Abstract
Evidence-based thresholds for risk stratification based on pulse pressure (PP) are currently unavailable. To derive outcome-driven thresholds for the 24–h ambulatory PP, we analyzed 9938 people randomly recruited from 11 populations (47.3% women). After age stratification (<60 vs. ≥60 years) and using average risk as reference, we computed multivariable-adjusted hazard ratios (HRs) to assess risk by tenths of the PP distribution or risk associated with stepwise increasing (+1 mm Hg) PP levels. All adjustments included mean arterial pressure. Among 6028 younger participants (68,853 person-years), the risk of cardiovascular (HR, 1.58; P=0.011) or cardiac (HR, 1.52; P=0.056) events increased only in the top PP tenth (mean, 60.6 mm Hg). Using stepwise increasing PP levels, the lower boundary of the 95% confidence interval of the successive thresholds did not cross unity. Among 3910 older participants (39,923 person-years), risk increased (P≤0.028) in the top PP tenth (mean, 76.1 mm Hg). HRs were 1.30 and 1.62 for total and cardiovascular mortality, and 1.52, 1.69 and 1.40 for all cardiovascular, cardiac and cerebrovascular events. The lower boundary of the 95% confidence interval of the HRs associated with stepwise increasing PP levels crossed unity at 64 mm Hg. While accounting for all covariables, the top tenth of PP contributed less than 0.3% (generalized R2 statistic) to the overall risk among elderly. Thus, in randomly recruited people, ambulatory PP does not add to risk stratification below age 60; in the elderly, PP is a weak risk factor with levels below 64 mm Hg probably being innocuous.
Keywords: ambulatory blood pressure, cardiovascular risk, population science, pulse pressure
Introduction
The blood pressure wave consists of a steady and pulsatile component, mean arterial pressure and pulse pressure, respectively.1 Mean arterial pressure, the product of cardiac output with peripheral arterial resistance is the force driving blood flow.1 Pulse pressure, the difference between systolic and diastolic blood pressure, depends on left ventricular ejection, the elasticity of the central arteries, and the timing and intensity of the backward wave originating at refection sites in the peripheral circulation. Pulse pressure widens in the elderly, because with advancing age systolic blood pressure continues to rise, whereas the age-related increase in diastolic blood pressure levels off or even reverses in the fifth decade of life.2
Under the premise that systolic blood pressure and pulse pressure reflect arterial stiffness, the Framingham investigators demonstrated that with increasing age, a gradual shift occurs from diastolic to systolic pressure and then to pulse pressure as predictors of coronary heart disease.3 Several other studies showed that pulse pressure, derived from the conventionally measured blood pressure, predicts adverse outcomes in patients with cardiovascular4 or renal disease5,6 as well as in populations.7–11 Compared to the conventionally measured blood pressure, ambulatory monitoring substantially refines risk stratification, but the five studies that examined the predictive value of ambulatory pulse pressure only included hypertensive patient12–16 or patients with end-stage renal disease.5 Although described as a priority in 2006,17 to our knowledge, current guidelines for the management of hypertension18–20 do not propose outcome-driven thresholds for pulse pressure discriminating normal from abnormal values. We addressed these issues in a subject-level meta-analysis of 9938 people recruited from 11 populations and enrolled in the International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO).
Methods
Study Population
Previous publications described the construction of the IDACO database.21 All studies received ethical approval and qualified for inclusion if they involved a random population sample, contained baseline information on the ambulatory blood pressure and cardiovascular risk factors, and follow-up of fatal and nonfatal outcomes. All participants gave informed written consent. The IDACO database21 included 12 randomly recruited population cohorts and 12,725 participants, but at time of writing of this report, validated information on outcome was available in only 11 studies (details and references provided in the online Data Supplement), leaving 12,148 participants. Of those, we excluded 2210, because they were younger than 18 years (n=74); or because they had fewer than 10 daytime or 5 nighttime blood pressure readings (n=2136). Thus, the number of subjects included in the present analysis totaled 9938.
Blood Pressure Measurement
Methods used for conventional and ambulatory blood pressure measurement are described in detail in Data Supplement. Conventional blood pressure was the average of 2 consecutive readings. Hypertension was as a conventional blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic, or use of antihypertensive drugs. Portable monitors were programmed to obtain ambulatory blood pressure readings at 30-minute intervals throughout the whole day, or at intervals ranging from 15 to 30 minutes during daytime and from 15 to 60 minutes at night. Daytime ranged from 10 am to 8 pm in Europeans and South Americans and from 8 am to 6 pm in Asians. The corresponding nighttime intervals ranged midnight to 6 am and from 10 pm to 4 am. Pulse pressure was the difference between systolic and diastolic blood pressure and mean arterial pressure was diastolic blood pressure plus one third of pulse pressure.
Other Measurements
We used questionnaires to obtain information on each participant’s medical history and smoking and drinking habits. We measured serum cholesterol and blood glucose by automated enzymatic methods. Diabetes was the use of antidiabetic drugs, a fasting blood glucose concentration of ≥7.0 mmol/L,22 a random blood glucose concentration of ≥11.1 mmol/L,22 a self-reported diagnosis, or diabetes documented in practice or hospital records.
Ascertainment of Events
We ascertained vital status and the incidence of fatal and nonfatal diseases from the appropriate sources in each country, as described in previous publications21 and in Data Supplement. Fatal and nonfatal stroke did not include transient ischemic attacks. Coronary events encompassed death from ischemic heart disease, sudden death, nonfatal myocardial infarction, and coronary revascularization. Cardiac events comprised coronary endpoints and fatal and nonfatal heart failure. The composite cardiovascular endpoint included all aforementioned endpoints plus cardiovascular mortality. In all outcome analyses, we only considered the first event within each category.
As in previous IDACO analyses, we considered the composite cardiovascular endpoint as the main outcome, because it provides the largest number of events. We informed sample size calculations with the event rate of the composite cardiovascular endpoint in the IDACO cohort (10.7 per 1000 person-years). We used the one-sample test as implemented in the PROC POWER procedure of the SAS package. To demonstrate a 10% change in the relative risk associated with each–10 mm Hg increase in 24–h pulse pressure, approximately 7000 subjects would be needed with the 2-sided α-level set at 0.05 and power at 0.90.
