Abstract
Chronic neutrophilic inflammation is a manifestation of a variety of lung diseases including cystic fibrosis (CF). There is increasing evidence that fragments of extracellular matrix proteins, such as collagen and elastin, play an important role in inflammatory cell recruitment to the lung in animal models of airway inflammation. Unfortunately, the association of these peptides with human disease and the identification of therapeutic targets directed toward these inflammatory pathways have remained elusive. In this study, we demonstrate that a novel extracellular matrix-derived neutrophil chemoattractant, proline-glycine-proline (PGP), acts through CXC receptors 1 and 2 on neutrophils, similar to N-acetylated proline-glycine-proline (N-α-PGP). We describe the specific multistep proteolytic pathway involved in PGP generation from collagen, involving matrix metalloproteases 8 and 9 and prolyl endopeptidase, a serine protease for which we identify a novel role in inflammation. PGP generation correlates closely with airway neutrophil counts after administration of proteases in vivo. Using CF as a model, we show that CF sputum has elevated levels of PGP peptides and that PGP levels decline during the course of CF inpatient therapy for acute pulmonary exacerbation, pointing to its role as a novel biomarker for this disease. Finally, we demonstrate that CF secretions are capable of generating PGP from collagen ex vivo and that this generation is significantly attenuated by the use of inhibitors directed toward matrix metalloprotease 8, matrix metalloprotease 9, or prolyl endopeptidase. These experiments highlight unique protease interactions with structural proteins regulating innate immunity and support a role for these peptides as novel biomarkers and therapeutic targets for chronic, neutrophilic lung diseases.
Neutrophils are important mediators in a variety of chronic inflammatory diseases affecting the airways such as chronic obstructive pulmonary disease (COPD)4 (1) and cystic fibrosis (CF) (2). Neutrophil influx into these chronically inflamed airways propagates damage via multiple mechanisms including oxidant injury and the release of proteolytic enzymes, leading to parenchymal lung injury and end-organ dysfunction (3).
The major chemoattractants for neutrophils in these conditions are glutamic acid-leucine-arginine-positive (ELR+) CXC chemokines including IL-8, growth-related oncogene (GRO)-α, GRO-β, and GRO-γin humans and KC and MIP-2 in mice (4). Nonspecific collagen-derived fragments have also been reported to induce neutrophil chemotaxis in murine models (5). Senior et al. (6) have previously described elastin fragments ending with proline-glycine as having the capacity to cause fibroblast and monocyte chemotaxis and, to a lesser degree, neutrophil chemotaxis. We have recently described the role of a specific collagen-derived peptide, N-acetylated proline-glycine-proline (N-α-PGP), in neutrophilic lung inflammation (7). N-α-PGP, via structural homology to most ELR+ CXC chemokines, acts as neutrophil chemoattractants through CXCR1 and CXCR2 on neutrophils. This novel CXC ligand demonstrates the ability to not only induce neutrophil chemotaxis but also to induce superoxide release from neutrophils via CXCR1 binding. The cellular kinetic response to aerosolized LPS administration to mice demonstrates that initial neutrophil influx is dependent on traditional ELR+ chemokines (KC and MIP-2) but is maintained and augmented by N-α-PGP until neutrophils are cleared from the airways, concomitant with declining N-α-PGP levels in the airway.
Nonacetylated proline-glycine-proline (PGP) has also been previously described as a neutrophil chemoattractant in vitro, although it is four to seven times less potent than N-α-PGP (8). Recently, our group has described the presence of PGP as a prominent neutrophil chemoattractant in a murine model of pneumonic tularemia (9), although the mechanisms for inducing chemotaxis are not known. In addition, although N-α-PGP has been reported from clinical disease samples (7), PGP has not been reported in clinical samples. Despite the presence of PGP-containing peptides and other structural proteins in animal models of inflammation, no specific ECM-derived peptide has been consistently shown as a biomarker in clinical disease. Although the biological properties of some of these peptides are becoming increasingly understood (7), the specific mechanism of generation of these peptides remains unclear, although proteolytic enzymes are thought to play an important role (9, 10).
Recent models of airway inflammation indicate that protease/ antiprotease imbalance is a prime feature in several pulmonary diseases including COPD (10) and CF (11). One class of proteases recently felt to play an important role in airway remodeling in lung disease are matrix metalloproteases (MMPs), a family of zinc-containing endopeptidases with the capacity to degrade multiple components of the extracellular matrix (12). Recently, our laboratory has shown the presence and enhanced activity of discrete MMPs in the sputum of patients with CF, including MMP-8 and MMP-9 (13). Despite recent evidence that implicates MMP-9 as involved in the generation of PGP (9), MMP-9 does not demonstrate the substrate specificity to liberate PGP directly from collagen by itself. Thus, one or more other proteases are likely involved. As such, the specific proteolytic mechanism for the release of this peptide from collagen is unknown.
The aim of this study was to determine the mechanism of PGP-induced neutrophil chemotaxis, the specific proteolytic mechanisms involved in PGP generation from collagen, their importance in neutrophilic inflammation using CF as a disease model, and whether these fragments might serve as a biomarker of the inflammatory condition. In this report, we use a novel mass spectrometry (MS) technique to now simultaneously detect both N-α-PGP and PGP from clinical samples. We describe the presence of PGP-containing peptides in significantly increased quantities in the sputum of CF individuals compared with healthy subjects and demonstrate the capacity of CF sputum to generate PGP from intact collagen using a novel ex vivo system. We further describe the proteolytic system involved in PGP generation as a two-step process using the coordinated efforts of MMPs (MMP-8 and MMP-9) and prolyl endopeptidase (PE), a serine protease herein described for the first time with a role related to airway inflammation. PGP levels are elevated during CF exacerbation, pointing to its role as a novel disease biomarker. Finally, we demonstrate that inhibition of MMP-8, MMP-9, and PE blocks ex vivo generation of PGP by CF sputum, pointing to a possible role of these inhibitors as therapeutics in chronic neutrophilic lung diseases, including CF.
Materials and Methods
Patient populations
The University of Alabama-Birmingham Institutional Review Board approval was obtained before all studies involving human subjects and samples. All patients granted written informed consents and collected with a unique patient identifier to maintain patient confidentiality.
