Abstract
Antigen-presenting cells (APCs) are important in the initiation of productive antigen-specific T-cell responses and in the induction of T-cell anergy. The inflammatory status of the APC at the time of encounter with antigen specific T-cells plays a central role in determining such divergent T-cell outcomes. A better understanding of the regulation of pro-inflammatory and anti-inflammatory genes in its natural setting, the chromatin substrate, might provide novel insights to overcome anergic mechanisms mediated by APCs. Here we show for the first time that treatment of BALB/c murine macrophages with the histone deacetylase inhibitor (HDI) LAQ824, induces chromatin changes at the level of the IL-10 gene promoter that leads to enhanced recruitment of the transcriptional repressors HDAC11 and PU.1. Such an effect is associated with diminished IL-10 production and induction of inflammatory cells able of priming naïve antigen-specific T-cells, but more importantly, capable of restoring the responsiveness of anergized antigen-specific CD4+ T-cells.
INTRODUCTION
The potency of an immune response is dictated in large part by the potency of the antigen-presenting cell (APC) and its ability to optimally prime the T-cell response. This in turn, is influenced by such factors as the particular APC cell type as well as the context –inflammatory versus non-inflammatory- in which the APC acquires the antigen for processing and presentation to antigen-specific T-cells(1, 2). Not surprisingly, APCs isolated from a non-inflammatory tumor microenvironment are relatively inefficient at priming protective responses, inducing instead T-cell anergy(3-5).
During the past several years, numerous studies in experimental models as well as in humans have provided sufficient evidence supporting the conclusion that the induction of T-cell anergy to tumor antigens represents a significant barrier to harness antitumor immunity(5-9). Important lessons learned from these studies point to manipulation of the inflammatory status of the APC as an enticing strategy to overcome anergic mechanisms in cancer(10-13). A better understanding of the molecular/signaling mechanism(s) regulating pro- and/or anti-inflammatory genes in the APC would likely provide important insights into how these cells influence T-cell responses and might unveil novel targets to overcome anergy to tumor antigens.
Recently, a significant effort is being devoted to better understand the regulation of pro-inflammatory and anti-inflammatory genes in their natural setting, the chromatin substrate(14). Chromatin modification by acetylation/deacetylation of histone tails is an important mechanism of regulation of gene transcription, including genes involved in the inflammatory response(15). In general, histone acetylation mediated by histone acetyl transferases (HATs) results in transcriptionally active chromatin. In contrast, histone deacetylation mediated by histone deacetylases (HDACs) leads to an inactive chromatin and gene repression(16).
HDACs exist as large multimeric complexes and are recruited to gene promoters by co-repressors or by multiprotein transcriptional complexes. Eighteen HDACs have been identified and they have been grouped into four principal classes(17, 18). HDACs are the molecular target of several structurally diverse compounds known as histone deacetylase inhibitors (HDI). Existing HDIs inhibit proliferation of malignant cells in vitro by inducing cell cycle arrest and apoptosis, and some of them have already demonstrated significant antitumor activity in cancer patients(19, 20). In contrast to their well-known effects upon cancer cells, little is still known about the immunological effects of HDIs. While some studies have shown that HDIs have anti-inflammatory properties(21, 22), promote the expression of the suppressive factor, indoleamine 2,3-dioxygenase (IDO) in dendritic cells(23) and diminish the morbidity and mortality of graft-versus-host disease(24), others have highlighted the pro-inflammatory effects of these compounds. For instance, Tomasi’s group has shown that treatment of melanoma cells with HDIs augments their antigen-presenting capabilities leading to activation of IFN-γ secreting T-cells via the Class I pathway(25, 26). Vo et al. have recently demonstrated that in vivo treatment of tumor bearing mice with the hydroxamic acid analogue pan-HDI LAQ824, significantly enhances the anti-tumor activity of adoptively transferred antigen-specific T-cells(27). Needless to say, the underlying molecular mechanism(s) by which HDIs influence inflammatory responses remain to be fully elucidated.