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.3 (SAS Institute, Carey, NC). We compared means and proportions using the large-sample z-test, and χ2 statistic, respectively. After stratification for cohort and sex, we interpolated missing values of body mass index (n=46) and total serum cholesterol (n=683) from the regression slope on age. In subjects with unknown drinking (n=813) or smoking habits (n=69), we set the design variable to the cohort- and sex-specific mean of the codes (0,1). Statistical significance was a 2-sided P value of ≤0.05.
To relate outcome to pulse pressure, while adjusting for covariables, we applied Cox regression. The baseline characteristics used for adjustment included: cohort, sex, age (continuous), mean arterial pressure, heart rate, body mass index (continuous), current smoking and drinking (0,1), serum cholesterol (continuous), history of cardiovascular disease (0,1) and diabetes mellitus (0,1), and antihypertensive drug treatment (0,1). For adjustment, mean arterial pressure and heart rate were derived from the same recordings as pulse pressure (24–h, daytime, nighttime or conventional measurements).
Because of the Framingham results23 and the lower age boundary in several randomized clinical trials on antihypertensive treatment in the elderly,24 we stratified our analyses by 60 years of age. Exploratory analyses demonstrated that the association of endpoints with 24–h pulse pressure was not always log linear. To account for this nonlinear association, we applied the deviation from mean coding25 to compute hazard ratios (HRs) in tenths of the 24–h pulse pressure distribution. This approach expresses the risk in each tenth relative to the overall risk in the whole study population and allows computing 95% confidence intervals (CIs) for the hazard ratios in all tenths without definition of an arbitrary reference group. Hazard ratios relating endpoints to mean arterial pressure expressed the risk associated with a 1–SD increase in the level. We tested heterogeneity in the hazard ratios across subgroups by introducing the appropriate interaction term in the Cox model. We applied the generalized R2 statistic to assess the risks additionally explained by 24–h pulse pressure over and beyond mean arterial pressure and other covariables.26 To assess whether collinearity between pulse pressure and mean arterial pressure affected our estimates, we applied penalized Cox regression as applied in the ridging=relative model option of the PROC PHREG procedure of the SAS package.
In an attempt to refine the level of pulse pressure that was associated with significantly increased risk, we did a stepwise analysis. We calculated hazard ratios for 1–mm Hg increments in pulse pressure for thresholds ranging from the 10th to the 90th percentile. These hazard ratios expressed the risk in participants whose pulse pressure exceeded the cutoff point vs. average risk. We plotted these hazard ratios and their 95% confidence limits vs. the increasing cutoff points with the goal to determine at which level the lower confidence limit of the hazard ratios crossed unity.
Results
Characteristics of Participants
The whole study population comprised 6623 Europeans (66.6%), 1877 Asians (18.9%) and 1438 South Americans (14.5%). Of the 9938 participants, 4703 were women (47.3%), 4058 (40.8%) had hypertension on conventional blood pressure measurement, and 1946 (19.6%) were taking blood pressure–lowering drugs. Mean age was 52.7±15.8 years. In the whole study population, average 24–h blood pressure levels were 123.5±14.0 mm Hg systolic, 73.6±8.3 mm Hg diastolic, 49.9±9.6 mm Hg for pulse pressure, and 90.2±9.5 mm Hg for mean arterial pressure. At enrolment, 2789 participants (28.1%) were current smokers, and 4759 (47.9%) reported intake of alcohol.
Table 1 shows the characteristics of the participants by age group. The median number of readings averaged to estimate the 24–h pulse pressure was 50 (5th to 95th percentile interval, 35–81; range, 21–95) in younger participants and 56 (5th to 95th percentile interval, 35–82; range, 20–99) in the elderly. Table S1 additionally lists the conventional blood pressure and the daytime and nighttime blood pressures by age group. All of the differences between the age groups were significant (P≤0.0012) with the exception of the proportion of Asians (P=0.057).
Table 1.
Baseline Characteristics of Participants by Age Group
| Characteristic | <60 years (N=6028) |
≥60 years (N=3910) |
|---|---|---|
| Ethnicity | ||
| Asian | 953 (15.8) | 924 (23.6) |
| European | 4061 (67.4) | 2562 (65.5) |
| South American | 1014 (16.8) | 424 (10.8) |
| Women | 3239 (53.7) | 1464 (37.4) |
| Cardiovascular risk factors | ||
| Smoking | 1857 (30.9) | 932 (24.1) |
| Drinking alcohol | 2795 (47.7) | 1964 (60.1) |
| Diabetes mellitus | 247 (4.1) | 411 (10.5) |
| Cardiovascular disease | 269 (4.5) | 521 (13.3) |
| Hypertension | 1529 (25.4) | 2529 (64.7) |
| Antihypertensive drug treatment | 619 (10.3) | 1327 (34.1) |
| Age, years | 42.5±11.1 | 68.6±5.6 |
| Body mass index, kg/m2 | 25.1±4.3 | 25.7±4.0 |
| Serum total cholesterol, mmol/L | 5.45±1.14 | 5.83±1.15 |
| Blood glucose, mmol/L | 5.01±1.14 | 5.52±1.60 |
| 24-h blood pressure measurements | ||
| Systolic blood pressure, mm Hg | 119.4±12.0 | 129.8±14.5 |
| Diastolic blood pressure, mm Hg | 73.0±8.3 | 74.6±8.3 |
| Pulse pressure, mm Hg | 46.4±7.2 | 55.2±10.5 |
| Mean arterial pressure, mm Hg | 88.5±9.1 | 93.0±9.6 |
| Heart rate, beats per minute | 73.6±8.9 | 69.9±9.1 |
Data are No. (%) or mean±SD. Hypertension is a conventional blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic or use of antihypertensive drugs. To convert glucose and cholesterol from mmol/l to mg/dl, multiply by 18.01 and 38.61, respectively. All of the differences between age groups were significant (P<0.0001) with the exception of the proportion of Asians (P=0.057).
Analyses of Younger Participants
Incidence of Endpoints
Among 6028 younger participants (<60 years), median follow-up was 12.1 years (5th to 95th percentile interval, 2.5 to 18.2 years). Over 68,853 person-years, 228 participants died (3.3 per 1000 person-years) and 221 experienced a fatal or nonfatal cardiovascular complication (3.2 per 1000 person-years). The Data Supplement provides information on the overall and cause-specific number of fatal and nonfatal events.
