Abstract
Purpose
To determine whether the mechanical stretching renders modulation of the peptidyl arginine deiminase 2 (PAD2) expression in cultured astrocytes.
Methods
Isolated rat brain astrocytes were subjected to mechanical stretching using a glass bead set-up and polyethylene set-up with or without immobilization. Activity assays and ELISA were performed to detect PAD2 expression. Protein deimination levels in the cells were measured using an anti-citrulline ELISA. PAD2 expression in cells subjected to mechanical stretching was compared with controls. Astrocytes were also subjected to pressure treatment and compared to control cells for PAD2 expression and deimination levels.
Results
Astrocytes subjected to mechanical stretching with or without immobilization showed elevated PAD2 expression. Pressure treatment of astrocytes (25–100 mmHg) also resulted in elevated PAD2 expression and deimination.
Conclusion
These results suggest mechanical stretching induced PAD2 expression and consequent protein deimination.
Keywords: astrocyte, citrullination, collagen, deimination, immobilization, mechanical stretching, peptidyl arginine deiminase 2, riboflavin, UV cross-linking
INTRODUCTION
Glaucoma is a group of late-onset and progressive, irreversible blinding diseases usually with onset at the third decade of life.1 New approaches of microarray2–5 and proteomic analyses have been applied to glaucomatous tissues6,7 in order to obtain information in a reagent (such as antibodies) independent manner. Proteomic mass spectrometric analyses of optic nerve identified peptidyl arginine deiminase 2 (PAD2), and, subsequently, elevated presence of its catalytic product, protein-bound citrulline, was also found in the glaucomatous optic nerve.6 The process of generation of protein-bound citrulline from arginines catalyzed by peptidyl arginine deiminases (PADs) is referred to as deimination or, often interchangeably, as citrullination.8 The PADs do not catalyze conversion of free arginines. The elevation of PAD2 in neurodegenerative diseases has been shown to be due to aberrant regulation both at the level of transcription9 and translation.6 The presence of PAD2 mRNA has been shown in astrocytes10,11 and in oligodendrocytes.12 We have previously shown elevation of PAD2 and consequent deimination in isolated astrocytes using pressure chambers.6,13 The pressure chamber model with astrocytes has been used in a number of previous studies.6,14–17 However, this model may reflect a mixed effect of hypoxia and changes in hydrogen ion concentration in addition to incremental small pressure changes.13 Cells immobilized on a surface as it occurs in the ocular optic nerve tissue in the eye are more likely to experience mechanical stretching in response to incremental changes in pressure. In this investigation we have utilized two different mechanical stretching methods, including one with immobilized astrocytes to determine whether stretching induces PAD2 expression and results in protein deimination.
MATERIALS AND METHODS
Primary Astrocyte Cultures
Astrocytes were derived from Sprague Dawley rats (Harlan, Indianapolis, IN, USA) brain cortex and were prepared as described previously.13 Briefly, mixed glial cell suspensions were prepared from the P3 rat cortex regions following established procedures18 from which enriched glial fibrillary acidic protein (GFAP) and neural cell adhesion molecule (NCAM) positive cells were obtained using immunopanning.19 A set of experiments was also performed with human optic nerve head astrocytes from 7- to 12-year old male Caucasian donors. Eight donor eyes were received from regional eye banks within 24–36 hours of death, and the optic nerve head (ONH) to lamina cribrosa (LC) was dissected from the remaining ocular tissue. ONH and LC tissue from each donor eye wasere cut into four explants and placed in culture plates containing DMEM plus 8% fetal bovine serum (FBS; Gibco Laboratories, CA, USA). Cells were then cultured in Ham’s F-10 medium with 8% FBS and passaged with a 0.25% trypsin solution and subsequently plated in serum-free astrocyte growth medium (AGM).20 After 24 hr in culture, the medium was changed to AGM containing 5% FBS. The majority of the cells that attached in serum-free medium were astrocytes. A sequential panning procedure was adopted to further purify the astrocytes. Briefly, the suspension of primary cells was first panned on a Petri dish coated with C5 anti-neuroepithelial monoclonal antibody. Subsequently, the nonadherent cells from the C5 dish were passed onto a dish coated with the anti-Thy1 monoclonal antibody to deplete microglia, fibroblasts, and meningeal cells. The nonadherent cells from the Thy1 dish were discarded.19 The purity of the culture was assessed by GFAP immunostaining.
Mechanical Stretching with or without Immobilization
Astrocytes were subjected to mechanical stretching using two different methods. In contrast to the first method (glass bead set-up), the second method (polyethylene membrane set-up) did involve immobilization of cells prior to mechanical stretching, as described below.