CF subjects
All subjects carried the diagnosis of CF based on accepted diagnostic criteria, including a minimum of two clinical features consistent with the diagnosis and either two sweat Cl− values >60 mM or two disease-causing CF transmembrane conductance regulator mutations (2). Those CF individuals deemed as having exacerbation (inpatients) and hospitalized had at least three of the following symptoms: increased cough/ sputum production, fever, weight loss, tachypnea, findings on chest x-ray consistent with pneumonia, or a 10% or greater drop in pulmonary function testing.
Normal controls
All normal subjects were nonsmoking individuals without known lung disease; sputum was collected via hypertonic saline induction.
Materials
Recombinant MMP-9, MMP-8, MMP-12, CXC receptor Abs and isotype control Ab were purchased from R&D Systems. Recombinant human neutrophil elastase (HNE), HNE-specific inhibitor, MMP-9- specific inhibitor, MMP-8-specific inhibitor, MMP-2-specific inhibitor, and PE inhibitor were purchased from Calbiochem. PE was purchased from US Biologicals. PE substrate was purchased from Chem-Impex. Types I and II collagen were purchased from Sigma-Aldrich.
Chemotaxis assay
Chemoattractant is placed in the bottom wells of a 3-μm, 96-well poly-carbonate filter plate (Millipore) in 150 μl of DMEM. Neutrophils (2 × 105) were added in 100 μl of DMEM to the top portion. These were incubated for 1 h at 37°C in 5% CO2. The upper portion of the plate was removed and micrographs of the migrated cells were made with an Olympus IX70 microscope.
Enzyme inhibitors
The enzyme inhibitors used are as listed in Table I.
Table I.
Enzyme Inhibitors
| Enzyme Inhibited | Chemical Composition (manufacturer) | Specificity | Ref. |
|---|---|---|---|
| PE | Z-prolyl prolinal (Calbiochem) | KI =500 pM | 14 |
| MMP-8 | (3R)-(+)-[2– 4-methoxybenzenesulfonyl)-1,2,3,4-tetrahydroisoquinoline-3-hydroxamate] (Calbiochem) | IC50 =4 nM | 15 |
| MMP-9 | C27H33N3O5S (Calbiochem) | IC50 =5 nM | 16 |
| MMP-2 | cis-9-octadecenoyl-N-hydroxylamide (Calbiochem) | Ki = 1.7 μM | 17 |
| HNE | N-(2-(4-(2,2-dimethylpropionyloxy) phenylsulfonylamino)benzoyl) aminoacetic acid N-(o-( p-pivaloyloxybenzene) sulfonylaminobenzoyl) glycine (Calbiochem) | IC50 =50 nM | 18 |
| Nonspecific MMP | Doxycycline (Calbiochem) | Nonspecific MMP inhibitor | 19 |
Electrospray ionization liquid chromatography-MS/MS (ESI-LC/MS/MS) for PGP detection
PGP and N-α-PGP were measured for in vitro and sputum samples using a MDS Sciex (Applied Biosystems) API-4000 spectrometer equipped with a Shimadzu HPLC. HPLC was done using a 2.1 × 150-mm Develosi C30 column (with buffer A: 0.1% formic acid, and buffer B: acetonitrile plus 0.1% formic acid); at 0 – 0.6 min, 80% buffer A/20% buffer B and at 0.6 –5 min, the gradient is up to 0% buffer A/100% buffer B. Background was removed by flushing with 100% isopropanol plus 0.1% formic acid. Positive electrospray mass transitions were at 270-70 and 270-116 for PGP and 312-140 and 312-112 for N-α-PGP.
In vivo murine administration
Animal protocol for protease administration was approved by the University of Alabama-Birmingham Institutional Animal Care and Use Commission. Mice underwent intratracheal protease administration as described in Fig. 5 legend. MMPs were preactivated using 1 mM aminophenylmercuric acetate for 2 h at 37°C. The concentrations of proteases administered were: MMP-8, MMP-9, and MMP-12: 55.6 μg/kg; HNE: 200 μg/kg; and PE: 18.4 mg/kg. The relative enzyme activities are: PE enzyme activity: 1 unit = 1 μM p-nitroaniline (pNA)/min at 30°C, pH 7; sp. act, 22.6 U/mg PE; MMP-9 enzyme activity: 10 μM ES001 (substrate) and 20 ng of MMP-9 = 1300 pmol/min per μg at 37°C; MMP-8 enzyme activity: 10μM ES001 (substrate) and 50 ng of MMP-8 = 250 pmol/min per μg at 37°C; MMP-12 enzyme activity: 10 μM ES001 (substrate) and 20 ng of MMP-12 = 500 pmol/min per μg at 37°C; and HNE enzyme activity: 22 U/mg protein. (1 unit = hydrolysis of 1.0 μmol pNA/min at 25°C, pH 8.0.
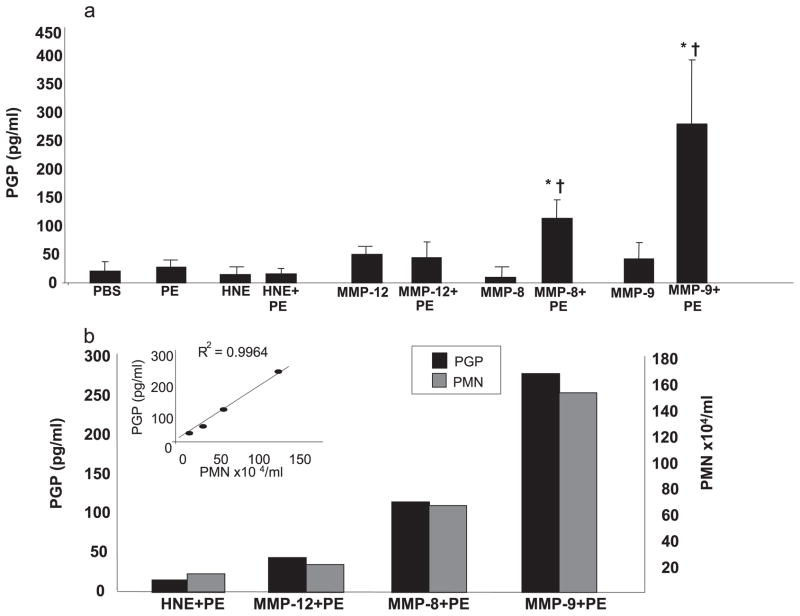
FIGURE 5.
a, In vivo PGP generation: in vivo PGP production was examined using MMPs or HNE with or without PE. Various proteases (50 μl) were administered intratracheally into murine (4- to 6-wk-old BALB/c mice) airways and bronchoalveolar lavage fluid was collected 24 h later. PGP levels were determined using ESI LC-MS/MS. PGP production was significantly increased in MMP-9 with PE (*, p < 0.05 vs PBS control; †, p < 0.05 vs MMP-9 alone) and MMP-8 with PE (*, p <0.05 vs PBS control; †, p <0.05 vs MMP-8 alone) compared with other proteases with or without PE. PBS control and PE alone had similar PGP production. Of note, aminophenylmercuric acetate alone (or in combination with PE) also did not generate PGP (data not shown). Number of mice per group = 6. b, PGP production correlated with neutrophil influx: PGP production levels (■) were compared with PMN counts (
 ) in mice treated with a combination of the indicated protease and PE from Fig. 3a. There is a notable correlation between PGP production and PMN counts for each condition (R2 = 0.996, inset).