In this study we show that the pan-HDI LAQ824 induces several chromatin changes in macrophages that resulted in enhanced recruitment of the transcriptional repressors HDAC11 and PU.1 to the IL-10 gene promoter. Such an effect is associated with inhibition of IL-10 production and induction of cells able of priming naïve antigen-specific T-cells and capable of restoring the responsiveness of anergized CD4+ T-cells.
MATERIALS AND METHODS
Mice
Male BALB/c mice (6- to 8-weeks old) were obtained from the National Institutes of Health (Frederick, MD). TCR transgenic mice expressing an αβ T-cell receptor specific for amino acids 110-120 from influenza hemagglutinin (HA) presented by I-Ed were a generous gift of H. von Boehmer (28). All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the University of South Florida College of Medicine.
Cell lines
The macrophage cell line RAW264.7 has been described previously(29) and the B-cell lymphoma cell line A20 was obtained from the American Type Culture Collection (ATCC). A20HA was generated by electroporation-mediated plasmid transfection, and transfected cells were selected as previously reported (3, 6). Cells were cultured in vitro in RPMI 1640 media, supplemented with 10% FBS, penicillin/streptomycin (50 U/ml), L-glutamine (2 mM), and 2-mercaptoethanol (50 mM) (complete media), and grown at 37°C and 5% CO2. A20 media was also supplemented with additional sodium pyruvate (1mM) and nonessential amino acids (1x, Mediatech, Manassas, VA).
Isolation of peritoneal elicited macrophages (PEM)
BALB/c mice were injected intraperitoneally (ip) with 1 mL of thioglycollate (DIFCO Laboratories, Detroit, MI). Four days later, peritoneal elicited macrophages (PEM) were isolated by peritoneal lavage as previously described (30).
Isolation of splenic macrophages
BALB/c mice were injected intravenously with 1×106 A20 lymphoma cells. Three weeks later, animals were sacrificed and their spleens were removed. Splenocytes were isolated, washed with PBS and then suspended in complete RPMI medium. Cells were then cultured at 37°C and 5% CO2 for 2 hours to allow macrophage’s to adhere to the plate. Non-adherent cells were then removed with 2 washes of cold PBS. Splenic macrophages were then incubated for an additional hour and the cells were treated as indicated.
Reagents
LPS (Escherichia coli 055:B5, Catalog # L-2880) was purchased from Sigma (St. Louis, MO). The HDAC inhibitors LAQ824 and LBH589 were provided by Dr. K. Bhalla (University of Kansas Medical Center). TSA was obtained from Sigma (St. Louis, MO) and MS-275 is from Calbiochem (San Diego, CA). HDI were first reconstituted in DMSO for stock preparation (10 mM). Solutions were then diluted in RPMI for in vitro use in cell cultures.
Antibodies and Immunoblottings
Histone H3 (D1H2) monoclonal antibody (4499), Histone H4 (Lys8) polyclonal antibody (2592), Acetyl-Histone H3 (Lys9) polyclonal antibody (9671) and Acetyl-Histone H4 (Lys8) polyclonal antibody (2594) were purchased from Cell Signaling (Danvers, MA) for western blotting. The GAPDH (FL-335, sc-25778), Pol II (H-224, sc-9001), and PU.1 (Spi-1/T-21, sc-352) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Hyperacetylated H3 (06-599), hyperacetylated H4 (06-598), Sp1 (07-645), and STAT3 (06-596) polyclonal antibodies were purchased from Millipore (Billerica, MA). Two different polyclonal antibodies against HDAC11 were used; ab47036 from ABCAM (Cambridge, MA) for immunoblots, and Ab H4539 from Sigma was used for ChIP analysis. Neutralizing anti-mouse IL-10 (AB-417-NA) monoclonal antibody was purchased from R&D Systems, Inc (Minneapolis, MN) Recombinant mouse IL-10 (550070) was purchased from BD Bioscience (San Diego, CA).