In the first method (glass bead set-up), when cells attained a confluency of about 80%, they were placed in serum-free medium for 24 hr before and during stretching experiments. To apply mechanical stretch, as described in previous reports for trabecular meshwork cells, a smooth, 5.25-mm glass bead was placed in the dish beneath the center of the insert membrane. A weight was then applied to the lid of the plate, which forced the insert down onto the bead.31,32 This process created a defined upward distortion of the membrane (maximum 10% stretch), which increased the surface area and produced mechanical stretching of the cells.
In the second mechanical stretching method (polyethylene membrane set up), astrocytes were immobilized on a polyethylene membrane in an approach similar to that performed previously for enzymatic proteins21 but using a different immobilization method. Briefly, a polyethylene membrane (Birla Textiles Ltd., Amritsar, India) was coated with purified collagen (Sigma Chemical Co., St. Louis, C9879–1G) solution on one surface (1–5mg protein per 5 sq cm) and subjected to microwave treatment for 10 seconds. The collagen coated surface of the polyethylene membrane was then spread or coated with riboflavin (vitamin B2; Sigma Chemical Co., St. Louis, R7649–25G) solution (100 μg/ml), subsequently about 5000 astrocytes were placed on the membrane and subjected to cross-linking using 365 nm UV light (hand held lamp) for 5–10 seconds. This immobilization method has been adopted from a technique used for cross-linking live cornea22,23. Astrocytes immobilization was performed due to the fact that in vivo they remain immobilized in the optic nerve. The polyethylene membrane bearing immobilized cells was bathed in AGM medium with 5% FBS such that two edges of the membrane remain outside the Petri dish. No loss in viability was found after immobilization when determined using Trypan blue exclusion method. The astrocytes with or without immobilization were subjected to mechanical stretching by pulling from both sides, as previously described21. The maximum stretch of 1mm was applied on both sides for a duration of 1 minute and relaxed for 1 minute was considered one cycle. The cells were maintained in a sterile and 5% CO2 environment under constant temperature (37°C), the polyethylene membrane was placed in a Petri dish during the entire course of reaction in serum free medium (AGM) with 5% FBS as described above.
Astrocytes and pressure treatment
As described in our previous report13, astrocytes were exposed to hydrostatic pressure for five hours15,24. The cells at a density of ~3–10 × 104 cells/well were plated in 4 cm plates and were incubated with serum free medium overnight. A closed pressurized chamber (5% carbon dioxide) equipped with a manometer (constructed at Washington University Instrument Machine Shop, St. Louis, MO) was used to subject the cells to elevated pressure. Cells were placed in the chamber and the pressure was elevated to 25–100 mm Hg and the chamber was placed in a tissue culture incubator at 37°C. The cells were incubated for 16 hours after pressure treatment. Control cells from identical passage of cell lines were simultaneously incubated in a tissue culture incubator at atmospheric pressure at 37°C. After incubation, cells were trypsinized and subjected to ELISA analyses for PAD2 and protein deimination as well as assay for PAD2 activity.
Assay of PAD2 enzyme
In vitro determination of PAD enzymatic activity25 was performed using a spectrophotometer. Purified PAD2 or astrocyte extract was added to the reaction mixture for initiation of the assay. Briefly, the reaction mixtures containing 100 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 2.5 mM DTT, 10 BAEE and an appropriate amount of cell lysates (5 μg/sample) in a final volume of 50 μl were incubated at 37°C for 3 h. The reaction of enzyme activity was stopped by adding 10 μl of 5 M perchloric acid and the perchloric acid-soluble fraction was subjected to 150 μl of carbidino detection reagent, which was assembled freshly from its two components with one of part A containing 0.5% diacetyl monoximine and 0.01% thiosemicarbazide added to two of parts B containing 0.25 mg of FeCl3/ml in 24.5% sulfuric acid (H2SO4) and 17% phosphoric acid (H3PO4). The reaction was mixed vigorously and heated at 95°C for 5 min. After heating, samples were cooled to room temperature and the absorbance was measured at 535 nm in an Agilent spectrophotometer. A solution of L-citrulline solution (10–100 mM) served as standard for quantification. Activity levels of 5 μg of BSA and recombinant purified PAD2 under identical conditions were used as 0 and 100 percent respectively.