) in mice treated with a combination of the indicated protease and PE from Fig. 3a. There is a notable correlation between PGP production and PMN counts for each condition (R2 = 0.996, inset).
Bronchoalveolar lavage
After mice were euthanized with phenobarbital, mice underwent bilateral thoracotomy and were lavaged with four 1-ml aliquots of cold PBS.
Sputum processing
Sputum was obtained by spontaneous expectoration. Sputum was collected on ice and diluted 1/2 with 0.9% normal saline, centrifuged at 1000 rpm for 15 min, and supernatant was collected. Protein concentration was measured and then separate aliquots were saved for measurements (MMP, HNE, PE) and MS (N-α-PGP/PGP).
PE activity assay
Twenty microliters of sputum was incubated with a specific substrate (2 mM Z-glycine-proline-pNA) at 37°C and 5% CO2 and cleavage of pNA) from the substrate by PE was detected using a spectrophotometer at 410 nm and compared with a generated standard curve for PE activity.
Ex vivo collagen assay
One hundred microliters of saline-diluted sputum was incubated with extensively dialyzed, intact type I or II collagen (50 μl, 1 mg/ml) for 24 h at 37°C and 5% CO2. The samples were filtered through a 10-kDa filter, washed with 20 μl of 1 N HCl, and analyzed using ESI- LC-MS/MS for levels of PGP and N-α PGP. Amounts of PGP and N-α PGP generated by each sputum sample were determined by subtracting basal levels already present in each sample.
Thereafter, CF sputum samples were individually evaluated and the most active samples were pooled. For the inhibitor experiments, these pooled sputa were treated with noted inhibitor and allowed to incubate for 6 h. At 6 h, dialyzed collagen was added to the sample and sample was further incubated for 18 h. Inhibitor concentrations used were: MMP-8, -9, and -2 at 50 μM, PE at100 μM, and doxycycline at 1 mM.
Statistical testing
Descriptive statistics including mean and SEM were made for all quantitative measures. The two-tailed Student t test was used for comparisons between two groups and ANOVA was used for comparing means of three or more groups. Pearson’s correlation was used to compare the relationship between 1) PE activity and PGP generation, 2) change in FEV1 and change in PGP levels, and 3) change in FVC and change in the PGP (4) relationship between PGP and N-α-PGP in clinical CF samples. Means presented are ±SEM; statistical significance is considered for p < 0.05. Calculations were made using Instat software (GraphPad) and SPSS version 14. Values of p < 0.05 were determined to be statistically significant.
Results
PGP acts via CXC receptors to induce neutrophil chemotaxis
As previously mentioned, we have recently characterized PGP as a neutrophil chemoattractant in a murine model of airway inflammation (9). However, it is unknown whether this nonacetylated peptide acts through similar mechanisms as N-α-PGP. To test this possibility, we used mAbs to block CXCR1 and CXCR2 on neutrophils. When neutrophil migration is examined via chemotaxis assay, either a CXCR1 or CXCR2 Ab used individually causes only a partial reduction in chemotaxis (data not shown). However, when both Abs are used in conjunction, there is a dose-dependent effect leading to complete blockade of neutrophil influx (Fig. 1a). Isotype-matched control mAb was without effect. Thus, as with N-α-PGP, PGP apparently acts through CXCR1- and CXCR2-dependent mechanisms to induce neutrophil chemotaxis.
FIGURE 1.
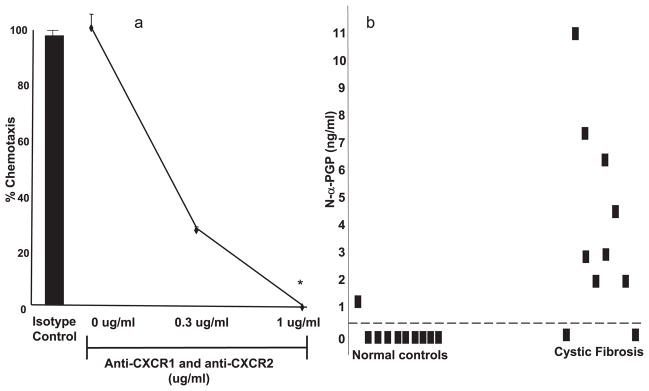
a, PGP acted via a CXCR-dependent mechanism to cause neutrophil chemotaxis: PMN are pretreated with CXCR1 and CXCR2 Abs or IgG2a isotype control Ab (2 μg/ml) for 1 h at 22°C. PGP (at 10 μg/ml) is placed in the bottom of chemotaxis plate. The isotype Ab demonstrated no change in neutrophil chemotaxis compared with untreated cells (■). However, at 1 μg/ml concentration of each CXCR Ab, PGP chemotaxis is completely blocked (*, p < 0.01 compared with no Ab and isotype Ab control). b, N-α-PGP is increased in CF samples compared with normal control samples: CF (n = 10) and normal control (n = 10) sputum samples were analyzed using ESI-LC/MS/MS for N-α-PGP detection. CF samples demonstrated 8 (80%) of 10 positive for N-α-PGP vs normal controls having 1(10%) of 10 positive for N-α-PGP. The threshold for positivity (0.825 ng/ml) was determined as two SDs above mean (95% confidence interval) for control sputum values.