Phenotypic and functional analysis of macrophages
The expression of B7.2 in PEMs was determined by staining with Biotin-conjugated anti-CD86 (GL1 antibody, BD Pharmingen, San Jose, CA) and followed by Strep-Avidin-PE (Caltag, Carlsbad,CA) antibodies. Ten thousand gated events were collected on a FACScan (Becton Dickinson) and analyzed using FlowJo software. In a parallel plate, PEMs were treated with HDI alone, LPS alone or a combination thereof. Then supernatants were collected and production of IL-10, IL-12, IL-1α, IL-1β, IL-6, RANTES, TNF-α and GM-CSF were determined by ELISA (R&D Systems, Minneapolis, MN).
Antigen-presentation studies
PEMs or splenic macrophages (1×105/well) were treated with LPS (2mcg/ml) or with LPS plus HDI for 24 hours. Cells were then washed and 5×104 purified naïve antigen-specific CD4+ T cells (isolated from the spleen of HA TCR transgenic mice) or a similar number of anergized antigen-specific CD4+ T cells (isolated from the spleen of A20HA lymphoma bearing mice) were added to macrophage’s monolayer in the presence –or not– of cognate peptide (HA peptide110-120 SFERFEIFPKE). After 48 hours supernatants were collected and stored at –20°C until assayed for IL-2 and IFN-γ production by ELISA. Values for T cells cultured in media alone are usually less than 10% of the values for antigen-stimulated T cells. The amount of cytokine production is expressed as pg/ml per 100 clonotype-positive CD4+ T cells.
Real time (RT)-PCR analysis
Macrophage cell line or primary murine macrophages were plated at 2×106 cells per 35mm well and cultured under conditions detailed for each experiment. Total RNA was extracted using TriZol reagent (Qiagen,Valencia,CA) and cDNA obtained with the iScript cDNA synthesis kit (Bio-Rad,Hercules,CA). Target mRNA was quantified using MyIQ single color real time PCR detection system (Bio-Rad) and iQ SYBR green Supermix (Bio-Rad,Hercules,CA). Mouse IL-12p40 primers (left GCAACGTTGGAAAGGAAAGA, right AAAGCCAACCAAGCAGAAGA), mouse IL-10 primers (left CAGGGATCTTAGCTAACGGAAA, right GCTCAGTGAATAAATAGAATGGGAAC), were used for PCR amplification (cycling parameters 3 min 95°C, 15 secs 95 °C, 30 secs 60°C 40 reps, 1 min 95°C). Single product amplification was confirmed by melt curve analysis and primer efficiency was near to 100% in all the experiments performed. Quantification is expressed in arbitrary units and target mRNA levels were normalized to GAPDH expression using the method of Pfaffl(31).
Chromatin Immunoprecipitation (ChIP) assays
ChIP studies were performed as previously described(32). The primers used were; IL-10 (proximal region) sense 5’-GGAGGAGGAGCCTGAATAAC-3’ and antisense 5’-CTGTTCTTGGTCCCCCTTTT-3’, IL-10 (distal region) sense 5’-AACTCAGCCTGGAACTGACC-3’ and antisense 5’-GCCTCTCCTCCTGACACTCTT-3’, IL-12 sense 5’-GTGGAGCCAAACAGGGAGGTA and antisense 5’-GACGTCGAAATCCCAGTTTA-3’. All samples and Inputs were quantified using MyIQ single color real time PCR detection system (Bio-Rad) and iQ SYBR green Supermix (Bio-Rad). Single product amplification was confirmed by melt curve analysis and primer efficiency was near or close to 100% in all experiments performed. Target sequence levels were normalized to the input signal using the method of Pfaffl(31). Quantification is expressed in arbitrary units as the ratio between treated and untreated cells. All ChIP experiments were repeated three times, and final quantitative real time PCR was done in triplicates.
T-cell anergy model
We used a well-established experimental model of tumor induced antigen-specific CD4+ T-cell anergy (3, 6). Briefly, 2.5×106 naïve CD4+ transgenic T-cells specific for an MHC Class II epitope of influenza hemagglutin (HA) were injected intravenously into A20HA lymphoma-bearing mice. Twenty-one days after T-cell transfer, animals were sacrificed and T-cells were re-isolated from their spleens. Cytokine production by clonotypic CD4+ T-cells in response to HA-peptide110-120 presented by untreated, LPS-treated or PEM treated with LPS plus LAQ824 was then determined as described under antigen presentation studies.