Enzyme-Linked ImmunoSorbent Assay (ELISA)
About 5 μg of purified in vitro deiminated bovine serum albumin (BSA) or 10 μg of astrocyte lysate was centrifuged at 10000 × g for 10 minutes and clear solution was transferred and placed per well in a plate (Costar 9018 plate) and incubated for 20 min at room temperature. Recombinant cellular retinaldehyde binding protein (CRALBP) produced in E. coli was used as non-deiminated control. The recombinant CRALBP and in vitro fully deiminated BSA (5 μg) was used to set the level 0 and 100 respectively. Proteins were incubated with 40 μl/well of 1:1 reagent A and B from a citrulline kit (Millipore Corporation, Billerica, MA; 17–347) at 37°C for 90 min. The supernatant was discarded and the plate was washed with PBS. The plates were blocked with 1% Ovalbumin for 1 hour, washed with PBS add incubated for 1 hour with rabbit polyclonal antibody against modified citrulline. After subsequent washes with PBS, plates were incubated with the secondary antibody coupled with alkaline phosphatase for 1 hour, washed with PBS and incubated with phosphatase substrate (100 μl/well) in diethanolamine buffer pH 7.5. The absorbance was then measured at 405 nm on a plate reader. For detection of PAD2, the reagent A and B incubation step was omitted and a rabbit polyclonal antibody against PAD2 (Abcam Inc., CA, ab16478) was used for detection using an identical procedure. Purified BSA and recombinant purified PAD2 (5 μg) under identical conditions were used as 0 and 100 percent respectively. ELISA analyses results are expressed as mean ± standard deviation, statistical analysis was performed for each sample at every experimental point shown, compared to 0.0 using the two tailed one-sample t-test and was found significantly different from 0.0 at each time point by the one-sample t-test: *p < 0.05.
Western analyses
To confirm the presence of PAD2 and deiminated proteins detected using the ELISA analysis, a select set of cell lysates were subjected to analyses by immunoblotting. Astrocyte lysates were separated on a 4–20% tris-glycine gradient gel (Invitrogen Corporation, CA) and transferred onto Polyvinylidene fluoride (PVDF) membrane. Western analysis utilized established protocols26,27 and the antibodies (~5 μg/ml) detailed below were used. For these analyses previously described mouse monoclonal antibody (mAb) against PAD228,29 was used. Mouse mAbs for human glyceraldehyde dehydrogenase (GPDH) and GFAP were procured from Chemicon International and Sigma Chemical Co., St. Louis, MO. For detection of citrulline, the blots were treated with 2,3-butanedione monooxime and antipyrine in a strong acid atmosphere enabling chemical modification of citrulline into ureido groups which ensures detection of citrulline-containing proteins regardless of neighboring amino acid sequences30. Polyclonal antibody (pAb) to citrulline (Citrulline kit) procured from Millipore corporation was utilized to detect citrulline. This kit was also used in ELISA analyses for detection of citrulline described above.
Immunocytochemical analyses
For immunocytochemical analyses about 3–10 × 104 astrocytes were plated on 4 cm plates and were fixed with calcium acetate buffered 4% para-formaldehyde. Cover slips or polyethylene membranes were blocked with 2% BSA in phosphate buffered saline (PBS), then incubated with 20 ng mAb to PAD229 or GFAP for 2 hours at 4°C and subsequently with 10 ng Alexa 594 conjugated secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for one hour at room temperature. The nuclei were stained with DAPI. Sections were sealed with vectashield and were analyzed with a scanning confocal Leica TCS-SP5 AOBS microscope.
Results
Increased PAD2 expression in response to mechanical stretching
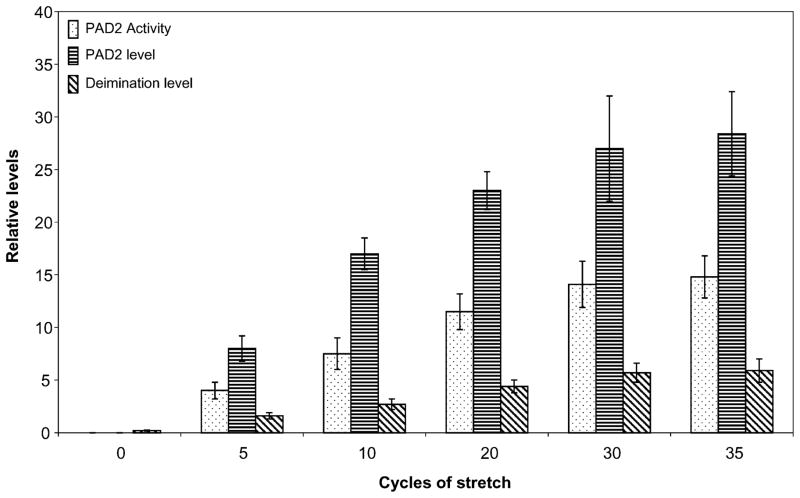
In our previous studies, when subjected to hydrostatic pressure, isolated astrocytes from P3 rat brain cortex were shown to express PAD2 and undergo protein deimination in a manner similar to that of human optic nerve derived astrocytes6,13. We subjected astrocytes to mechanical stretch or distortion that they are likely to experience in optic nerve head using a previously published glass bead set up31,32. We subjected the cells to multiple cycles of stretch because in vivo cells are subjected to dynamic mechanical stretch33 and also due to the fact that optic nerve head astrocytes experience IOP fluctuations which will be akin to multiple cycles of stretch rather than single or static stretch34. The application of pressure on the bead induced deformity on the insert on which cells rested. Repeated application of pressure on the bead for 1 minute with a relaxation period of 1 minute was considered one cycle. We found that a minimum of 5 cycles were necessary to result in detectable difference in PAD2 level, activity and change in level of deimination. Although, in the fresh isolated cells our assay method, found no detectable deiminase activity, albeit a very low level of protein deimination was present (Fig. 1). Between 5–20 stretch cycles, there was doubling of PAD2 level and activity for every doubling of stretch cycles. The PAD2 level commensurate with PAD2 activity and level of protein deimination increased with pressure up to 30 stretch cycles. The PAD2 level, activity and protein deimination showed very little increase between 30–35 cycles and almost reached a plateau between cycles 30–35 (Fig. 1). We also observed that increased mechanical stretching results in elevated PAD2 expression but not cell death. Trypan blue exclusion showed no cell death for application of repeated mechanical stretch up to even 70 cycles (data not shown).