N-α-PGP and PGP are elevated in sputum from CF individuals
To examine N-α-PGP and PGP in sputum from CF individuals and normal controls (see Materials and Methods for description of populations), we modified our published MS technique of ESI LC-MS/MS for simultaneous detection of these peptides in clinical samples. These clinically stable CF patients (60% female/40% males; mean age, 26.6 years) had moderately severe lung disease with mean forced expiratory volume 1 s (FEV1) of 34% predicted and a mean forced vital capacity (FVC) of 45% of predicted. The majority of these individuals were either ΔF508 heterozygous (40%) or homozygous (50%). Eighty percent of these individuals were Pseudomonas aeruginosa-positive via sputum culture.
Fig. 1b shows that 8 (80%) of 10 CF sputum samples had N-α-PGP above our threshold for positivity vs 1(10%) 10 normal controls with mean values of each group 3.78 ng/ml (±1.84) and 0.13 ng/ml (±0.12), respectively ( p < 0.01). The mean values for PGP in the CF samples were 204.8 ng/ml (±83.9) vs 16.2 ng/ml (±19.8) in normal controls ( p < 0.05; data not shown), highlighting an elevation of PGP-containing peptides seen in the CF population. These samples demonstrated a correlation coefficient (R2) of 0.76 between their N-α-PGP and PGP levels ( p < 0.01), demonstrating a strong relationship of the presence of these peptides in clinical samples. The above results led to an inquiry regarding the specific proteases involved in the generation of PGP in vivo.
PE activity is elevated in CF sputum and correlates with PGP
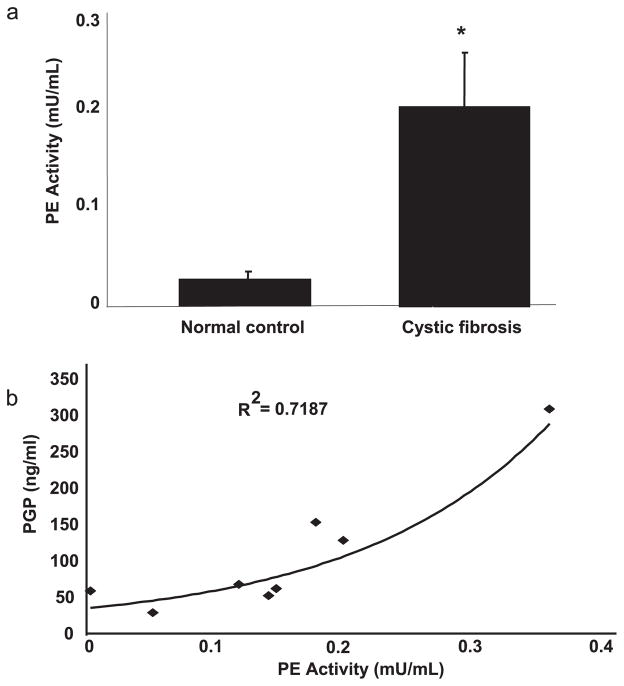
To our knowledge, the only enzyme directly capable of cleaving PGP from the often repeated “PPGP” motif in collagen is PE, a serine protease which provides specific cleavage at the C-terminal side of a proline (20). PE is an enzyme implicated in neuropeptide processing and specific neurological conditions (21). This enzyme has also been reported previously in human T cells (22). Although its location has been reported to be cytosolic (20), many reports also describe extracellular activity of the enzyme (21). This enzyme has been previously identified in lung parenchyma (23), pulmonary macrophages (24), and bronchoalveolar lavage fluid, although its function in the lung is unknown (25).
To determine whether PE may be playing a role in PGP generation in the CF lower airway, we attempted to assay its presence in CF sputum. Using a very specific substrate for PE (Z-glycine-proline-pNA) (26), we found a 5-fold increase in PE activity in CF patients (n = 10) compared with normal control samples (Fig. 2a) (n = 10). When we correlated PGP production and PE activity in the CF samples capable of generating PGP (n = 8), the correlation coefficient (R2) was 0.72 (Fig. 2b; p < 0.01). Together, these results implicate increased PE activity with PGP generation in vivo. However, PE is only capable of cleaving substrates 30 –100 aa or less (27); therefore, PE alone could not directly cleave collagen to a tripeptide and would require an initial cleavage of collagen before liberating PGP. Therefore, we hypothesized that PGP generation from intact collagen was a stepwise process involving initial proteolytic cleavage of collagen with subsequent activity by PE.
FIGURE 2.
a, PE activity was elevated in CF samples compared with normal controls: CF (n = 10) and normal control (n = 10) sputum samples were examined for PE activity using a colorimetric assay. CF samples demonstrated a 5-fold increase in PE activity compared with normal controls (*, p < 0.01). b, PE activity correlated with PGP: concentration of PGP was correlated with PE activity in CF samples (n = 8). The samples demonstrated a correlation coefficient (R2) of 0.718 (p < 0.01).
CF sputum is capable of generating PGP from collagen
Recently, our laboratory reported increased protease activity in CF sputum; human neutrophil elastase (HNE) was elevated along with the MMP isoforms collagenase-2 (MMP-8), gelatinase B (MMP-9), stromelysin-3 (MMP-11), and macrophage metalloelastase (MMP-12) (13). Since these enzymes and PE were found to have elevated activity in CF sputum, we hypothesized that CF sputum had the necessary components to generate PGP from collagen. We used type I collagen for these studies, because this is the prominent form of collagen seen in the airways (28). However, to demonstrate the potential generalizability of this process, we also examined type II collagen. Both CF and normal control sputum samples were incubated with either type I or type II collagen and examined via MS for PGP or N-α-PGP. Fig. 3a shows that sputum samples from CF individuals generated PGP from type I collagen (average, 148 ng/ml; range, 130 –530% of basal PGP) compared with normal patient samples (average, 1.3 ng/ml; range, 0 –117% of basal PGP; p < 0.05). Similarly, Fig. 3b demonstrates CF sputum samples generated PGP from type II collagen (average, 240 ng/ml; range, 140 – 675% of basal PGP) compared with PGP generation from normal controls (average, 9.8 ng/ml; range. 0 –155% of basal PGP; p < 0.05). Slightly lower but statistically significant increases in N-α-PGP levels were seen in CF sputum incubated with both type I and type II collagen (data not shown). These data demonstrate that CF sputum contains the proteolytic enzymes necessary to generate PGP from collagen.
FIGURE 3.