Categorical Analysis of 24-H Pulse Pressure
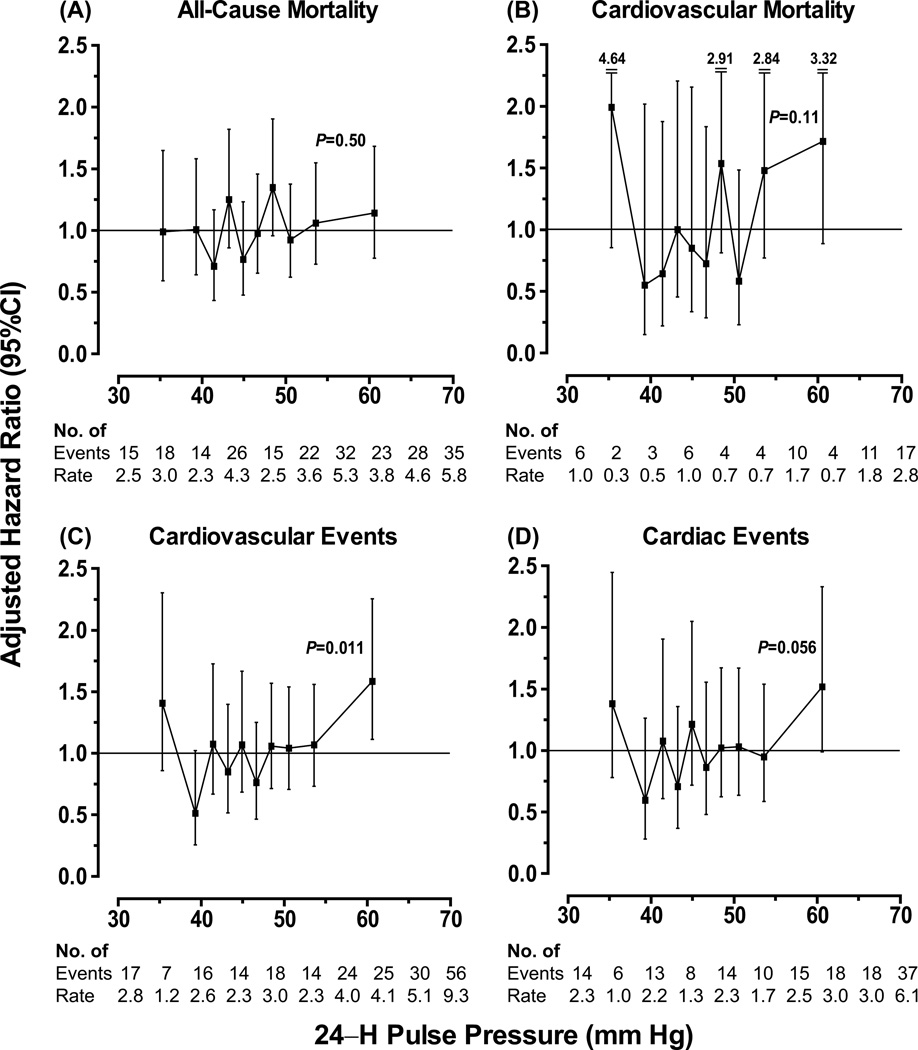
Figure 1 shows the hazard ratios expressing the risk in each tenth of the distribution of the 24–h ambulatory pulse pressure vs. average risk. Only in the highest tenth of the pulse pressure distribution (threshold, ≥55.6 mm Hg; mean 60.1 mm Hg), the risk of the composite cardiovascular endpoint was elevated (HR, 1.58; 95% CI, 1.11 to 2.25; P=0.011) with a similar trend for cardiac endpoints (HR, 1.52; CI, 0.99 to 2.33; P=0.056). Otherwise, the risks across tenths of the pulse pressure distribution (Figure 1) did not deviate from average (P≥0.058). For stroke, Cox models across tenths of the pulse pressure distribution did not converge, because of the low number of events (n=63). The HRs expressing the risk associated with a 1–SD increase in mean arterial pressure were 1.11 (CI, 0.95 to 1.29; P=0.19) for total mortality, 1.40 (CI, 1.09 to 1.80; P=0.009) for cardiovascular mortality, 1.37 (CI, 1.19 to 1.59; P<0.0001) for a composite cardiovascular endpoint, and 1.40 (CI, 1.18 to 1.66; P=0.0001) for a cardiac event.
Figure 1. Hazard ratios in tenths of the distribution of 24–h pulse pressure in 6028 younger participants.
Hazard ratios for total (A) and cardiovascular (B) mortality and for cardiovascular (C) and cardiac (D) events express the risk in each tenth compared with average risk. Hazard ratios were adjusted for cohort, sex, age, 24–h mean arterial pressure, 24–h heart rate, body mass index, smoking and drinking, serum cholesterol, history of cardiovascular disease and diabetes, antihypertensive drug treatment. Vertical bars denote 95% confidence intervals. For each tenth, the number of events and unadjusted incidence rates (in percent) are given. The P value refers to the significance of the hazard ratio in the top tenth of the 24–h pulse pressure distribution.
Stepwise Analysis of Pulse Pressure
Figure S1 shows the HRs of 24–h pulse pressure levels that stepwise increased by 1 mm Hg from the 10th to the 90th percentile. For all endpoints under study, the lower boundary of the confidence interval of the successive HRs did not cross unity.
Analyses of Older Participants
Incidence of Endpoints
Among 3910 older participants (≥60 years), median follow-up was 10.7 years (5th to 95th percentile interval, 2.5 to 16.1 years). Over 39,923 person-years, 1160 participants died (29.0 per 1000 person-years) and 940 experienced a fatal or nonfatal cardiovascular complication (23.5 per 1000 personyears). The Data Supplement lists the number of fatal and nonfatal events.