Figure 1.
Mechanical stretching (glass bead set up) induced over-expression of PAD2 and consequent deimination in primary astrocytes. The astrocytes (5000 cells) were subjected stretch-cycles by application of pressure on the bead for number of cycles as indicated and subjected to analyses for PAD2 activity, expression of PAD2 (ELISA), and level of deimination (ELISA) assay as described in methods. PAD2 activity, PAD2 level and level of deimination are represented by bars with dots, bars with horizontal and bars with diagonal strips respectively. The results are standard deviation of three independent experiments.
Increased PAD2 expression in response to cycles mechanical stretching with or without immobilization of astrocytes
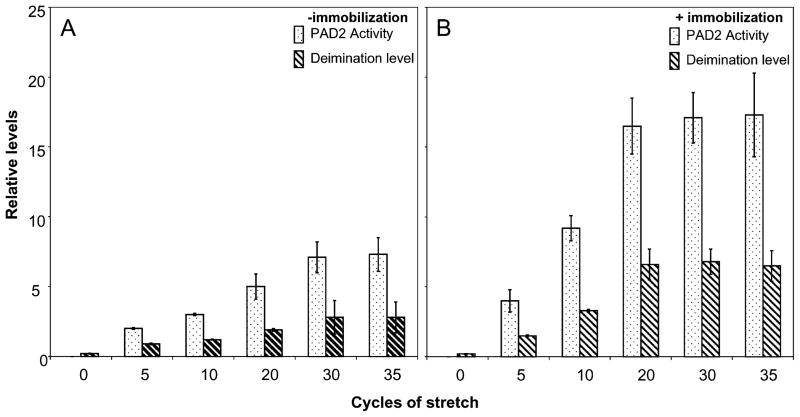
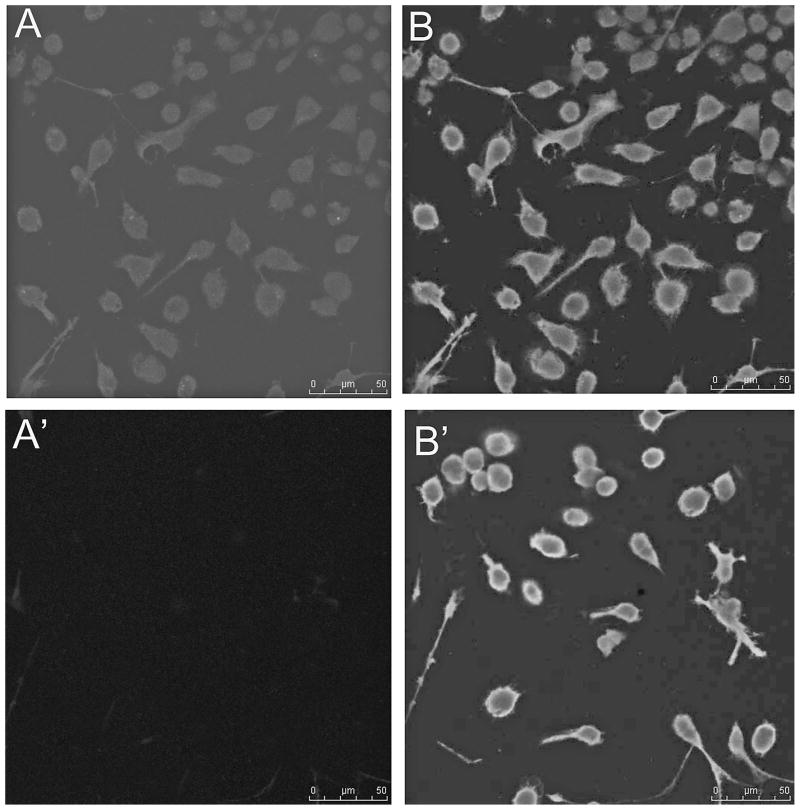
The astrocytes placed on a polyethylene membrane were subjected to mechanical stretch without (Fig. 2A) or with (Fig. 2B) immobilization. Without immobilization, the PAD2 activity and level of deimination follows a similar pattern comparable to that with mechanical stretch using the glass bead set up (Fig. 1), however, the PAD2 activity as well as level of protein deimination was substantially reduced compared to that attained with glass bead set up (Fig. 2A). Although there was doubling of PAD2 activity and deimination level for doubling in stretch cycles between cycles of 5–20 but the levels were much less than glass bead set up method (Figs. 1 and 2A). The levels attained a plateau between cycles 30–35 and again levels were much lower than glass bead set up. In contrast the levels reached a plateau beyond 20 cycles when the cells were immobilized and doubling of PAD2 activity and protein deimination level was observed between cycles 5–20 (Fig. 2B). We did not detect any appreciable alteration in PAD2 activity or protein deimination due to immobilization alone (data not shown). Western analysis demonstrated elevated PAD2 levels compared to controls for astrocytes subjected to mechanical stretching (Fig. 3) commensurate with the PAD2 activity (Figs. 1 and 2). The PAD2 level was relatively higher for immobilized astrocytes in polyethylene set up compared to astrocytes not subjected to immobilization or in glass bead set up. The astrocytes in polyethylene set up without immobilization showed relatively lowest PAD2 levels after being subjected to 35 cycles of stretching compared to all other astrocytes subjected to stretching (Fig. 3). Previously we have shown lack of immunocytochemical detection of PAD2 immunoreactivity in freshly isolated astrocytes6,13, however, repeated