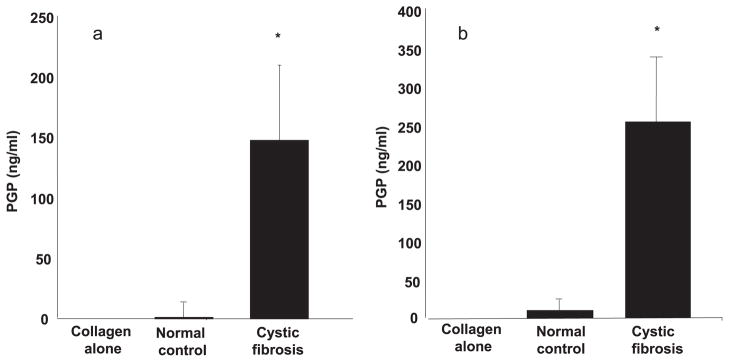
a, PGP production was significantly increased in CF samples compared with normal control samples on type I collagen: CF sputum (n = 10) and normal control sputum (n = 10) were each incubated on extensively dialyzed type I collagen for 24 h at 37°C. PGP values of the samples on PBS were subtracted from PGP values of samples incubated on type I collagen to determine PGP production. PGP generated from CF samples were significantly increased compared with normal control samples on type I collagen (*, p < 0.05). b, PGP production was significantly increased in CF samples compared with normal control samples on type II collagen: CF sputum (n = 10) and normal control sputum (n = 10) were each incubated on extensively dialyzed type II collagen for 24 h at 37°C. PGP values of the samples on PBS were subtracted from PGP values of samples incubated on type II collagen to determine PGP production. PGP generated from CF samples were significantly increased compared with normal control samples on type II collagen (*, p < 0.05).
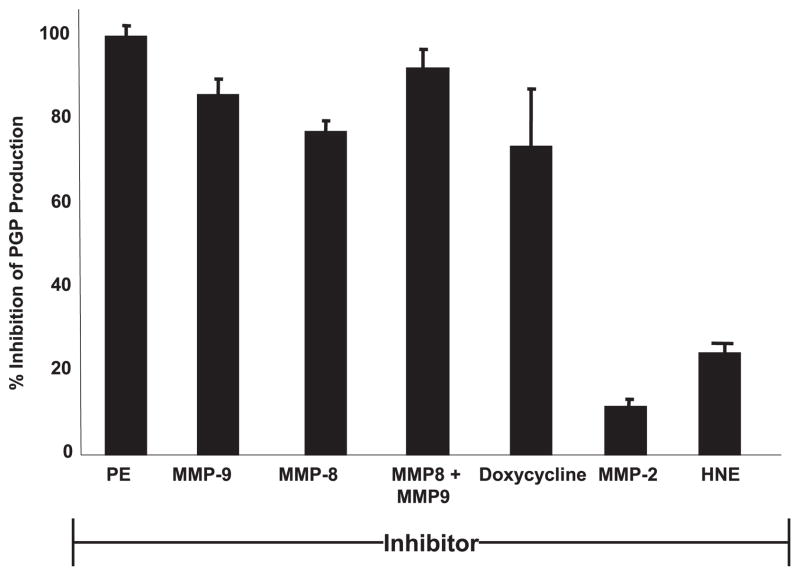
PGP generation is significantly abrogated by use of specific protease inhibitors
To identify the specific proteases involved in PGP generation, we used the CF sputum ex vivo assay for PGP production from intact collagen in the presence of various protease inhibitors. CF sputum was incubated with known specific enzyme inhibitors (see Materials and Methods) and then incubated with type I collagen. The results were reported as percent inhibition compared with PGP generation by CF sputum alone (Fig. 4). PE inhibition completely blocked generation of PGP, indicating its central importance to PGP generation. PGP production was also significantly inhibited by both MMP-8 and MMP-9 antagonists individually, and this inhibition is augmented when MMP-8 and MMP-9 antagonists were combined. That MMP-8 and -9 inhibitors alone block PGP production suggested that the two proteases act in concert to generate an optimal substrate for PE. MMP-2 and HNE inhibitors had minimal effects on PGP generation. Doxycycline, a clinically used antimicrobial which also acts as a nonspecific small molecule MMP inhibitor (29), caused ~75% inhibition of PGP production and may serve as a potential therapeutic agent with the capability to modify this pathway of inflammation in CF patients. To confirm the implied role for MMP-8, MMP-9, and PE in PGP generation by CF sputum, we attempted to recapitulate such PGP production by in vivo administration of proteases into murine airways.
FIGURE 4.
PE, MMP-8, and MMP-9 inhibitors can block the production of PGP: inhibitors were incubated for 6 h with pooled CF sputum and these sputa were placed on type I collagen for 24 h as previously described. PGP concentrations from these groups were compared with pooled CF sputum on type I collagen not treated with inhibitor. PE inhibitor demonstrated complete blockade of PGP production and MMP-8- and -9-specific inhibitors individually demonstrated 80–90% inhibition, with their combination resulting in complete blockade of PGP generation. Doxycycline, a nonspecific MMP inhibitor, demonstrated comparable PGP inhibition as MMP-8 alone. Neither MMP-2- nor HNE-specific inhibitors resulted in significant changes in PGP production.
PGP generation correlates with polymorphonuclear leukocyte/ neutrophil (PMN) influx and is found to be a stepwise process involving MMPs and PE
Fig. 5a shows that following intratracheal delivery to murine lungs, MMPs alone, HNE alone, or PE alone did not generate PGP. However, when either MMP-8 or MMP-9 was combined with PE, PGP was generated at levels significantly higher than PBS control or either enzyme alone. Of note, MMP-12 and HNE, two prominent proteases found in a variety of chronic neutrophilic lung diseases including CF (13, 30), generated no PGP in the presence of PE. Fig. 5b demonstrates a tight correlation (R2 = 0.996) between PMN influx and PGP generation following exposure to enzyme combinations. These results verified the stepwise generation of PGP with specific MMPs in combination with PE and completely recapitulated our ex vivo human findings.
