Abstract
Many antipsychotics cause weight gain in humans, but usually not in rats, when injected once or twice daily. Since blood antipsychotic half-lives are short in rats, compared to humans, chronic administration by constant infusion may be necessary to see consistent weight gain in rats. Male and female rats were implanted with minipumps for constant infusion of olanzapine (5 mg/kg/day), clozapine (10 mg/kg/day) or vehicle for 11 days. Food intake and body weight were measured; blood drug levels were measured by HPLC. Olanzapine increased food intake and body weight in female, but not male rats. Serum olanzapine concentrations were 30-35 ng/ml. Clozapine had no effect on food intake or body weight in female or male rats. Serum clozapine concentrations were about 75 ng/ml. Single-dose pharmacokinetic analysis revealed a serum terminal half-life of 1.2-1.5 hr for each drug, with no sex differences. Despite the fact that olanzapine and clozapine promote weight gain in humans, these drugs appear to have minimal effects on body weight and food intake in rats, except for a modest effect of olanzapine in female rats, even though therapeutic levels of olanzapine are achieved in serum during chronic infusion. Hence, the rapid clearance of drug following single administration in previous studies cannot explain the weak or absent effects of antipsychotics on weight gain in this species. The rat thus appears to be an inadequate model of weight gain produced by some antipsychotics in humans.
Keywords: olanzapine, clozapine, rat, body weight, food intake, pharmacokinetics
Introduction
Atypical antipsychotics offer significant clinical advantages over first-generation antipsychotics (Freedman, 2003). However, many of these drugs, notably olanzapine and clozapine, produce the side-effect of chronic weight gain, which is a cause for concern, because of the serious co-morbidities associated with obesity (Allison et al., 1999;Ganguli, 1999). The mechanism(s) by which atypical antipsychotics cause this unwanted side-effect is (are) presently unknown, in part because no animal model exists that reliably reproduces the phenomenon observed in humans. That is, while studies exist showing that weight gain can occur in rats given repeated daily injections of one or another atypical antipsychotic, the potency of the effect does not vary from drug to drug in the same manner as that seen in humans (Baptista et al., 1987;Allison et al., 1999), and when observed, weight gain is usually restricted to females, something not typically seen in humans (Ganguli, 1999;Bhana et al., 2001;Frankenburg et al., 1998;de Leon et al., 2007).
One possible reason for the variable ability of atypical antipsychotics to cause weight gain in rats might be related to the different pharmacokinetics of these drugs in rats and humans, and the difference in the manner in which they are administered. Humans typically take antipsychotics once or twice a day, in keeping with the fact that the drug half-lives are fairly long (e.g., olanzapine, 30 hr; clozapine, 14 hr) (Callaghan et al., 1999;Byerly and Devane, 1996;Bhana et al., 2001;Schulte, 2003). Rats have also been given these drugs once or twice a day, by injection or intubation, but the half-lives are considerably shorter (1-3 hr) (Baldessarini et al., 1993;Chiu and Franklin, 1996). Hence, blood drug levels no doubt fall to very low values soon after injections (these have rarely been measured), and steady-state therapeutic levels are undoubtedly never achieved (therapeutic levels by human standards). This scenario of low chronic drug exposure may explain why current rodent models do not faithfully reproduce effects seen in humans.
One way to bypass this pharmacokinetic difficulty is to administer drug by constant infusion, at a rate that allows blood drug levels to rise chronically into the therapeutic window for humans. We have examined this issue, using the two atypical antipsychotics most associated with chronic weight gain in humans, olanzapine and clozapine (Allison and Casey, 2001;Ganguli, 1999). Drug was administered chronically using surgically-implanted minipumps.