cycles of mechanical stretching alone was sufficient to cause over-expression of PAD2 (Fig. 4). The astrocytes showing elevated PAD2 (red fluorescence) also stained for GFAP (green fluorescence; Fig. 4A, B). Control astrocytes not subjected to stretch did not show red fluorescence (Fig. 4A′). As shown previously for pressure induced over-expression of PAD26, the PAD2 immunoreactivity, activity and level of protein deimination can be reduced by application of shRNA against the coding region of PAD2 (data not shown).
Figure 2.
Mechanical stretching (polyethylene membrane set up) induced over-expression of PAD2 and consequent elevated deimination in primary astrocytes. The astrocytes (5000 cells) were subjected stretch-cycles by pulling the polyethylene membrane 1 cm for number of cycles as indicated and subjected to analyses for PAD2 activity, expression of PAD2 (ELISA), and level of deimination (ELISA) assay as described in methods. One cycle is defined as 1 cm stretch from both sides of membrane and holding period for 1 minute followed by relaxation to the original point and holding up for 1 minute. During incubation the membrane was held at relaxed position. PAD2 activity and level of deimination are represented by bars with dots and bars with diagonal strips respectively. A, B, PAD2 activity, PAD2 level and level of deimination of astrocytes without and with immobilization as indicated respectively. The results are standard deviation of three independent experiments.
Figure 3.
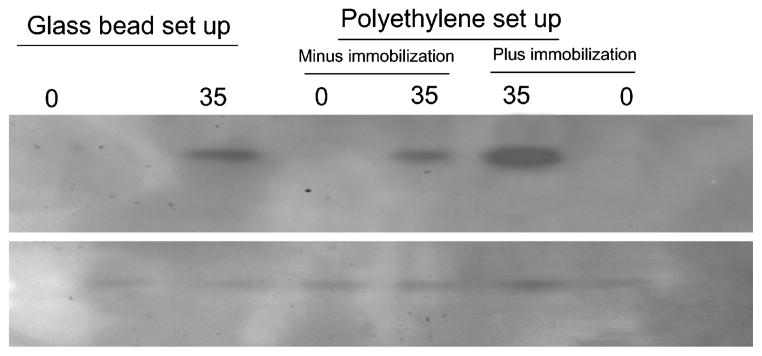
Representative Western analysis for PAD2 expression in astrocytes. About 5 μg of total protein from astrocytes lysates were separated on a 4–20% SDS-PAGE, transferred on to a PVDF membrane and subjected to probing with anti-PAD2 or anti-actin respectively. The astrocytes were subjected to stretching using glass bead set up and polyethylene membrane set up as indicated, 0 and 35 represents control (without stretching) and at the end of 35 cycles of stretching respectively. Plus and minus immobilization are as indicated.
Figure 4.
Immunohistochemical analysis of PAD2 expression in response to mechanical stretching. A, Astrocyte subjected to mechanical stretching showed robust expression of PAD2 as revealed with staining with a monoclonal antibody to PAD2 (secondary coupled to Alexa 594, red fluorescence), B, merged picture shows anti-GFAP staining (secondary Alexa 488, green) and DAPI staining in addition to PAD2 staining, A′ and B′, Control astrocytes not subjected to mechanical stretch stained with anti-PAD2 and anti-GFAP respectively. Bars = 50 μm.