Statistical Analysis
Unpaired t-tests were performed using Microsoft Excel with significance at p<0.05.
RESULTS
Phenotypic and functional changes in macrophages treated with the HDI LAQ824
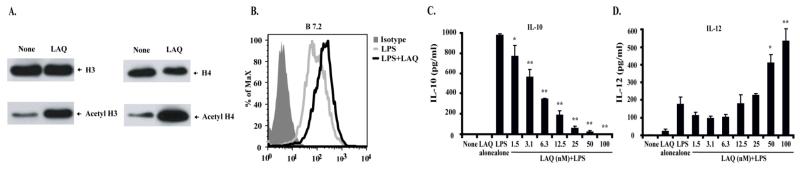
First, we asked whether treatment of macrophages with the hydroxamic acid analogue pan-HDI LAQ824 would result in changes in histone acetylation and the induction –or not– of inflammatory cells. As shown in Figure 1A, treatment of peritoneal elicited macrophages (PEM) with LAQ824 was associated with increased acetylation of histones H3 and H4 relative to their acetylated status in untreated PEM (None). No changes in the expression of MHC molecules or co-stimulatory molecules were observed in PEM treated with LAQ824 alone (data not shown). However, higher expression of B7.2 was observed in PEMs treated with LPS plus LAQ824 as compared to cells treated with LPS alone (Figure 1B). Furthermore, treatment of PEMs with LPS in the presence of increasing concentrations of LAQ824 resulted in a dose-dependent inhibition of the anti-inflammatory cytokine IL-10 (Figure 1C). Of note, this inhibitory effect was accompanied by an increased production of the pro-inflammatory cytokine IL-12 (Figure 1D). It should be pointed out that LAQ824 was not toxic to PEMs since these cells were viable (as determined by trypan blue exclusion) and capable of producing IL-12 even when they were exposed to the highest concentration of HDI (100nM) used in our experiments.
Figure 1. Phenotypic and functional changes in macrophages treated with the HDI LAQ824.
(A) PEMs were treated –or not– with LAQ824 (12.5nM) for 24 hours. Then, cell lysates were obtained and acetylation of histones H3 and H4 was determined by immunoblotting using anti-acetyl H3 and H4 antibodies respectively. (B) PEMs were treated with LPS (2mcg/ml) or with LPS plus LAQ824 (12.5nM) for 24 hours. Cells were then stained with an anti-B7.2 antibody or an isotype control. In a parallel experiment, PEMs were treated with LPS alone (2mcg/ml) or LPS plus increasing concentrations of LAQ824 as indicated. After 24 hours, supernatants were collected and the levels of IL-10 (C) and IL-12 (D) were determined by ELISA. Shown is a representative experiment of five experiments with similar results. * p<0.05, ** p<0.01
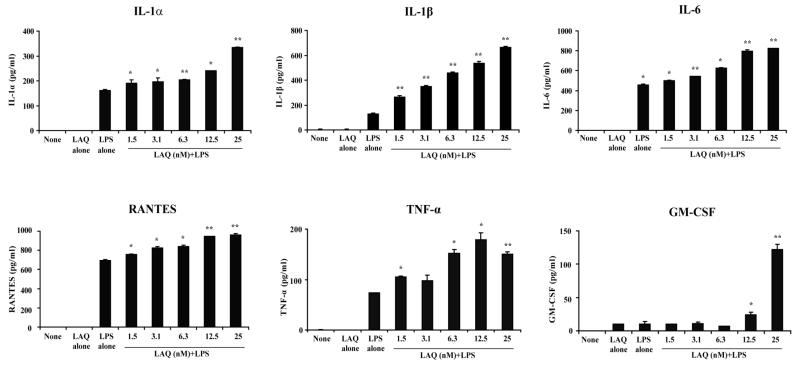
