila S. Gain in 1q is a common abnormality in phyllodes tumours of the breast. Anal Cell Pathol. 2003;25:89–93. doi: 10.1155/2003/803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer. 2008;8:714–24. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ertel A, Dean JL, Rui H, et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–63. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witkiewicz AK, Ertel A, McFalls J, et al. RB-pathway disruption is associated with improved response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2012;18:5110–22. doi: 10.1158/1078-0432.CCR-12-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]