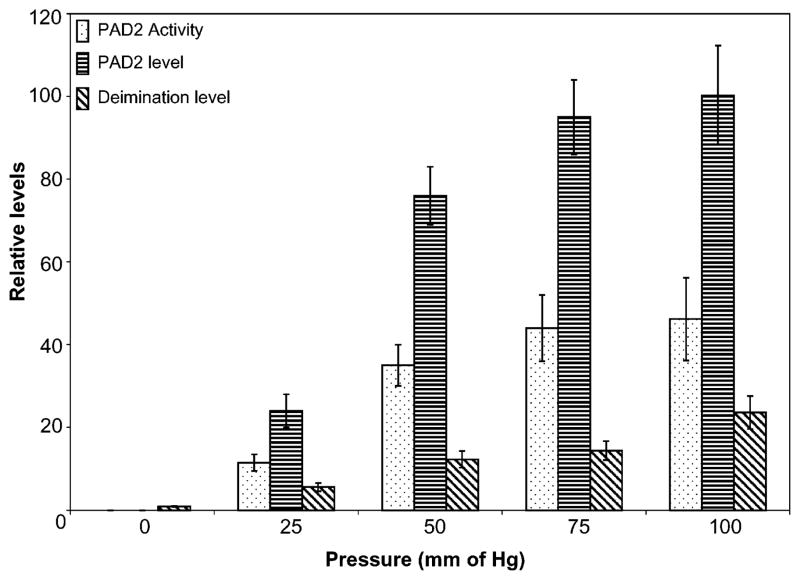
Elevation in PAD2 and deimination level has been observed in astrocytes subjected to mechanical stretching cycles (Fig. 1 and 2). Astrocytes subjected to hydrostatic pressure also shows elevated levels of PAD2 expression demonstrated by elevated levels of PAD2 activity, immunoreactivity and protein deimination immunoreactivity (Fig. 5) consistent with the results of our previous studies13. Our previous studies13 have demonstrated treatment of astrocytes to elevated pressure resulting in biphasic death kinetics with a relatively fast cell death within physiologically relevant pressure range (20–100 mm of Hg) and relatively slower kinetics at pressures beyond physiological range in contrast to lack of cell death due to mechanical stretching. Application of pressure beyond physiological range leads to increased PAD2 level but the expression level is not increased any further than observed at about 100 mm of Hg.13
Figure 5.
Effect of pressure in vitro on PAD2 expression, activity and level of deimination. The astrocytes (5000 cells) were subjected to 25–100 mm Hg as indicated for 5 hours and restored to normal pressure or kept untreated (controls: 0). The PAD2 activity and level, and level of deimination in astrocytes subjected to pressure treatment has been shown by dots, horizontal and diagonal bars as indicated.
DISCUSSION
The late onset and progressive diseases that occur due to intrinsic changes are complex, often multifactorial, difficult to understand, investigate and develop therapeutic intervention strategies compared to the diseases that occur due to foreign pathogens. The latter have been very effectively addressed often due to inherent metabolic differences between the host and pathogens. Protein methylation and deimination are among several post translational modifications (PTMs) that have important consequences in the function of multicellular organisms. These modifications play a role in protein processing and signaling, protein-protein, protein-organelle, protein-cells and cell-cell interactions. Physiological role of deimination is yet remains to be defined.8 A very limited repertoire of proteins: keratin, myelin basic protein (MBP), glial fibrillary acidic protein (GFAP), vimentin, trichohyalin, histones (H2A, H3 and H4), filaggrin and fibrinogen are currently known to undergo deimination.35 Modulation in levels of deimination has not been ascribed to a specific physiological condition as yet.
The hydrostatic pressure treated astrocytes is an imperfect model as it deviates from the real situation at the optic nerve head. Whether the applied hydrostatic pressure paradigm is effective on isolated cells has not been investigated from a basic pressure physics standpoint and other factors may also influence the observed alterations of cells subjected to the pressure, for example, potential change in the gas composition resulting in hypoxia or changes in medium pH. Glaucoma is a chronic disease and short term hydrostatic pressure exposure to astrocytes (not mixed cultures) in our previous studies captures only limited events relevant to the disease paradigm. Studies in DBA/2J mice optic nerve6 suggests that PAD2 expression and deimination in vivo parallels observations with astrocytes subjected to hydrostatic pressure in vitro. It has been conjectured that the cells in the optic nerve head actually experience mechanical stretching. The actual stretching and/or pressure experienced by cells are difficult to model. Results presented here demonstrate over-expression of PAD2 and elevated deimination in cells subjected to mechanical stretching (Fig. 1, 2). Immobilization on a membrane has an effect on the cells subjected to stretching (Fig. 2 A, B). Immobilization leads to a higher level of PAD2 expression and deimination level under identical conditions suggests that without immobilization not all cells in the population perhaps experience the same effect of stretching. The differences between glass bead set up (Fig. 1) and polyethylene set up without immobilization (Fig. 2A) compared to with immobilization (Fig. 2B) suggests lack of exertion of uniform effect on cells resulting in relatively less pronounced PAD2 expression and level of deimination. It is likely that in vivo, cells experience a complex combinatorial effect of both parameters and future models combining these aspects might enable providing greater insight. In addition, in vivo cells are surrounded by other mixed cell population and a matrix which also are expected to modulate the environment in a fashion not captured by in vitro mechanical stretching and hydrostatic pressure model. In the future, more sophisticated models will help capture the mechanistic differences due to each of these factors.