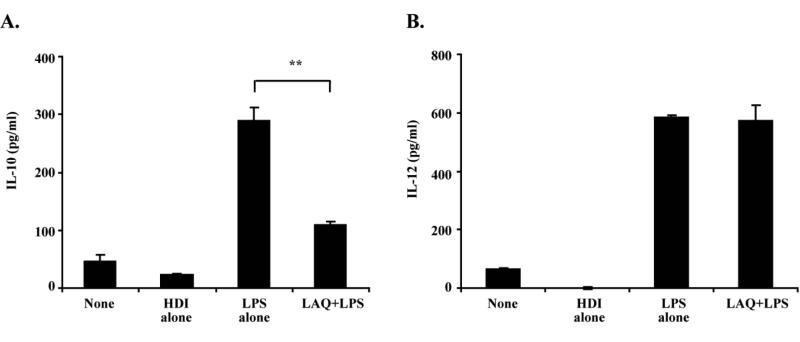
To further confirm the pro-inflammatory effects of LAQ824, we determined next the production of cytokines/chemokines by PEMs treated with HDI. As shown in Figure 2, PEMs treated with LPS and LAQ824 produced higher levels of IL-1α, IL-1β, IL-6, RANTES, TNF-α and GM-CSF as compared to PEMs treated with LPS alone. Of note, LAQ824 also inhibits the production of IL-10 by macrophages isolated from the spleen of tumor-bearing mice (Figure 3A). No changes in IL-12 production were observed in tumor bearing macrophages treated with HDI plus LPS as compared to macrophages treated with LPS alone (Figure 3B).
Figure 2. Production of pro-inflammatory mediators by macrophages treated with HDI LAQ824.
PEMs were treated with LPS (2mcg/ml), LAQ824 (12.5nM) or LPS (2mcg/ml) plus increasing concentrations of LAQ824 as indicated. After 24 hours, supernatants were collected and cytokine/chemokine levels were determined by ELISA. Shown is a representative experiment of three experiments with similar results.
* p<0.05, ** p<0.01
Figure 3. LAQ824 inhibits IL-10 production by macrophages from tumor bearing mice.
(A) Splenic macrophages from A20 bearing mice were treated with LPS (2mcg/ml), LAQ824 alone (12.5 nM) or LPS (2mcg/ml) plus LAQ824 for 24 hours. The supernatants were collected and production of IL-10 and IL-12 were determined by ELISA. Show is a representative experiment of two experiments with similar results.
** p<0.01
Pan-HDI LBH589 and TSA, but not the Class I selective HDI MS-275, inhibit IL-10 production by macrophages
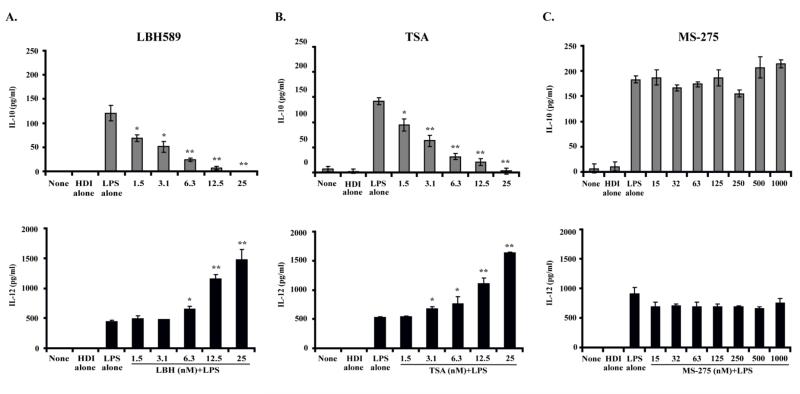
HDIs represent a family of structurally diverse compounds that inhibit the enzymatic function of HDACs. LAQ824 along with LBH589, TSA and SAHA are pan-HDIs that belong to the hydroxamic acid family of compounds. To determine whether the other members of this family share the pro-inflammatory effects of LAQ824, we treated PEMs with either LBH589 or TSA. Reminiscent of our findings with LAQ824, LBH589 and TSA also inhibit in a dose-dependent manner the production of IL-10 by PEMs in response to LPS (Figure 4A-B, left). Such an inhibition was associated with increased production of IL-12 (Figure 4A-B, right). Similar changes, although of a lesser magnitude, were observed in PEMs treated with SAHA (data not shown).