Materials and Methods
Sixty day-old (adult) male and female Sprague-Dawley rats (315-345g [males], 275-300g [females], Hilltop Laboratories, Scottdale, PA) were housed singly and given free access to food (Purina Rodent Laboratory Chow 5001, Purina Mills, St Louis MO) and water. They were exposed to 12 hr of light daily (lights off 1100-2300 hr), and an ambient temperature of 22 °C. For all experiments, animals were allowed to adapt to our housing facility for at least one week prior to experimentation. All experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
In chronic minipump studies, males and females were briefly anesthetized with isoflurane and implanted either subcutaneously (SC) between the shoulder blades or intraperitoneally (IP) with a 14-day osmotic mini-pump (Alzet model 2002, Durect Corp, Cupertino CA). The pumps contained olanzapine (5 mg/kg/day, dissolved in vehicle; a gift of Eli Lily, Indianapolis IN), clozapine (10 mg/kg/day, dissolved in vehicle; Sigma-Aldrich Corp, St. Louis MO) or vehicle (1 M lactic acid, 2.5% dimethyl sulfoxide [DMSO]). Experiments with olanzapine and clozapine were performed separately, each with a vehicle group (n = 7/group). Food consumption and body weights were measured daily, just prior to the onset of darkness. Daily food intake was calculated as the difference in the amount of food placed in the hopper and that remaining 24 hr later, less the amount recovered as spillage. After 11 days, all rats were killed by decapitation, and trunk blood collected and spun (2000 rpm for 20 minutes, 4 °C) to isolate serum. The experiments were terminated at 11 days, to allow measurement of circulating drug levels at a time when blood levels would still be at steady-state. In olanzapine studies, 10 μ1/ml serum of 25% ascorbic acid in water was added to each sample (Olesen and Linnet, 1998); in clozapine studies, no additions were made to serum samples. Sera were frozen at −80 °C until analyzed by HPLC.
In pharmacokinetic studies, groups of male and female rats (n = 6/group) received olanzapine (5 mg/kg IP) or clozapine (10 mg/kg IP), and were killed 15, 30, 60, 180 or 420 minutes later. Sera were prepared and stored as above.
Serum samples were assayed for olanzapine and clozapine following extraction. For the olanzapine assay (Olesen and Linnet, 1998), an internal standard (trifluoperazine; Sigma Aldrich Corp.) was added, the pH was adjusted with NaOH, and the samples were extracted with heptane:isoamylalcohol. Following centrifugation, the heptane:isoamylalcohol layer was removed, and dried under a nitrogen stream. The residue was reconstituted in a small volume of mobile phase, and an aliquot was injected onto the HPLC. The HPLC consisted of a Waters 510 pump, a Waters Spherisorb S5W column (4.6 × 150 mm), and a Waters 441 Absorbance Detector (254 nm filter; Waters Corp, Milford MA). The mobile phase was 50 mM ammonium acetate (pH 9.9) in methanol (15:85; v:v), run at 1 ml/min at room temperature (Olesen and Linnet, 1998). For the clozapine assay, an internal standard (triprolidine; Sigma Aldrich Corp) was added to serum samples, which were then extracted with ethyl acetate. Following centrifugation, the ethyl acetate was removed and extracted with a small volume of 0.1 M HCl (Volpicelli et al., 1993). Following centrifugation, the aqueous layer was removed, and an aliquot injected directly onto the HPLC. The HPLC system was the same as that described above, but employed a Spherisorb-C6, 4.6 × 250 mm, 5 μm column (Waters Corp). The mobile phase was: 450 ml acetonitrile (Fisher Scientific, Pittsburgh PA), 550 ml 30 mM KH2PO4 (Sigma Aldrich Corp), 2 g hexane sulfonic acid (Sigma Aldrich Corp), adjusted to pH 2.7 with phosphoric acid (Volpicelli et al., 1993), and was run at 1.5 ml/min and 30°C. The column stream was monitored by the absorbance detector at 254 nm (Volpicelli et al., 1993;Baldessarini et al., 1993).
Data are presented as means ± standard errors of the mean. They were analyzed statistically by analysis of covariance and analysis of variance (repeated measures, when appropriate). Individual post hoc comparisons were evaluated using the Scheffé test, following 1-way ANOVA (non-repeated measures) (StatView, SAS Institute, Inc; Carey, NC). The serum drug data from the pharmacokinetic studies were subjected to noncompartmental analysis to estimate terminal half-lives (Winnonlin, v. 4.1; Pharsight Corporation, Mountain View CA).