Categorical Analysis of 24-H Pulse Pressure
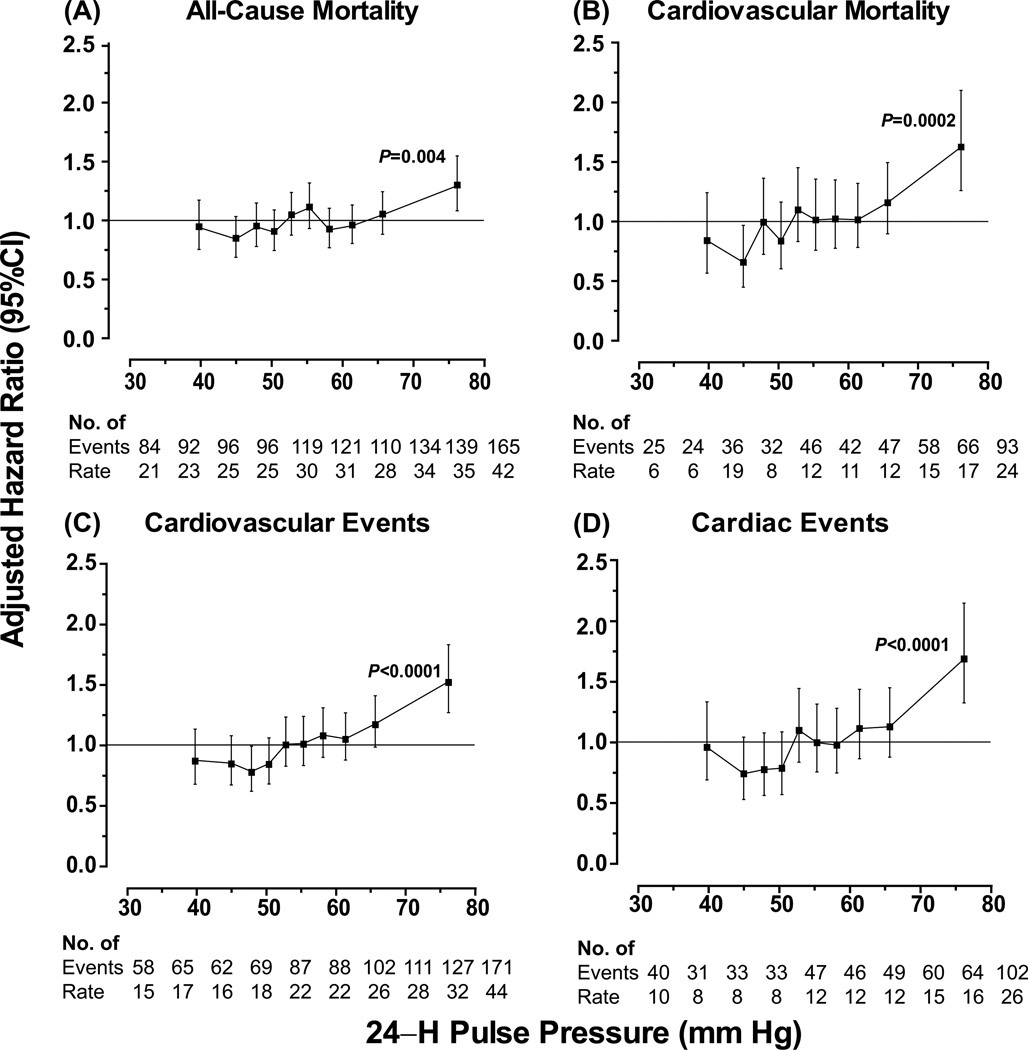
Figure 2 shows the hazard ratios expressing the risk in each tenth of the distribution of the 24–h ambulatory pulse pressure vs. average risk. The risk of any death, cardiovascular mortality, a composite cardiovascular endpoint or a cardiac event was consistently elevated in the top tenth of the pulse pressure distribution (threshold, ≥68.8 mm Hg; mean 76.1 mm Hg). The HRs were 1.30 (CI, 1.09 to 1.55; P=0.004), 1.62 (CI, 1.26 to 2.10; P=0.0002), 1.52 (CI, 1.26 to 1.83; P<0.0001), and 1.69 (CI, 1.33 to 2.15; P<0.0001), respectively. The HR for stroke in the top tenth of the pulse pressure distribution was 1.40 (CI, 1.04 to 1.89; P=0.028; Figure S2). For cardiovascular mortality and the composite cardiovascular endpoint, the HRs were 0.66 (CI, 0.45 to 0.97; P=0.033) and 0.78 (CI, 0.61 to 0.99; P=0.040) in the second and third tenth of the pulse pressure distribution, respectively (Figure 2). Otherwise, the risks across tenths of the pulse pressure distribution (Figure 2 and Figure S2) did not deviate from average (P>0.05). The HRs expressing the risk associated with a 1–SD increase in mean arterial pressure were 1.04 (CI, 0.96 to 1.12; P=0.31) for total mortality, 1.15 (CI, 1.02 to 1.29; P=0.02) for cardiovascular mortality, 1.19 (CI, 1.10 to 1.29; P<0.0001) for a composite cardiovascular endpoint, 1.07 (CI, 0.96 to 1.19; P=0.22) for a cardiac event, and 1.39 (CI, 1.23 to 1.58; P<0.0001) for stroke. The R2 statistic for adding a design variable coding for the top tenth of the 24–h pulse pressure distribution to Cox models including all other covariables were 0.10% and 0.12% for total and cardiovascular mortality, 0.27%, 0.21%, 0.09% for the composite cardiovascular endpoint, all cardiac events and stroke, respectively.
Figure 2. Hazard ratios in tenths of the distribution of 24–h pulse pressure in 3910 older participants.
Hazard ratios for total (A) and cardiovascular (B) mortality and for cardiovascular (C) and cardiac (D) events express the risk in each tenth compared with average risk. The hazard ratios were adjusted as in Figure 1. Vertical bars denote 95% confidence intervals. For each tenth, the number of events and unadjusted incidence rates (in percent) are given. The P value refers to the significance of the hazard ratio in the top tenth of the 24–h pulse pressure distribution.