Figure 4. Production of IL-10 and IL-12 by PEMs treated with the pan-HDIs LBH589 or TSA, or with the class I selective HDI MS-275.
PEMs were treated with LPS (2mcg/ml), HDI alone or LPS (2mcg/ml) plus increasing concentrations of the HDI LBH589, TSA or MS-275 as indicated. After 24 hours, supernatants were collected and production of IL-10 (left) and IL-12 (right) were determined by ELISA. Shown is a representative experiment of two experiments with similar results. * p<0.05, ** p<0.01
In contrast to the above results, treatment of PEMs with a more selective HDI, compound MS-275, which inhibits the enzymatic function of Class I HDACs (HDAC 1, 2, 3 and 8) did not result in inhibition of IL-10 or augmentation of IL-12 production by treated cells (Figure 4C). These findings argue against a role for Class I HDACs in regulating IL-10 production in PEMs and point to Class II and/or Class IV HDACs as potential molecular targets involved in the inhibitory effect on IL-10 mediated by pan-HDIs.
LAQ824-treated PEMs efficiently prime naïve antigen-specific T-cells and restore the responsiveness of anergized T-cells
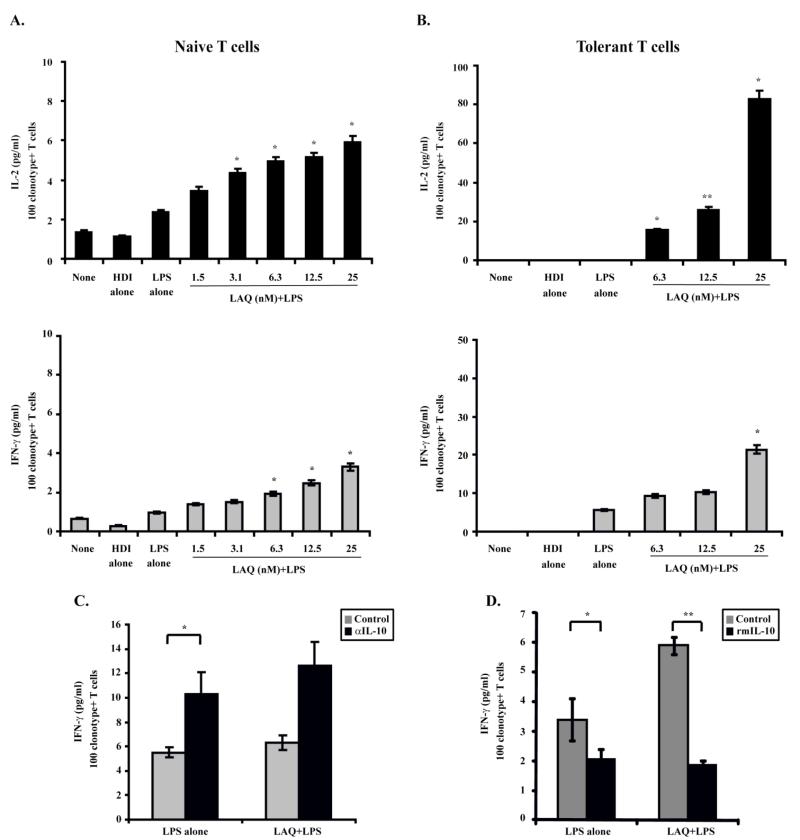
Next we determined whether the acquisition of inflammatory properties by LAQ824-treated macrophages renders these cells better activators of antigen-specific CD4+ T-cells. PEMs were therefore treated with LAQ824, LPS or a combination of LPS plus LAQ824 for 24 hours. Following this treatment, naïve CD4+ T-cells specific for a MHC Class II restricted epitope of influenza hemagglutinin (HA) were added to the PEM monolayer and stimulated, or not, with cognate HA-peptide. First, clonotypic T-cells encountering cognate peptide on untreated PEM (None) or on PEM treated with LAQ824 (HDI alone) produce similar levels of IL-2 (Figure 5A-top). A slightly increased production of IL-2 was observed in T-cells encountering antigen in LPS-treated PEMs (Fig. 5A-top, LPS alone). IL-2 production was further enhanced in CD4+ T-cells that encountered HA-peptide in PEMs treated with LPS plus increasing concentrations of LAQ824 (Figure 5A-top, LAQ+LPS). LAQ824-treated PEMs also triggered an enhanced effector function of clonotypic CD4+ T cells, as determined by their capacity to produce higher levels of IFN-γ in response to cognate peptide (Figure 5A-bottom).