Results
Olanzapine
In female, but not male rats, chronic infusion by minipump increased food intake and body weight, compared to placebo. In females, significant main effects of treatment (P < 0.001) and time (P < 0.001) were noted on food intake in groups receiving olanzapine (5 mg/kg/day) or vehicle SC by minipump for 11 days; the interaction term did not quite reach statistical significance (P = 0.07; repeated measures ANOVA; figure 1A). A significant effect of time (P < 0.001), but not treatment (olanzapine, vehicle; P = 0.29), was also found on body weight; the interaction term was highly significant (P < 0.001; figure 1C). In males, a significant main effect of time (P < 0.001), but not treatment (P = 0.19) was noted on food intake in groups receiving olanzapine (5 mg/kg/day) or vehicle SC; the interaction term was not significant (P = 0.41; repeated measures ANOVA; figure 1B). A significant effect of time (P < 0.001), but not treatment (P = 0.46), was also found on body weight; the interaction term was not significant (P = 0.90; figure 1D). Olanzapine concentrations, measured in serum samples collected at the conclusion of the study (day 11), were 38.4 ± 8.97 in females, and 30.02 ± 5.83 ng/ml in males implanted SC with olanzapine-containing pumps (not significantly different, P = 0.41, ANOVA).
Figure 1.
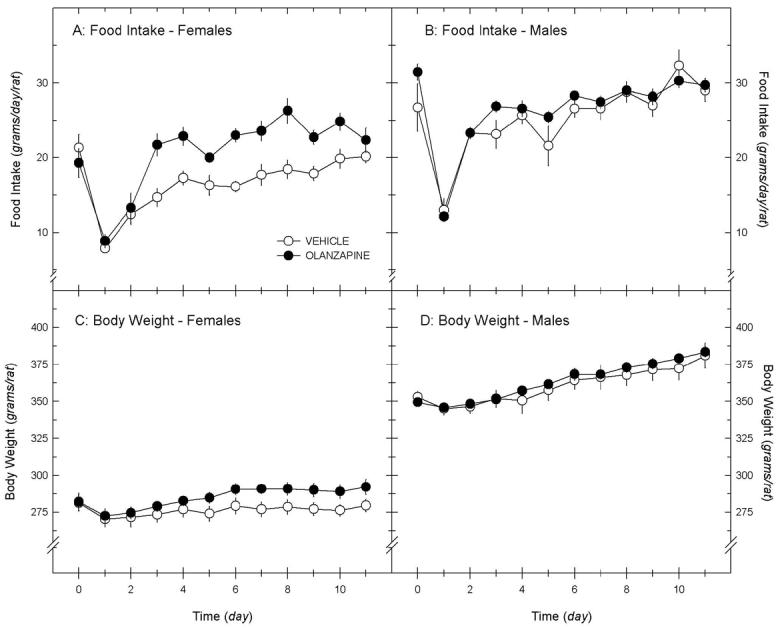
Daily food intake (panels A & B) and body weight (C & D) for female and male rats implanted subcutaneously with osmotic mini-pumps containing olanzapine (5 mg/kg/day, black circles) or vehicle (white circles). Mini-pumps were inserted on day 0. Data are means ± sem (n = 7/group).
A study of similar design, in which the pumps were inserted into the peritoneal cavity, produced similar results: relative to vehicle, females receiving olanzapine (5 mg/kg/day) showed a significant increase in body weight (Δ weight (grams/11 days): vehicle, 14.7 ± 2.4; olanzapine, 37.7 ± 3.6; P < 0.01) and an almost significant increase in food intake (cumulative intake (grams/11 days): vehicle, 150 ± 10; olanzapine, 193 ± 13, P = 0.07), while males showed no increase in body weight (Δ bodyweight (grams/11 days): vehicle, 26.2 ± 2.6; olanzapine, 23.9 ± 2.5, P = 0.54) or food intake (cumulative food intake (grams/11 days): vehicle, 206 ± 17; olanzapine, 225 ± 9, P = 0.41). In this study, serum olanzapine levels were about half those obtained when pumps were implanted subcutaneously (17.0 ± 1.9 ng/ml, females; 18.7 ± 2.9 ng/ml, males).
A pharmacokinetic study was conducted to ascertain if male and female rats eliminated olanzapine at similar rates. Following acute injection, the terminal half-life for the drug was found to be similar in males (T1/2= 1.12 hr) and females (T1/2= 1.39 hr; figure 2A).
Figure 2.