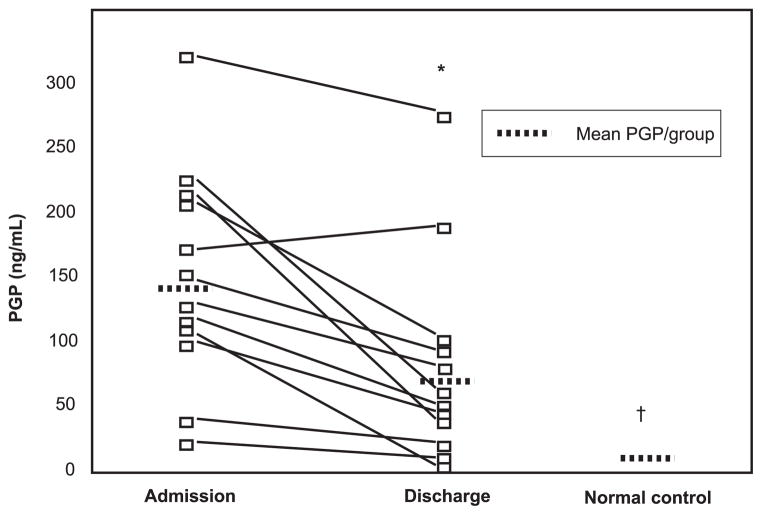
PGP serves as a unique biomarker during CF exacerbation
The presence of PGP in CF secretions led to the question of whether this peptide may serve as a novel inflammatory biomarker in CF lung disease. PGP measurements were taken in CF inpatients at the beginning of CF exacerbation (within 48 h of admission) and at the end of hospitalization (day 13/14). All subjects were treated with standard CF inpatient therapy (including two antibiotics (aminoglycoside and either β-lactam or fluoroquinolone), intensive airway clearance techniques, and nebulized therapies (i.e., recombinant human DNase, albuterol) during the hospitalization. These patients were 60% female, 40% male, and had a mean age of 18 years. These individuals were either ΔF508 heterozygous (33%) or homozygous (66%). Eighty-three percent of these individuals were P. aeruginosa-positive via sputum culture. They also demonstrated moderately severe lung disease at admission (mean FEV1 = 43% of predicted and mean FVC = 60% of predicted) which improved after inpatient therapy (mean FEV1 = 51% of predicted and mean FVC = 67% of predicted).
Fig. 6 shows a significant reduction in the PGP levels during the course of hospitalization in aggregate and a notable decline in 11 of the 12 subjects ( p < 0.01). Of note, there was a correlation seen in decline of PGP levels with improvement in FEV1 and FVC during hospitalization and these results trended toward statistical significance (R = 0.53 for FEV1 and PGP, p = 0.12; R = 0.52 for FVC and PGP, p = 0.11). Despite the reduction in PGP levels during hospitalization in CF subjects, the PGP levels upon discharge remained 5-fold higher than that seen in normal controls ( p < 0.01), indicating that even after resolution of CF exacerbation, these patients demonstrated ongoing inflammation and matrix degradation which may be mediated in part by proteases and PGP.
FIGURE 6.
PGP levels decline during inpatient therapy for CF exacerbation: sputum PGP levels from CF individuals (n = 12) were examined within 48 h of admission and at discharge (day 13/14) for CF exacerbation. The mean levels of PGP decreased during hospitalization (146.4 ± 24.4 vs 80.0 ± 22.5; *, p < 0.01), although these levels are still 5-fold elevated compared with secretions from normal controls (†, p < 0.01).
Discussion
In this report, we describe multiple novel aspects of PGP biology. PGP induces neutrophil chemotaxis through a CXC receptor-dependent mechanism. To our knowledge, this article describes the first specific protease cascade generating a chemotactic ECM fragment. We use a unique ex vivo assay system to demonstrate the capability of CF sputum to generate PGP from intact collagen; this type of model has not previously been described for other structural protein fragments. Finally, by using a novel MS technique, we can simultaneously detect both N-α-PGP and PGP in sputum samples, which are less invasive than using bronchoscopy and bronchoalveolar lavage.
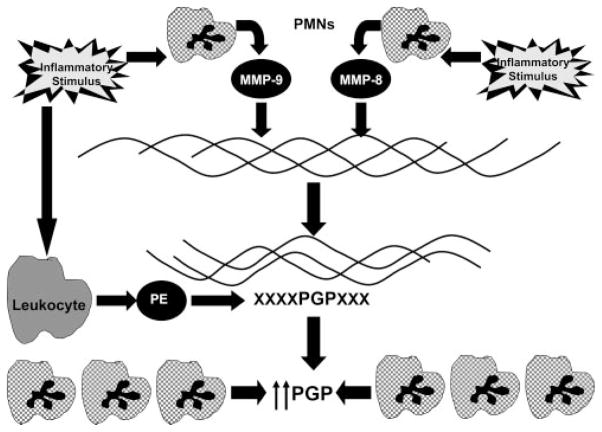
Our experimental results point to the generation of PGP as a multistep process involving both members of the MMP family (MMP-8, MMP-9) and the serine protease family (PE). These findings comprise a growing body of data implicating MMPs in regulation of inflammation (31, 32). Although MMP-8 and MMP-9 are present in a variety of cells, their highest concentrations are found in neutrophils. MMP-8 and MMP-9 likely work in concert to create an optimal substrate for PE activity. The release of these specific MMPs into the airway lead to the cleavage of collagen fragments as modeled in Fig. 7. These collagen fragments are derived from well-documented cleavage sites for these proteases (33, 34) and conform well to the size specification for a PE substrate.
FIGURE 7.
PGP generation is a multistep process: the generation of PGP is a multistep process initially involving release of MMP-8 or MMP-9 from activated neutrophils. These proteases denature and proteolytically cleave collagen to fragments 30 –100 aa in length. These collagen fragments are then further cleaved to PGP by PE. The PGP generated then acts as a neutrophil chemoattractant and allows for an environment of ongoing proteolytic damage and PGP generation.
Unlike MMPs, PE is an enzyme that has not been implicated in inflammation biology or lung pathology. PE has been described in both macrophages and lymphocytes (T cells), although its presence in other immune cell populations is unknown. Our current data suggest that PE has a central place in a mechanism of pulmonary neutrophilic inflammation, demonstrating a unique role in the generation of PGP from collagen. CF clinical samples demonstrate a strong correlation between PE activity and PGP production, recapitulating with fidelity our in vivo murine and ex vivo data. This is the first report of this enzyme as a modulator of the inflammatory response in any organ system. Further investigation of this enzyme and its role in pulmonary immunology and host defense certainly appears warranted.
The increased levels of N-α-PGP and PGP seen in CF sputum suggest an important role of these peptides in conditions with prominent ECM remodeling and neutrophilic inflammation. The generation of PGP in CF would require access of proteases to the underlying collagen within the airways. Initially in CF, the airway epithelium may serve as a barrier to prevent the actions of these proteases on collagen. However, as the disease progresses, the ongoing damage to the epithelium (either apically or loss of epithelial-epithelial junctions) will allow the proteases access to the underlying ECM and lead to the generation of PGP (2). We would, therefore, hypothesize that this collagen fragment would play a more prominent role in CF-related inflammation in airways with loss of the integrity of the epithelial cell layer, leading to ongoing matrix remodeling.