Acknowledgments
This study was supported by NIH grant P30 EY014801, a career award (SKB) and an unrestricted grant to department of ophthalmology, University of Miami from Research to Prevent Blindness. We thank Bharathi Govindarajan, Nanthawan, Avishai and Gabriel Gaidosh for their assistance and Dr. Vera Bonilha for her critical comments on the manuscript.
Contributor Information
Mabel E. Algeciras, Bascom Palmer Eye Institute, University of Miami, Miami, Florida, USA
Sanjoy K. Bhattacharya, Bascom Palmer Eye Institute, University of Miami, Miami, Florida, USA
Hidenari Takahara, Ibaraki University, School of Agriculture, Ami, Inashiki, Ibaraki, Japan.
References
- 1.Coleman AL. Epidemiology of Glaucoma. In: Morrison JC, Pollack IP, editors. Glaucoma Science and Practice. New York: Thieme Medical Publishers Inc; 2003. pp. 2–11. [Google Scholar]
- 2.Steely HT, Jr, English-Wright SL, Clark AF. The similarity of protein expression in trabecular meshwork and lamina cribrosa: implications for glaucoma. Exp Eye Res. 2000;70:17–30. doi: 10.1006/exer.1999.0764. [DOI] [PubMed] [Google Scholar]
- 3.Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- 4.Borras T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003;22:435–463. doi: 10.1016/s1350-9462(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, Agapova O, Parker A, Shannon W, Pecen P, Duncan J, Salvador-Silva M, Hernandez MR. DNA microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure. Physiol Genomics. 2004;17:157–169. doi: 10.1152/physiolgenomics.00182.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya SK, Crabb JS, Bonilha VL, Gu X, Takahara H, Crabb JW. Proteomics implicates peptidyl arginine deiminase 2 and optic nerve citrullination in glaucoma pathogenesis. Invest Ophthalmol Vis Sci. 2006;47:2508–2514. doi: 10.1167/iovs.05-1499. [DOI] [PubMed] [Google Scholar]
- 7.Steely HT, Jr, Clark AF. The use of proteomics in ophthalmic research. Pharmacogenomics. 2000;1:267–80. doi: 10.1517/14622416.1.3.267. [DOI] [PubMed] [Google Scholar]
- 8.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 9.Mastronardi FG, Noor A, Wood DD, Paton T, Moscarello MA. Peptidyl argininedeiminase 2 CpG island in multiple sclerosis white matter is hypomethylated. J Neurosci Res. 2007;85:2006–2016. doi: 10.1002/jnr.21329. [DOI] [PubMed] [Google Scholar]
- 10.Asaga H, Akiyama K, Ohsawa T, Ishigami A. Increased and type II-specific expression of peptidylarginine deiminase in activated microglia but not hyperplastic astrocytes following kainic acid-evoked neurodegeneration in the rat brain. Neurosci Lett. 2002;326:129–132. doi: 10.1016/s0304-3940(02)00334-8. [DOI] [PubMed] [Google Scholar]
- 11.Sambandam T, Belousova M, Accaviti-Loper MA, Blanquicett C, Guercello V, Raijmakers R, Nicholas AP. Increased peptidylarginine deiminase type II in hypoxic astrocytes. Biochem Biophys Res Commun. 2004;325:1324–1329. doi: 10.1016/j.bbrc.2004.10.173. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama K, Sakurai Y, Asou H, Senshu T. Localization of peptidylarginine deiminase type II in a stage-specific immature oligodendrocyte from rat cerebral hemisphere. Neurosci Lett. 1999;274:53–55. doi: 10.1016/s0304-3940(99)00678-3. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya SK, Bhat MB, Takahara H. Modulation of peptidyl arginine deiminase 2 and implication for neurodegeneration. Curr Eye Res. 2006;31:1063–1071. doi: 10.1080/02713680600991437. [DOI] [PubMed] [Google Scholar]
- 14.Ju WK, Liu Q, Kim KY, Crowston JG, Lindsey JD, Agarwal N, Ellisman MH, Perkins GA, Weinreb RN. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest Ophthalmol Vis Sci. 2007;48:2145–2151. doi: 10.1167/iovs.06-0573. [DOI] [PubMed] [Google Scholar]
- 15.Yang JL, Neufeld AH, Zorn MB, Hernandez MR. Collagen type I mRNA levels in cultured human lamina cribrosa cells: effects of elevated hydrostatic pressure. Exp Eye Res. 1993;56:567–574. doi: 10.1006/exer.1993.1070. [DOI] [PubMed] [Google Scholar]