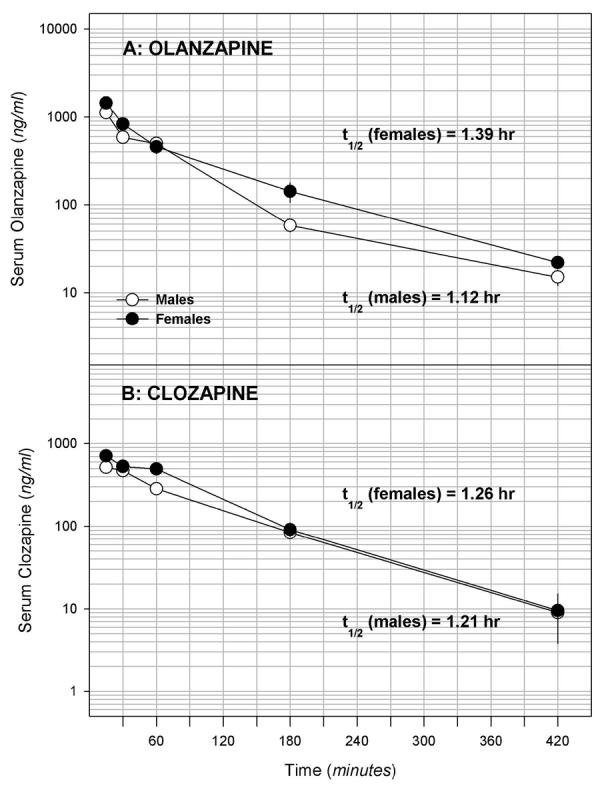
Changes in serum concentrations of olanzapine (A; 5 mg/kg) and clozapine (B; 10 mg/kg) following intraperitoneal administration to male and female rats. The data were used to calculate terminal half-lives for each drug. Females, black circles; males, white circles. Data are means ± sem (n = 6/group).
Clozapine
Chronic clozapine infusion failed to modify food intake or body weight significantly in female or male rats, compared to placebo. In females, a significant main effect of time (P < 0.001) was noted on food intake in groups receiving clozapine (10 mg/kg/day) or vehicle SC by minipump for 11 days; the treatment effect was borderline significant (P = 0.055), while the interaction term was not significant (P = 0.80; repeated measures ANOVA; figure 2A). A significant effect of time (P < 0.001), but not treatment (clozapine, vehicle; P = 0.96), was found on body weight; the interaction term was not significant (P = 0.98; figure 2C). In males, a significant main effect of time (P < 0.001), but not treatment (P = 0.33) was noted on food intake in groups receiving clozapine (10 mg/kg/day) or vehicle; the interaction term was not significant (P = 0.93; figure 2B). A significant effect of time (P < 0.001), but not treatment (P = 0.63), was also found on body weight; the interaction term was not significant (P = 1.00; figure 2D). Clozapine concentrations, measured in serum samples collected at the conclusion of the study (day 11), were 84.7 ± 10.0 ng/ml in female rats, and 69.4 ± 18.2 ng/ml in male rats implanted sc with clozapine-containing pumps (sex difference not significant, P = 0.44, ANOVA). These values are well below therapeutic levels needed for clinical efficacy in humans (350 ng/ml) (Greenwood-Smith et al., 2003;Schulte, 2003). Attempts to increase the dose of drug delivered by the mini-pumps were not successful, because of the limited solubility of the drug, and the need to limit pump size (larger pumps proved uncomfortable for the animal).
A study of similar design, in which the pumps were inserted into the peritoneal cavity, revealed insignificant effects of treatment and time on food intake and body weight in both female and male rats (data not shown). In this study, serum clozapine levels were about half those obtained when pumps were implanted subcutaneously (33 ± 5 ng/ml, females; 31 ± 5 ng/ml, males).
A pharmacokinetic study was conducted to ascertain if male and female rats eliminated olanzapine at similar rates. Following acute injection, the terminal half-life for the drug was found to be similar in males (T1/2= 1.21 hr) and females (T1/2= 1.26 hr; figure 2B).
Discussion
This study demonstrates that continuous exposure of rats to atypical antipsychotics via implanted mini-pumps does not increase food intake and body weight in a manner like that seen in humans. It also shows that the vagary of effects in rats cannot consistently be explained by varying exposure to drug. Hence, in olanzapine studies, chronic serum drug concentrations exceeded the therapeutic threshold for humans. But, food intake and body weight increases were seen only in female rats, despite the fact the serum olanzapine concentrations were comparable and the terminal half-life of drug elimination was almost the same in males and females. In clozapine studies, no significant effects on food intake or body weight were observed in male or female rats. But, serum drug concentrations did not reach the therapeutic threshold in human blood, perhaps explaining the absence of effects.