The reduction of PGP levels over the course of inpatient therapy demonstrates that changes in PGP are specific for treatment effects expected to reduce acute airway inflammation during CF exacerbation. Previous examination of airway inflammatory markers in sputum during CF exacerbations demonstrates modest declines in PMN counts, IL-8, and elastase levels (35). Another examination of biomarkers in adults and children with CF exacerbation demonstrated that sputum myeloperoxidase levels only changed in some patients and did not correlate well with other biomarkers of CF exacerbation (36). None of these markers have demonstrated consistent changes during the course of CF exacerbation, suggesting the need for the development of other novel disease biomarkers. Our data demonstrate a robust decrease in PGP levels between individuals during the course of inpatient therapy not observed from these other biomarkers. It is also notable that, even in a small population of patients, there are significant differences in sputum PGP levels over the course of CF exacerbation. Since not all patients may be expected to expectorate sputum, we have also recently modified our MS technique to successfully measure PGP in serum from patients; these results demonstrate a 3-fold increase ( p < 0.05) in PGP levels in CF individuals (n = 5) compared with normal controls (n = 5; data not shown). These findings suggest that PGP may serve as an important inflammatory biomarker to be measured during the course of acute CF exacerbation.
Although some studies have examined the inflammatory cell influx through elastin fragmentation in murine models of inflammation (6), this is the first report to directly implicate a specific protease pathway in the generation of a specific neutrophil chemotactic ECM-derived peptide and to then describe these peptides in clinical disease. It is conceivable that the biological compartment of other chronic neutrophilic inflammatory conditions (i.e., arthritis, vasculitis, atherosclerotic disease) may have elevated levels of PE and MMP-8 or MMP-9, leading to the generation of PGP and augmenting inflammation.
Finally, the delineation of the proteases involved in the generation of PGP may point to new future therapeutic targets in the treatment of the unrelenting inflammation seen in conditions such as CF and COPD. Regulation of collagen turnover and reduction of inflammatory peptides may help identify disease-specific end points and even alter the natural history of these conditions. Although most MMP or PE inhibitors are not currently available for testing in human trials, well-tolerated MMP inhibitors such as doxycycline may be useful in clinical trials as inhibitors of MMPs and of N-α-PGP and PGP generation. The findings presented here demonstrate a novel mechanism of inflammation seen in a chronic neutrophilic lung disease, showcase interactions between multiple inflammatory cells in a unique avenue of lung innate immunity, and underscore the role of PGP-containing peptides as both therapeutic targets of disease and as novel disease biomarkers.
Acknowledgments
We appreciate Heather Young and Ginger Reeves for their assistance in obtaining human samples. We thank Diane Weigent for editorial assistance. We thank Dr. Nathaniel Weathington and Dr. F. Shawn Galin for insight into aspects of protease biology. We also thank Drs. E. J. Sorscher and Chad Steele for thoughtful comments in review of this manuscript.
Footnotes
This work was supported by the University of Alabama Clinical Investigator Fellowship Award, the Cystic Fibrosis Foundation (Grant GAGGA07A0 to A.G.). S.M.R. is funded through the National Institutes of Health (Grant 1K23DK075788). J.P.C. is funded through The Thrasher Award. J.E.B. is funded through the Cystic Fibrosis Foundation (Grant R464-CR02) and National Institutes of Health (Grants HL07783 and HL090999). Funds for the purchase of mass spectrometers and the operation of the Mass Spectrometry Shared Facility came from the following National Institutes of Health grants to the University of Alabama at Birmingham: S10 RR19231, P30 CA13148, P50 AT00477, U54 CA100949, P30AR050948, and P30 DK74038. This project was supported in part by grants from the National Heart, Lung, and Blood Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations used in this paper: COPD, chronic obstructive pulmonary disease; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; ECM, extracellular matrix; ELR, glutamate-leucine-arginine; MS, mass spectrometry; ESI LC-MS/MS, electrospray ionization liquid chromatography-MS/MS; FEV1, forced expiratory volume 1 second; FVC, forced vital capacity; GRO, growth-related oncogene; HNE, human neutrophil elastase; PGP, proline-glycine-proline; N-α-PGP, N-acetylated PGP; PE, prolyl endopeptidase; PMN, polymorphonuclear leukocytes or neutrophils; pNA, paranitroaniline; MMP, matrix metalloprotease.
Disclosures
The authors have no financial conflict of interest.