Stepwise Analysis of Pulse Pressure
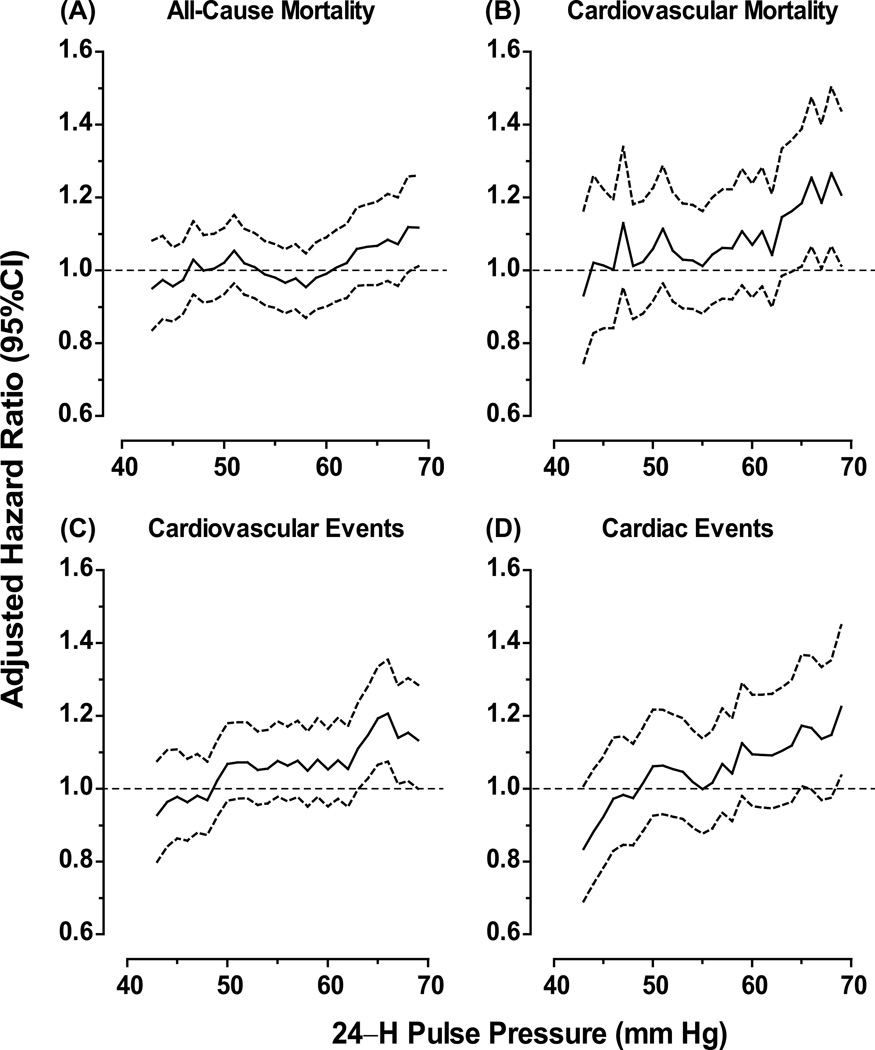
Figure 3 shows the hazard ratios for 24–h pulse pressure levels increasing by 1–mm Hg steps from the 10th up to the 90th percentile in older participants. For most endpoints under study (Figure 3) with the exception of stroke (Figure S2), the lower boundary of the confidence interval of the successive HRs crossed the reference line at levels ranging from 64 mm Hg (composite cardiovascular endpoint) to 69 mm Hg (total mortality and cardiac events).
Figure 3. Hazard ratios according to 24–h pulse pressure levels ranging from the 10th to the 90th percentile in 3910 older participants.
Hazard ratios for total mortality (A) and cardiovascular (B) mortality and for cardiovascular (C) and cardiac (D) events express the risk at each level of pulse pressure compared with average risk. Solid and dotted lined denote the point estimates and the 95% confidence intervals, respectively. The hazard ratios were adjusted as in Figure 1.
Sensitivity Analyses
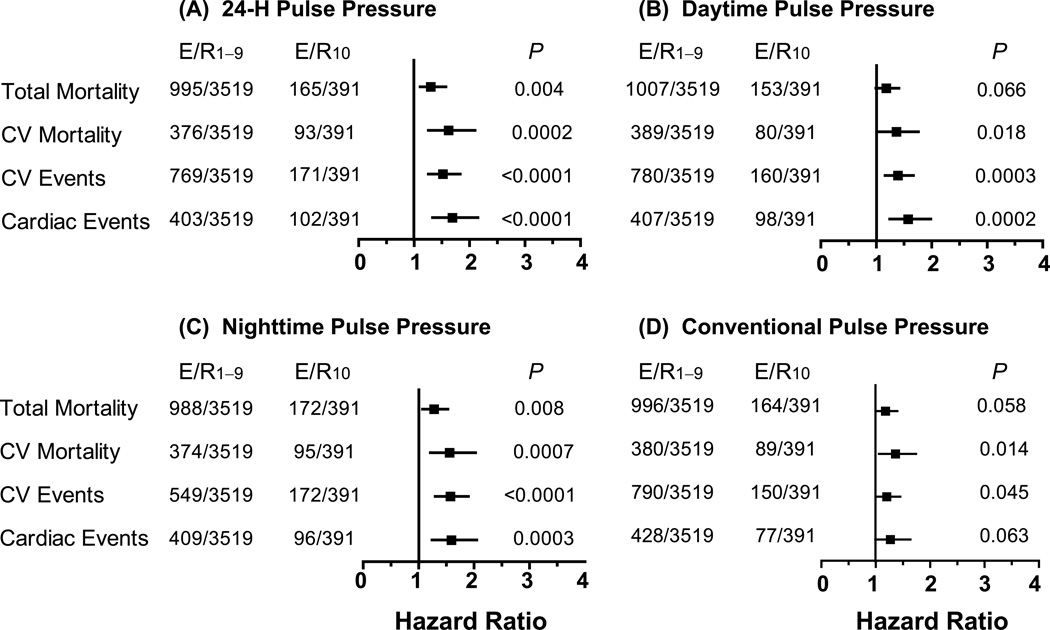
Excluding one cohort at a time produced confirmatory results as shown for cardiovascular mortality and the composite cardiovascular endpoint in the Data Supplement (Table S2). Similarly, when we stratified our analyses in older participants for ethnicity, sex, presence vs. absence of conventional hypertension, or use vs. nonuse of antihypertensive drugs, the results remained consistent (0.16≤P≤0.75 for interaction between subgroups, Table S3). After excluding 863 older participants with a history of cardiovascular disease or diabetes mellitus, the significance of these interaction terms did not materially change (0.37≤P≤0.97). Modeling cohort as a random effect in the Cox model instead of adjusting the model for cohort (Figures S3 and S4), applying ridge regression (Table S4) or adjusting for systolic or diastolic blood pressure instead of mean arterial pressure (Table S5) produced consistent results. Finally, when we substituted 24–h pulse pressure by daytime, nighttime or the conventionally measured pulse pressure, the results did not change (Figure 4).