Since antipsychotics promote weight gain in humans, attempts have been made to develop a model in rats, to explore for underlying mechanisms. While some investigators have found chronic effects of such drugs on food intake and body weight, positive responses have typically been seen in female, but not male rats (e.g., (Baptista et al., 1998;Baptista et al., 1987)). Among atypical antipsychotics, clozapine, the most potent promoter of weight gain in humans (Allison et al., 1999;Ganguli, 1999), causes no weight gain in male or female rats, and sometimes weight loss in males (Baptista et al., 1993;Albaugh et al., 2006). In most hands, olanzapine promotes weight gain in female, but not male rats (though occasionally weight gain (Minet-Ringuet et al., 2006b) or loss (Cooper et al., 2006) has been reported in males), when administered chronically by injection (Goudie et al., 2002;Pouzet et al., 2003;Arjona et al., 2004;Minet-Ringuet et al., 2006a). Food intake sometimes, but not always, parallels changes in body weight (Pouzet et al., 2003;Baptista et al., 1998;Arjona et al., 2004;Minet-Ringuet et al., 2006b;Cooper et al., 2006).
An explanation for this variability is lacking. One possible source is the manner of drug administration, taking drug half-lives into consideration. Whereas the half-lives of antipsychotic drugs are fairly long in humans, allowing drug to be given once or twice daily to achieve therapeutic exposure (Callaghan et al., 1999;Byerly and Devane, 1996;Bhana et al., 2001;Schulte, 2003), they are quite short in rats, on the order of a few hours ((Baldessarini et al., 1993;Chiu and Franklin, 1996); see also figure 2). Hence, the method of delivery to rats could dramatically influence drug exposure. In rat studies, the route of administration has been intraperitoneal, subcutaneous, and oral (Baptista et al., 1987), given once or twice a day (separated by different periods of time (Cooper et al., 2006;Goudie et al., 2002;Pouzet et al., 2003)), given over 30 minutes each day in a food vehicle (cookie dough) (Albaugh et al., 2006), continually as a component of food (Minet-Ringuet et al., 2006b;Minet-Ringuet et al., 2006a), or continuously by implanted mini-pump ((Benvenga, 2002); abstract only). This variety could reasonably be expected to influence drug exposure. For example, including drug in the rat's food would not afford constant exposure, since rats are nocturnal eaters. Drug intake during daylight hours would therefore be minimal (e.g., see table 2 in (Minet-Ringuet et al., 2006b)). One or two injections each day would also provide variable exposure to drug. Such can be seen in the study of Pouzet et al. (Pouzet et al., 2003), in which oral administration of olanzapine at 2.5 mg/kg twice daily produced peak serum olanzapine concentrations soon after each dosing, followed by a decline to low values between 4 and 7 hr. Hence, with intermittent administration of drug, blood levels presumably achieve values sufficient to stimulate food intake only during a portion of the 24-hr period, perhaps permitting compensatory changes in food intake at other times of day, when circulating drug levels are low. More generally, intermittent exposure of the animal to drug requires that care be taken to administer drug with sufficient frequency to insure that circulating drug concentrations exceed a therapeutic threshold at all times (e.g., about 20 ng/ml OLAN and 350 ng/ml CLOZ in humans, (Greenwood-Smith et al., 2003;Perry et al., 2001)), while at the same time not producing peak drug levels sufficiently high to induce adverse effects that might negatively affect food intake (e.g., reduced locomotor activity, suggestive of malaise or sedation (Arjona et al., 2004). Such appears to have occurred in the Pouzet study, when olanzapine was given at a high dose (10 mg/kg twice daily) (Pouzet et al., 2003).
The problem of short half-life is eliminated, if the drug is administered by constant infusion via minipump. In the present studies, using this approach, we observed that female, but not male rats, gained weight on olanzapine, dosed at 5 mg/kg/day via subcutaneously implanted mini-pump. Steady-state blood olanzapine concentrations ranged between 30 and 40 ng/ml in both males and females, values that are above the therapeutic threshold concentration in humans (Perry et al., 2001). When the pump was implanted into the peritoneal cavity, the same effect was observed on weight gain (increase in females, no change in males) at the same daily dose, but serum olanzapine concentrations were around 20 ng/ml, just at the human threshold concentration (the lower blood concentration with peritoneal infusion probably results from direct blood drainage through the liver, a principal site of olanzapine metabolism in the body (Bhana et al., 2001)). A preliminary study was also conducted using a higher dose of olanzapine (10 mg/kg/day in females; 7 mg/kg/day in males). Serum olanzapine concentrations approached 100 ng/ml in both sexes; males receiving olanzapine lost body weight at this dose, while females showed minimal gain, relative to animals receiving the vehicle (i.e., the weight-promoting effect seen at 5 mg/kg/day was not present at a higher dose).