- 16.Ricard CS, Kobayashi S, Pena JD, Salvador-Silva M, Agapova O, Hernandez MR. Selective expression of neural cell adhesion molecule (NCAM)-180 in optic nerve head astrocytes exposed to elevated hydrostatic pressure in vitro. Brain Res Mol Brain Res. 2000;81:62–79. doi: 10.1016/s0169-328x(00)00150-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Ju WK, Crowston JG, Xie F, Perry G, Smith MA, Lindsey JD, Weinreb RN. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 2007;48:4580–4589. doi: 10.1167/iovs.07-0170. [DOI] [PubMed] [Google Scholar]
- 18.Fuss B, Mallon B, Phan T, Ohlemeyer C, Kirchhoff F, Nishiyama A, Macklin WB. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev Biol. 2000;218:259–274. doi: 10.1006/dbio.1999.9574. [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Hernandez MR. Purification of astrocytes from adult human optic nerve heads by immunopanning. Brain Res Brain Res Protoc. 2003;12:67–76. doi: 10.1016/s1385-299x(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 20.Prasanna G, Krishnamoorthy R, Clark AF, Wordinger RJ, Yorio T. Human optic nerve head astrocytes as a target for endothelin-1. Invest Ophthalmol Vis Sci. 2002;43:2704–2713. [PubMed] [Google Scholar]
- 21.Agarwal PK, Bhattacharya SK. Construction of a multi RE module: exploitation of mechanochemistry of restriction endonucleases. Biotechnol Bioeng. 1999;65:233–239. [PubMed] [Google Scholar]
- 22.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 23.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 24.Salvador-Silva M, Aoi S, Parker A, Yang P, Pecen P, Hernandez MR. Responses and signaling pathways in human optic nerve head astrocytes exposed to hydrostatic pressure in vitro. Glia. 2004;45:364–377. doi: 10.1002/glia.10342. [DOI] [PubMed] [Google Scholar]
- 25.Liu GY, Liao YF, Chang WH, Liu CC, Hsieh MC, Hsu PC, Tsay GJ, Hung HC. Overexpression of peptidylarginine deiminase IV features in apoptosis of haematopoietic cells. Apoptosis. 2006;11:183–196. doi: 10.1007/s10495-006-3715-4. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya SK, Crabb JS, West KA, Gu X, Sun J, Bonilha VL, Smejkal G, Shadrach K, Hollyfield JG, Crabb JW. Optic nerve fractionation for proteomics. In: Smejkal GB, Lazarev A, editors. Separation Methods in Proteomics. New York: Marcel Dekker; 2005. in press. [Google Scholar]
- 27.Bhattacharya SK, Rockwood EJ, Smith SD, Bonilha VL, Crabb JS, Kuchtey RW, Robertson NG, Peachey NS, Morton CC, Crabb JW. Proteomics reveals cochlin deposits associated with glaucomatous trabecular meshwork. J Biol Chem. 2005;280:6080–6084. doi: 10.1074/jbc.M411233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koike H, Shiraiwa M, Sugawara K, Kohsaka T, Takahara H. Existence and differential changes of peptidylarginine deiminase type II in mouse yolk-sac erythroid cells. Biosci Biotechnol Biochem. 1995;59:552–554. doi: 10.1271/bbb.59.552. [DOI] [PubMed] [Google Scholar]
- 29.Koike H, Sase K, Uchida H, Sudo T, Shiraiwa M, Sugawara K, Takahara H. Production and epitope specificity of monoclonal antibody against mouse peptidylarginine deiminase type II. Biosci Biotechnol Biochem. 1994;58:2286–2287. doi: 10.1271/bbb.58.2286. [DOI] [PubMed] [Google Scholar]
- 30.Senshu T, Sato T, Inoue T, Akiyama K, Asaga H. Detection of citrulline residues in deiminated proteins on polyvinylidene difluoride membrane. Anal Biochem. 1992;203:94–100. doi: 10.1016/0003-2697(92)90047-b. [DOI] [PubMed] [Google Scholar]
- 31.Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- 32.Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- 33.Balestrini JL, Billiar KL. Equibiaxial cyclic stretch stimulates fibroblasts to rapidly remodel fibrin. J Biomech. 2006;39:2983–2990. doi: 10.1016/j.jbiomech.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Shuba LM, Doan AP, Maley MK, Zimmerman MB, Dinn RB, Greenlee EC, Alward WL, Kwon YH. Diurnal fluctuation and concordance of intraocular pressure in glaucoma suspects and normal tension glaucoma patients. J Glaucoma. 2007;16:307–312. doi: 10.1097/IJG.0b013e3180316736. [DOI] [PubMed] [Google Scholar]
- 35.Algeciras ME, Bhattacharya SK. Targeting optic nerve citrullination in glaucoma: a role for protein-arginine deiminase 2 (PAD2) inhibitors. Drugs of the Future. 2007;32:999–1006. [Google Scholar]