Figure 4. Multivariable-adjusted hazard ratios for outcomes in relation to 24–h (A), daytime (B), nighttime (C), and conventional (D) pulse pressure in 3910 older participants.
The hazard ratios, presented with 95% confidence interval (CI), express the risk in the top tenth compared with the average risk in the participants. Pulse pressure thresholds delineating the top tenth were ≥68.8, ≥71.3, ≥66.8, and ≥80.0 mm Hg for 24–h, daytime, nighttime and conventional blood pressure measurement; the corresponding mean levels of pulse pressure in the top tenth were 76.1, 78.8, 75.6 and 89.0 mm Hg, respectively. All models were adjusted for cohort, sex, age, mean arterial pressure and heart rate (on 24–h, daytime, nighttime, conventional measurement in panels A, B, C, and D, respectively), body mass index, smoking and drinking, serum cholesterol, history of cardiovascular disease and diabetes, and antihypertensive drug treatment. P values are for the risk in the top tenth relative to the overall risk in the whole study population. CV denotes cardiovascular. E/R1–9 and E/R10 indicate the number of events and participants at risk below the 90th percentile of the pulse pressure distribution and in the top tenth, respectively.
Analyses of Younger and Older Participants Combined
We tested the interaction between 24–h pulse pressure and age modeled as a categorical or continuous variable in 9938 participants. In the categorical analyses (Table S6), using as age cutoff limits 50, 55 or 60 years, none of the interaction terms reached significance (0.14≤P≤0.72) except for cardiovascular events with 50 years as age cut-off (P=0.005). In the continuous analyses, The hazard ratios associated with 10–mm Hg higher 24–h pulse pressure in younger and older participants (<60 vs. ≥60 years) were 1.12 (CI, 0.91–1.38) vs. 1.08 (1.00–1.15) for total mortality (P-value for difference, 0.085), 1.24 (0.86–1.81) vs. 1.13 (1.01–1.25) for cardiovascular mortality (P= 0.020), 1.28 (1.05–1.57) vs. 1.13 (1.05–1.21) for the composite cardiovascular endpoint (P= 0.009), and 1.21 (CI, 0.96–1.53) vs. 1.17 (1.06–1.28) for cardiac events (P= 0.22).
Discussion
After more than 2 decades of research7 pulse pressure remains an elusive cardiovascular risk factor with findings being inconsistent across studies. Indeed, previous cohort studies found that peripheral pulse pressure, as measured by conventional sphygmomanometry, was an independent risk factor in populations,3,7–11 or in patients with hypertension,4,27–29 coronary heart disease4 or severe renal dysfunction.5,6 Other population studies failed to confirm the risk associated with pulse pressure30,31 or reported that it was present only in women7 or in diabetic10 or treated hypertensive patients.29 In addition to the conventional method of blood pressure measurement, the aforementioned studies had limitations, because they recorded only fatal endpoints,5–8,10,11,30,31 or applied recruitment criteria confined to high-risk patients,4,6,27–29,31 a narrow age range7,8 or elderly.11,28 To address these drawbacks, we applied ambulatory blood pressure monitoring, the current state-of-the-art for blood pressure measurement,32 and we recorded both fatal and nonfatal endpoints in randomly recruited populations with age ranging from 18 to 93 years. The key finding of our study was that 24–h pulse pressure did not substantially add to risk stratification below age 60; in the elderly, 24–h pulse pressure was a weak risk factor with levels below 64 mm Hg probably being innocuous. For all endpoints under study, 24–h pulse pressure remained a significant predictor of outcome with either 24–h mean arterial pressure or 24–diastolic blood pressure as covariable in the Cox models, but it lost significance for all-cause mortality and stroke with 24–h systolic blood pressure in the model. These findings suggest that systolic blood pressure might be the major blood pressure component driving the risk associated with pulse pressure. Using continuous analyses in our current study, the hazard ratios expressing the risk associated with 10–mm Hg wider 24–h pulse pressure, where higher in younger than older participants for the composite cardiovascular endpoint (1.28 vs. 1.13). However, these hazard ratios only reflect relative risk. The composite cardiovascular endpoint was running at rates of 3.2 and 23.5 events per 1000 person-year in younger and older participants, respectively. In terms of absolute risk, 24–h pulse pressure therefore was a more important predictor in older than younger participants.
Already in 1971, the Framingham investigators33 demonstrated that the role of diastolic and systolic blood pressure as risk indicators depend on age. In 2001, they reported that with increasing age, there was a gradual shift from diastolic blood pressure to systolic blood pressure and then to pulse pressure as predictors of coronary heart disease.3 In 1989, the Multiple Risk Factor Trial researchers demonstrated that both systolic and diastolic blood pressure determine cardiovascular risk.34 Also in 1989, Darne and coworkers, by applying principal component analysis, established a steady and pulsatile component of blood pressure, which were unrelated to one another, but strongly correlated with mean arterial pressure and pulse pressure, respectively.7 Guided by these seminal publications,3,7,33,34 we stratified our main analyses by age (<60 vs. ≥60 years) and we modeled pulse pressure as risk factor, while accounting for mean arterial pressure.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure18 proposed that pulse pressure is only marginally stronger than systolic blood pressure for risk stratification in individuals over age 60, and that under age 60, pulse pressure is not predictive. According to the 2007 European guideline19 pulse pressure is a derived measure, which combines the imprecision of the original systolic and diastolic measurements. The 2007 guideline stated that, although levels of 50 to 55 mm Hg have been suggested,17 no practical cutoff values separating pulse pressure normality from abnormality is available. The 2013 European guideline increased this threshold to 60 mm Hg without any justification.20 Our current analyses established that below age 60, a 24–h pulse pressure level around 60 mm Hg might be associated with increased risk, but that a safe threshold could not be established. Among the elderly, a 24–h pulse pressure of around 76 mm Hg was definitely associated with higher risk and levels below 64 were probably safe. Using intra-arterial monitoring, Khattar and colleagues observed that survival rates were highest below age 60, if the 24–h pulse pressure was less than 70 and highest among elderly with a 24–h pulse pressure of 70 mm Hg or more.14 To our knowledge, Khattar’s report14 is the only other study proposing an outcome-driven threshold for 24–h pulse pressure. However, this article does not include any justification why 70–mm Hg was chosen as threshold in a dichotomized analysis. The results rested on an unadjusted Kaplan-Meier survival function analysis, and the study population consisted of patients with essential hypertension, in whom treatment had been withdrawn for 8 weeks.14 To our knowledge, all other proposals for pulse pressure thresholds relied on conventional blood pressure measurement. In analyses adjusted but not stratified for age, 2 studies9,27 derived a threshold from the 66th percentile of the pulse pressure distribution. Madhavan proposed 63 mm Hg based on the incidence of myocardial infarction in 2207 hypertensive patients aged 55 years,27 and Borghi suggested 67 mm Hg based on the incidence of cardiovascular disease among 2939 Italian people (14 to 84 years). Asmar derived a threshold of 65 mm Hg from the mean pulse pressure plus 2 SDs in 61,724 French people (16 to 90 years).35
Our current results must be interpreted within the context of some limitations. First, because of the low event rates below middle age, our analyses did not answer the issue whether pulse pressure has a different prognostic impact in young compared with older people. However, as reviewed elsewhere,36 our current study along with the Framingham report3 is one of the few with long-term follow-up of subjects younger than 40 years. Second, we did not account for regression dilution bias.30 However, we previously demonstrated that such correction is not necessary for blood pressure indexes obtained by 24–h ambulatory monitoring.37 Third, we had no information on central pulse pressure. As demonstrated in Europeans38 and Chinese,39 with advancing age, systolic augmentation increases and pressure amplification, the difference between peripheral and central systolic blood pressure, decreases. By measuring pulse pressure at a peripheral site, we might have underestimated its prognostic significance, in particular in younger people. Finally, oscillometric and auscultatory estimates of pulse pressure might differ. However, our findings were consistent regardless of the interval over which the ambulatory blood pressure was measured and on ambulatory (oscillometric) and conventional (predominantly auscultatory) measurement.