These findings suggest, therefore, that when female, but not male, rats are chronically and continuously exposed to a blood olanzapine concentration of 20-40 ng/ml, which is in the therapeutic blood concentration range for humans, they will eat more food and gain weight. But this sex difference in rats does not occur in humans, since both men and women gain weight on olanzapine (Bhana et al., 2001;Ganguli, 1999). Indeed, in one large study, men gained more weight than women (Basson et al., 2001). It is useful to note that treated female patients have higher circulating olanzapine concentrations than male patients (even when dose is corrected for the body mass difference), and a longer drug elimination time for the drug than males (Callaghan et al., 1999;Bhana et al., 2001;Weiss et al., 2005;Aichhorn et al., 2006). A lower exposure to drug thus cannot not explain why women do not gain more weight than men. In rats, chronic blood olanzapine concentrations did not differ between males and females, nor did the drug elimination half-life. Hence, drug exposure also cannot explain why female rats gained weight, while males did not. Overall, therefore, it appears that olanzapine-induced weight-gain, as seen in humans, is not reliably reflected in the rat.
Clozapine, administered at 10 mg/kg/day, did not influence food intake or body weight (relative to vehicle) in either male or female rats (figure 3). The absence of effect is consistent with findings using non-infusion models (Albaugh et al., 2006;Baptista et al., 1993;Benvenga, 2002). Serum clozapine levels in the present study were around 75 ng/ml, a value well below therapeutic serum concentrations in humans (350-400 ng/ml; (Schulte, 2003)). While the low blood level might explain the absence of an increase in food intake and body weight, the findings of Benvenga (Benvenga, 2002) may suggest otherwise: in his study, a dose of about 12 mg/kg/day, also administered by mini-pump, was not associated with weight gain. Moreover, a dose of about 6 mg/kg day elicited an increase in body weight, though the effect did not reach statistical significance. This finding suggests that doses higher than 12 mg/kg may suppress weight gain (relative to vehicle; a result observed by others at 20 mg/kg using a single daily injection paradigm (Baptista et al., 1993)). Because of this finding, and the difficulty of solubilizing clozapine at the concentrations needed for the mini-pump to raise serum clozapine values several-fold higher than that obtained in the present studies (to reach the human therapeutic range in serum), we did not pursue clozapine studies further.
Figure 3.
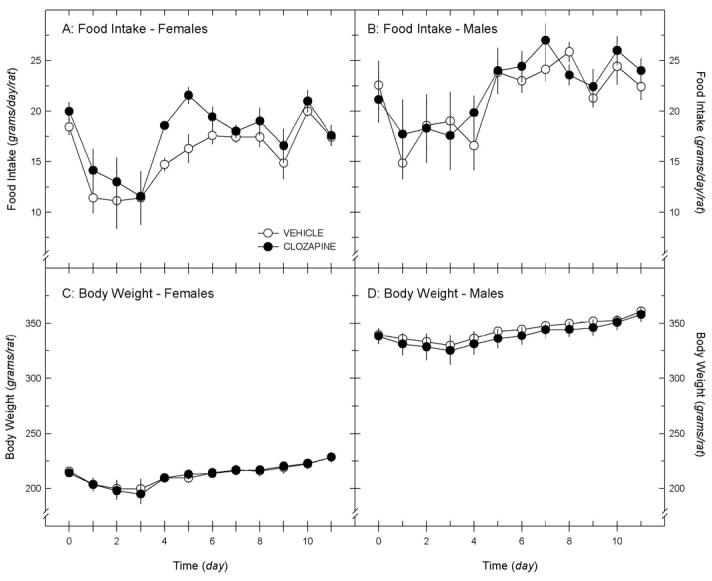
Daily food intake (A & B) and body weight (C & D) for female and male rats implanted subcutaneously with osmotic mini-pumps containing clozapine (10 mg/kg/day); black circles) or vehicle (white circles). Mini-pumps were inserted on day 0. Data are means ± sem (n = 7/group).