References
- 1.O’Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61:448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 3.Doring G, Worlitzsch D. Inflammation in cystic fibrosis and its management. Paediatr Respir Rev. 2000;1:101–106. doi: 10.1053/prrv.2000.0030. [DOI] [PubMed] [Google Scholar]
- 4.Weathington N, Blalock JE. The biology of CXC chemokines and their receptors. In: Schwiebert LM Jr, editor. Current Topics in Membranes. Vol. 55. Elsevier; New York: 2005. pp. 49–71. [Google Scholar]
- 5.Riley DJ, Berg RA, Soltys RA, Kerr JS, Guss HN, Curran SF, Laskin DL. Neutrophil response following intratracheal instillation of collagen peptides into rat lungs. Exp Lung Res. 1988;14:549–563. doi: 10.3109/01902148809087827. [DOI] [PubMed] [Google Scholar]
- 6.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859– 862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 8.Haddox JL, Pfister RR, Muccio DD, Villain M, Sommers CI, Chaddha M, Anantharamaiah GM, Brouillette WJ, DeLucas LJ. Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl-pro-line-glycine-proline. Invest Opthalmol Visual Sci. 1999;40:2427–2429. [PubMed] [Google Scholar]
- 9.Malik M, Bakshi CS, McCabe K, Catlett SV, Shah A, Singh R, Jackson PL, Gaggar A, Metzger DW, Melendez JA, et al. Matrix metalloproteinase-9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularemia. J Immunol. 2007;178:1013–1020. doi: 10.4049/jimmunol.178.2.1013. [DOI] [PubMed] [Google Scholar]
- 10.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004.7. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 11.Doring G. The Role of neutrophil elastase on chronic inflammation. Am J Respir Crit Care Med. 1994;150:S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 12.Greenlee K, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaggar A, Li Y, Weathington NM, Winkler M, Kong M, Jackson PL, Blalock JE, Clancy JP. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am J Physiol. 2007;293:L96–L104. doi: 10.1152/ajplung.00492.2006. [DOI] [PubMed] [Google Scholar]
- 14.Bakker AV, Jung S, Spencer RW, Vinick FJ, Faraci WS. Slow tight-binding inhibition of prolyl endopeptidase by benzyloxycarbonyl-prolyl-prolinal. Biochem J. 1990;271:559–562. doi: 10.1042/bj2710559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matter H, Schwab W, Barbier D, Billen G, Haase B, Neises B, Schudok M, Thorwart W, Schreuder H, Brachvogel V, et al. Quantitative structure-activity relationship of human neutrophil collagenase (MMP-8) inhibitors using comparative molecular field analysis and x-ray structure analysis. J Med Chem. 2007;42:1908–1920. doi: 10.1021/jm980631s. [DOI] [PubMed] [Google Scholar]
- 16.Levin JI, Chen J, Du M, Hogan M, Kincaid S, Nelson FC, Venkatesan AM, Wehr T, Zask A, Dijoseph D, et al. The discovery of anthranilic acid-based MMP inhibitors: part 2: SAR of the 5-position and P11 groups. Bioorg Med Chem Lett. 2001;11:2189–2192. doi: 10.1016/s0960-894x(01)00419-x. [DOI] [PubMed] [Google Scholar]
- 17.Berton A, Rigot V, Huet E, Decarme M, Eeckhout Y, Patthy L, Godeau G, Hornebeck W, Bellon G, Emonard H. Involvement of fibronectin type II repeats in the efficient inhibition of gelatinases A and B by long-chain unsaturated fatty acids. J Biol Chem. 2007;276:20458–20465. doi: 10.1074/jbc.M011664200. [DOI] [PubMed] [Google Scholar]
- 18.Kawabata K, Suzuki M, Sugatani M, Imaki K, Toda M, Miyamoto T. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem Bio-phys Res Commun. 1991;177:814– 820. doi: 10.1016/0006-291x(91)91862-7. [DOI] [PubMed] [Google Scholar]
- 19.Emingil G, Atilla G, Sorsa T, Luoto H, Kirilmaz L, Baylas H. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J Periodontol. 2004;75:106–115. doi: 10.1902/jop.2004.75.1.106. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblum JS, Kozarich JW. Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Curr Opin Chem Biol. 2003;7:496–504. doi: 10.1016/s1367-5931(03)00084-x. [DOI] [PubMed] [Google Scholar]
- 21.Morain P, Lestage P, De Nateuil G, Jochemsen R, Robin JL, Guez D, Boyer PA. S17092: a prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment: preclinical and clinical studies. CNS Drug Rev. 2002;8:31–52. doi: 10.1111/j.1527-3458.2002.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirasawa Y, Osawa T, Hirashima A. Molecular cloning and characterization of prolyl endopeptidase from human T cells. J Biochem. 1994;115:724–729. doi: 10.1093/oxfordjournals.jbchem.a124402. [DOI] [PubMed] [Google Scholar]
- 23.Sedo A, Krepela E, Kasafirek E. Dipeptidyl peptidase IV, prolyl endopeptidase, and cathepsin B activities in primary human lung tumors and lung parenchyma. J Cancer Res Clin Oncol. 1991;117:249–253. doi: 10.1007/BF01625433. [DOI] [PubMed] [Google Scholar]
- 24.Lesser M, Chang JC, Orlowski J, Kilburn KH, Orlowski M. Cathepsin B and prolyl endopeptidase activity in rat peritoneal and alveolar macrophages: stimulation of peritoneal macrophages by saline lavage. J Lab Clin Med. 1983;101:327–334. [PubMed] [Google Scholar]
- 25.Orlowski M, Orlowski J, Lesser M, Kilburn KH. Proteolytic enzymes in bronchopulmonary lavage fluids: cathepsin B activity and prolyl endo-peptidase. J Lab Clin Med. 1981;97:467– 476. [PubMed] [Google Scholar]
- 26.Makinen PL, Makinen KK, Syed SA. An endo-acting proline-specific oligopeptidase from Treponema denticola ATCC 35405: evidence of hydrolysis of human bioactive peptides. Infect Immun. 2007;62:4938– 4947. doi: 10.1128/iai.62.11.4938-4947.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan L, I, Mathews I, Khosla C. Structural and mechanistic analysis of two prolyl endopeptidases: role of interdomain dynamics in catalysis and specificity. Proc Natl Acad Sci USA. 2005;102:3599–3604. doi: 10.1073/pnas.0408286102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suki B, Ito S, Stamenovic D, Lutchen KR, Enginito EP. Biomechanics of lung parenchyma: critical role for collagens and mechanical forces. J Appl Physiol. 2005;98:1892–1899. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 29.Smith GN, Mickler EA, Hasty KA, Brandt KA. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: relationship to structure of the enzyme. Arthritis Rheum. 1999;42:1140–1146. doi: 10.1002/1529-0131(199906)42:6<1140::AID-ANR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sternlicht M, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by amino-terminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 33.Van den Steen PE, Proost P, Grillet B, Brand DD, Kang AH, Van Damme J, Opdenakker G. Cleavage of denatured natural collagen type II by neutrophil gelatinase B reveals enzyme specificity, post-translational modifications in the substrate, and the formation of remnant epitopes in rheumatoid arthritis. FASEB J. 2002;16:379–389. doi: 10.1096/fj.01-0688com. [DOI] [PubMed] [Google Scholar]
- 34.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ordonez CL, Henig NR, Mayer-Hamblett N, Accurso FJ, Burns JL, Chmiel JF, Daines CL, Gibson RL, McNamara S, Retsch-Bogart GZ, et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:1471–1475. doi: 10.1164/rccm.200306-731OC. [DOI] [PubMed] [Google Scholar]
- 36.Sloane A, Lindner RA, Prasad SS, Sebastian LT, Pedersen SK, Robinson M, Bye PT, Nielson DW, Harry JL. Proteomic analysis of sputum from adults and children with cystic fibrosis and from control subjects. Am J Respir Crit Care Med. 2005;172:1416–1426. doi: 10.1164/rccm.200409-1215OC. [DOI] [PubMed] [Google Scholar]