Perspectives
Based on our observations in randomly recruited people, pulse pressure adds little information on cardiovascular outcomes below age 60. In the elderly, ambulatory pulse pressure is a risk factor with levels below 64 mm Hg probably being harmless. However, using this threshold in clinical practice might be of little value, because ambulatory pulse pressure does not substantially enhance risk stratification over and beyond the steady component of the blood pressure level and other cardiovascular risk factors. We suggest that the threshold proposed for pulse pressure in the European 2013 guideline,20 irrespective of measurement technique, might be revised from the perspective of our current findings.
Supplementary Material
Novelty and Significance.
What is new?
This is the first population-based study to derive outcome-driven threshold for 24–h pulse pressure.
What is relevant?
-
▫
24–H pulse pressure does not substantially add to risk stratification below age 60.
-
▫
Starting from 60 years onwards, 24–h pulse pressure levels of 70 mm Hg or higher carry an increased cardiovascular risk, whereas levels below 64 mm Hg are probably innocuous. However, while accounting for all covariables, having a 24–h pulse pressure in the top tenth of the distribution contributed less than 0.3% to the overall risk among elderly.
-
▫
Findings for daytime, nighttime and conventional pulse pressure were similar to those for 24–ambulatory pulse pressure.
Summary
The present report is the first to provide outcome-driven thresholds for pulse pressure on 24–h ambulatory measurement. These findings could inform guidelines and be of help to clinicians in diagnosing and managing patients.
Acknowledgments
This study would not have been possible without the collaboration of the participants and the expert assistance of Mrs. Sandra Covens (Studies Coordinating Centre, Leuven, Belgium). The IDACO investigators are listed in reference.21
Source of funding
The European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006-037093 In-Genious HyperCare, HEALTH-F4-2007-201550 HyperGenes, HEALTH-F7-2011-278249 EU-MASCARA, HEALTH-F7-305507 HOMAGE and the European Research Council Advanced Research Grant 294713 EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0734.09, G.0881.13 and G.0880.13N) supported the Studies Coordinating Centre (Leuven, Belgium). The European Union (grants LSHM-CT-2006-037093 and HEALTH-F4-2007-201550) also supported the research groups in Shanghai, Kraków, Padova and Novosibirsk. The Danish Heart Foundation (grant 01-2-9-9A-22914), and the Lundbeck Fonden (grant R32-A2740) supported the studies in Copenhagen. The Ohasama study received support via Grant-in-Aid for Scientific Research (23249036, 23390171, 24591060, 24390084, 24591060, 24790654, 25461205, 25461083 and 25860156) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan; and a Health Labour Sciences Research Grant (H23-Junkankitou [Seishuu]-Ippan-005) from the Ministry of Health, Labour, and Welfare, Japan; the Japan Arteriosclerosis Prevention Fund; and the Grant from the Daiwa Securities Health Foundation. The National Natural Science Foundation of China (grants 30871360, 30871081, 81170245, and 81270373), Beijing, China, and the Shanghai Commissions of Science and Technology (grant 07JC14047 and the “Rising Star” program 06QA14043 and 11QH1402000) and Education (grant 07ZZ32 and the “Dawn” project 08SG20) supported the JingNing study in China. The Comisión Sectorial de Investigación Científica de la Universidad de la República (Grant I+D GEFA-HT-UY) and the Agencia Nacional de Innovación e Investigación supported research in Uruguay. The 1R01AG036469-01A1 from the National Institute on Aging and Fogarty International Center is supporting the Maracaibo Aging Study.
Footnotes
Disclosures
None.