In sum, the olanzapine results show that chronic administration by mini-pump places serum drug levels above the therapeutic threshold in both male and female rats, but produces an increase in food intake and body weight only in female rats. Such findings suggest that rats are not a useful human model for olanzapine-induced weight gain, since male humans gain weight on these drugs. The clozapine results show that the therapeutic threshold in serum drug level is unlikely to be reached using a mini-pump model: the levels achieved in this study were < 25% of the human therapeutic threshold, perhaps explaining why rats did not eat more or gain weight. However, delivering higher clozapine doses to rats to produce higher serum drug concentrations appears to suppress food intake and body weight, suggesting that the rat is also a poor model for clozapine-induced weight gain in humans. To date, studies in rats have employed diets that are not highly palatable (commercial rodent diets). Conceivably, the use of highly-palatable diets might improve the likelihood of observing increases in food intake and body weight in male rats treated with olanzapine. It's unclear if such would occur for clozapine, however, for either sex, given the need to use higher doses to achieve higher blood levels, and the emergence of adverse effects with increasing dose. Hence, at present, the bulk of evidence indicates that the rat is not a good model for examining antipsychotic-induced weight gain.
Acknowledgements
Supported by the National Institutes of Health (MH067815). The authors wish to thank George R. Rudloph and James M. Perel for assistance in the drug terminal half-life calculations, and Eli Lilly and Company for supplying olanzapine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aichhorn W, Whitworth AB, Weiss EM, Marksteiner J. Second-generation antipsychotics: is there evidence for sex differences in pharmacokinetic and adverse effect profiles? Drug Safety. 2006;29:587–598. doi: 10.2165/00002018-200629070-00004. [DOI] [PubMed] [Google Scholar]
- Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, Lynch CJ. Hormonal and Metabolic Effects of Olanzapine and Clozapine Related to Body Weight in Rodents. Obesity. 2006;14:36–51. doi: 10.1038/oby.2006.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J.Clin.Psychiat. 2001;62(Suppl 7):22–31. [PubMed] [Google Scholar]
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am.J.Psychiat. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Arjona AA, Zhang SX, Adamson B, Wurtman RJ. An animal model of antipsychotic-induced weight gain. Behav.Brain.Res. 2004;152:121–127. doi: 10.1016/j.bbr.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Centorrino F, Flood JG, Volpicelli SA, Huston-Lyons D, Cohen BM. Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology. 1993;9:117–124. doi: 10.1038/npp.1993.50. [DOI] [PubMed] [Google Scholar]
- Baptista T, Contreras Q, Teneud L, Albornoz MA, Acosta A, Paez X, de Quijada M, Lacruz A, Hernandez L. Mechanism of the neuroleptic-induced obesity in female rats. Prog.Neuropsychopharmacol.Biol.Psychiat. 1998;22:187–198. doi: 10.1016/s0278-5846(97)00101-2. [DOI] [PubMed] [Google Scholar]
- Baptista T, Mata A, Teneud L, de Quijada M, Han HW, Hernandez L. Effects of long-term administration of clozapine on body weight and food intake in rats. Pharmacol.Biochem.Behav. 1993;45:51–54. doi: 10.1016/0091-3057(93)90084-7. [DOI] [PubMed] [Google Scholar]
- Baptista T, Parada M, Hernandez L. Long term administration of some antipsychotic drugs increases body weight and feeding in rats. Are D2 dopamine receptors involved? Pharmacol.Biochem.Behav. 1987;27:399–405. doi: 10.1016/0091-3057(87)90340-6. [DOI] [PubMed] [Google Scholar]
- Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J.Clin.Psychiat. 2001;62:231–238. doi: 10.4088/jcp.v62n0404. [DOI] [PubMed] [Google Scholar]
- Benvenga MJ. Can clinical weight gain produced by atypical antipsychotic agents be modelled in the rat? Society for Neuroscience; Washington DC: 2002. Abstract 707.4. [Google Scholar]
- Bhana N, Foster RH, Olney R, Plosker GL. Olanzapine: an updated review of its use in the management of schizophrenia. Drugs. 2001;61:111–161. doi: 10.2165/00003495-200161010-00011. [DOI] [PubMed] [Google Scholar]
- Byerly MJ, Devane CL. Pharmacokinetics of clozapine and risperidone: A review of recent literature. J.Clin.Psychopharmacol. 1996;16:177–187. doi: 10.1097/00004714-199604000-00010. [DOI] [PubMed] [Google Scholar]
- Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine: pharmacokinetic and pharmacodynamic profile. Clin.Pharmacokinet. 1999;37:177–193. doi: 10.2165/00003088-199937030-00001. [DOI] [PubMed] [Google Scholar]
- Chiu JA, Franklin RB. Analysis and pharmacokinetics of olanzapine (LY170053) and two metabolites in rat plasma using reversed-phase HPLC with electrochemical detection. J.Pharm.Biomed.Anal. 1996;14:609–615. doi: 10.1016/0731-7085(95)01651-1. [DOI] [PubMed] [Google Scholar]
- Cooper GD, Pickavance LC, Wilding JP, Harrold JA, Halford G, Goudie AJ. Effects of olanzapine in male rats: enhanced adiposity in the absence of hyperphagia, weight gain or metabolic abnormalities. J.Psychopharm. 2006;21:405–413. doi: 10.1177/0269881106069637. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, Josiassen RC, Cooper TB, Simpson GM. Weight gain during a double-blind multidosage clozapine study. J.Clin.Psychopharmacol. 2007;27:22–27. doi: 10.1097/JCP.0b013e31802e513a. [DOI] [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC, Kando J, Centorrino F. Clozapine and body mass change. Biol.Psychiat. 1998;43:520–524. doi: 10.1016/S0006-3223(97)00488-5. [DOI] [PubMed] [Google Scholar]
- Freedman R. Schizophrenia. New Engl.J.Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- Ganguli R. Weight gain associated with antipsychotic drugs. J.Clin.Psychiat. 1999;60(Suppl 21):20–24. [PubMed] [Google Scholar]
- Goudie AJ, Smith J, Halford J. Characterization of olanzapine-induced weight gain in rats. J.Psychopharm. 2002;16:291–296. doi: 10.1177/026988110201600402. [DOI] [PubMed] [Google Scholar]
- Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: a review of their clinical utility. J.Psychopharm. 2003;17:234–238. doi: 10.1177/0269881103017002014. [DOI] [PubMed] [Google Scholar]
- Minet-Ringuet J, Even PC, Goubern M, Tome D, deBeaurepaire R. Long term treatment with olanzapine mixed with the food in male rats induces body fat deposition with no increase in body weight and no thermogenic alteration. Appetite. 2006a;46:254–262. doi: 10.1016/j.appet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Minet-Ringuet J, Even PC, Lacroix M, Tome D, deBeaurepaire R. A model for antipsychotic-induced obesity in the male rat. Psychopharmacology. 2006b;187:447–454. doi: 10.1007/s00213-006-0433-0. [DOI] [PubMed] [Google Scholar]
- Olesen OV, Linnet K. Determination of olanzapine in serum by high-performance liquid chromatography using ultraviolet detection considering the easy oxidability of the compound and the presence of other psychotropic drugs. J.Chromatog.B. 1998;714:309–315. doi: 10.1016/s0378-4347(98)00205-9. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Lund BC, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response: acute phase results of the North American Olanzapine Trial. J.Clin.Psychopharmacol. 2001;21:14–20. doi: 10.1097/00004714-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Mow T, Kreilgard M, Velschow S. Chronic treatment with antipsychotics in rats as a model for antipsychotic-induced weight gain in human. Pharmacol.Biochem.Behav. 2003;75:133–140. doi: 10.1016/s0091-3057(03)00042-x. [DOI] [PubMed] [Google Scholar]
- Schulte P. What is an adequate trial with clozapine?: therapeutic drug monitoring and time to response in treatment-refractory schizophrenia. Clin.Pharmacokinet. 2003;42:607–618. doi: 10.2165/00003088-200342070-00001. [DOI] [PubMed] [Google Scholar]
- Volpicelli SA, Centorrino F, Puopolo PR, Kando J, Frankenburg FR, Baldessarini RJ, Flood JG. Determination of clozapine, norclozapine, and clozapine-N-oxide in serum by liquid chromatography. Clin.Chem. 1993;39:1656–1659. [PubMed] [Google Scholar]
- Weiss U, Marksteiner J, Kemmler G, Saria A, Aichhorn W. Effects of age and sex on olanzapine plasma concentrations. J.Clin.Psychopharmacol. 2005;25:570–574. doi: 10.1097/01.jcp.0000185427.08268.db. [DOI] [PubMed] [Google Scholar]