References
- 1.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 2.Staessen J, Amery A, Fagard R. Editorial review. Isolated systolic hypertension. J Hypertens. 1990;8:393–405. doi: 10.1097/00004872-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 4.Bangalore S, Messerli FH, Franklin SS, Mancia G, Champion A, Pepine CJ. Pulse pressure and risk of cardiovascular outcomes in patients with hypertension and coronary artery disease: an INternational VErapamil SR-trandolapril STudy (INVEST) analysis. Eur Heart J. 2009;30:1395–1401. doi: 10.1093/eurheartj/ehp109. [DOI] [PubMed] [Google Scholar]
- 5.Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, Salvador M, Chamontin B. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Intern. 2000;57:2485–2491. doi: 10.1046/j.1523-1755.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu JH, Chen CC, Wang SM, Chou CY, Liu YL, Kuo HL, Lin HH, Wang IK, Yang YF, Huang CC. Association between pulse pressure and 30-month all-cause mortality in peritoneal dialysis patients. Am J Hypertens. 2008;21:1318–1323. doi: 10.1038/ajh.2008.286. [DOI] [PubMed] [Google Scholar]
- 7.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400. doi: 10.1161/01.hyp.13.4.392. [DOI] [PubMed] [Google Scholar]
- 8.Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieère P, Guize L. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- 9.Borghi C, Dormi A, Ambrosioni E, Gaddi A on behalf of the Brisighella Heart Study working party. Relative role of systolic, diastolic and pulse pressure as risk factors for cardiovascular events in the Brisighella Heart Study. J Hypertens. 2002;20:1737–1742. doi: 10.1097/00004872-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Schram MT, Kostense PJ, Van Dijk RAJM, Dekker JM, Nijpels G, Bouter LM, Heine RJ, Stehouwer CD. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens. 2002;20:1743–1751. doi: 10.1097/00004872-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch Intern Med. 2000;160:2765–2772. doi: 10.1001/archinte.160.18.2765. [DOI] [PubMed] [Google Scholar]
- 12.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C. Ambulatory pulse pressure. A potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32:983–988. doi: 10.1161/01.hyp.32.6.983. [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia P, Schillaci G, Reboldi G, Franklin SS, Porcellati C. Different prognostic impact of 24-hour mean blood pressure and pulse pressure on stroke and coronary artery disease in essential hypertension. Circulation. 2001;103:2579–2584. doi: 10.1161/01.cir.103.21.2579. [DOI] [PubMed] [Google Scholar]
- 14.Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001;104:783–789. doi: 10.1161/hc3201.094227. [DOI] [PubMed] [Google Scholar]
- 15.Staessen JA, Thijs L, O'Brien ET, Bulpitt CJ, de Leeuw PW, Fagard RH, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J, Safar ME for the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Ambulatory pulse pressure as predictor of outcome in older patients with systolic hypertension. Am J Hypertens. 2002;15(part 1):835–843. doi: 10.1016/s0895-7061(02)02987-4. [DOI] [PubMed] [Google Scholar]
- 16.Kao YT, Huang CC, Leu HB, Wu TC, Huang PH, Lin SJ, Chen JW. Ambulatory pulse pressure as a novel predictor for long-term prognosis in essential hypertensive patients. J Hum Hypertens. 2011;25:444–450. doi: 10.1038/jhh.2010.80. [DOI] [PubMed] [Google Scholar]
- 17.Laurent S, Cockcroft J, Van BL, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness : methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute, and the National High Blood Pressure Education Program Coordinating Commitee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker-Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Manolis A, Nilsson PM, Redon J, Struijker-Boudier HA, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, O'Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. 2007 guidelines for the management of arterial hypertension : The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 21.Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Li Y, Dolan E, Tikhonoff V, Sleidlerová J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang JG, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA on behalf of the IDACO Investigators. The International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255–262. doi: 10.1097/mbp.0b013e3280f813bc. [DOI] [PubMed] [Google Scholar]
- 22.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 23.Franklin SS, Gustin W, 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 24.Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J for the Systolic Hypertension in Europe Trial Investigators. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Jr, Leleshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. pp. 47–56. [Google Scholar]
- 26.Gillespie BW. Use of generalized R-squared in Cox regression. [accessed August 26, 2013];APHA Scientific Session and Event Listing. 2006 ( https://apha.confex.com/apha/134am/techprogram/paper_135906.htm). [Google Scholar]
- 27.Madhaven S, Ooi WL, Cohen H, Alderman MH. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension. 1994;23:395–401. doi: 10.1161/01.hyp.23.3.395. [DOI] [PubMed] [Google Scholar]
- 28.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg J. Antihypertensive treatment alters the predictive strength of pulse pressure and other blood pressure measures. Am J Hypertens. 2005;18:1033–1039. doi: 10.1016/j.amjhyper.2005.03.735. [DOI] [PubMed] [Google Scholar]
- 30.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 31.Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, Grimm R, Cohen J, Stamler J for the MRFIT Research Group. Pulse pressure and cardiovascular disease-related mortality. JAMA. 2002;287:2677–2683. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redón J, Staessen J, Stergiou G, Verdecchia P on behalf of the European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. Am J Cardiol. 1971;27:335–346. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- 34.Stamler J, Neaton JD, Wentworth DN. Blood pressure (systolic and diastolic) and risk of fatal coronary heart disease. Hypertension. 1989;13(suppl I):I-2–I-12. doi: 10.1161/01.hyp.13.5_suppl.i2. [DOI] [PubMed] [Google Scholar]
- 35.Asmar R, Vol S, Brisac AM, Tichet J, Topouchian J. Reference values for clinic pulse pressure in a nonselected population. Am J Hypertens. 2001;14:415–418. doi: 10.1016/s0895-7061(01)01284-5. [DOI] [PubMed] [Google Scholar]
- 36.Franklin SS. Pulse pressure as a risk factor. Clin Exp Hypertens. 2004;26:645–652. doi: 10.1081/ceh-200031962. [DOI] [PubMed] [Google Scholar]
- 37.Gasowski J, Li Y, Kuznetsova T, Richart T, Thijs L, Grodzicki T, Clarke R, Staessen JA. Is "usual" blood pressure a proxy for 24-hour ambulatory blood pressure in predicting cardiovascular outcomes? Am J Hypertens. 2008;21:994–1000. doi: 10.1038/ajh.2008.231. [DOI] [PubMed] [Google Scholar]
- 38.Wojciechowska W, Stolarz-Skrzypek K, Tikhonoff V, Richart T, Seidlerová J, Cwynar M, Thijs L, Li Y, Kuznetsova T, Filipovský J, Casiglia E, Grodzicki T, Kawecka-Jaszcz K, O'Rourke M, Staessen JA on behalf of the European Project on Genes in Hypertension (EPOGH) Investigators. Age dependency of central and peripheral systolic blood pressures: cross-sectional and longitudinal observations in European populations. Blood Press. 2012;21:58–68. doi: 10.3109/08037051.2011.593332. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Staessen JA, Sheng CS, Huang QF, O'Rourke M, Wang JG. Age dependency of peripheral and central systolic pressures: cross-sectional and longitudinal observations in a Chinese population. Hypertens Res. 2012;35:115–122. doi: 10.1038/hr.2011.160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.