Figure 5. PEMs treated with LAQ824 effectively activate antigen-specific CD4+ T-cells and restore the function of anergized T-cells.
PEMs (1×105/well) were treated with LPS alone (2mcg/ml), LAQ824 alone (12.5 nM) or LPS plus LAQ824 as indicated for 24 hours. Then, cells were washed with RPMI and 5×104 naïve anti-HA transgenic CD4+ T-cells (A) or 5×104 anergized CD4+ T-cells isolated from A20HA bearing mice (B) were added to the PEMs in the presence of cognate HA-peptide (12.5 mcg/ml). After 48 hours, supernatants were collected and IL-2 and IFN-γ production were measured by ELISA. The amount of cytokine production is expressed as pg/ml per 100 clonotype-positive CD4+ T-cells. Shown is a representative experiment of three experiments with similar results. PEMs (1×105/well) were treated with LPS alone (2mcg/ml) or LPS plus LAQ824 (12.5nM) in the presence or not, of recombinant murine IL-10 (C) or neutralizing anti-mouse IL-10 antibody (D) for 24 hours. Then, cells were washed with warmed RPMI and 5×104 naïve anti-HA transgenic CD4+ T-cells were added to the PEMs in the presence of cognate HA-peptide (12.5 mcg/ml). Recombinant murine IL-10 (C) or neutralizing anti-mouse IL-10 antibody (D) were replenished, or not, in the macrophages/T-cell cultures to maintain the respective pre-treated conditions, but without LPS. After 48 hours, supernatants were collected and IFN-γ production was measured by ELISA. * p<0.05, ** p<0.01
Utilizing a T-cell receptor transgenic model we have previously demonstrated that CD4+ T-cells specific for an MHC Class II epitope of influenza hemagglutinin (HA) are rendered anergic during the growth of a B-cell lymphoma expressing HA as a model tumor antigen (A20HA). Isolation of these clonotype-positive T cells from tumor bearing mice followed by their in vitro re-stimulation with HA-peptide plus APCs, demonstrated that these cells were anergic given their inability to produce IL-2 or IFN-γ(3, 6). However, in vitro incubation of these same anergic T-cells with LAQ824-treated PEMs resulted in restoration of T-cell responsiveness (Figure 5B). Indeed, anergized antigen-specific CD4+ T-cells encountering cognate antigen in PEMs treated with LAQ824 and LPS regained their ability to produce IL-2 (Fig. 5B-top) and IFN-γ (Figure 5B-botttom). In contrast, anergic T cells encountering cognate antigen on either untreated (none), HDI alone or LPS-treated PEM were unable to produce IL-2 (Fig. 5B-top) or produced minimal amounts of IFN-γ (Fig. 5B-bottom). Therefore, LAQ824-treated PEMs effectively prime naïve antigen-specific CD4+ T-cells and restore the responsiveness of anergic CD4+ T-cells.
To better define the role of IL-10 inhibition induced by LAQ824 in the augmentation of the antigen-presenting cell (APC) function of macrophages, we cultured these cells with naïve antigen-specific CD4+ T-cells in the presence of recombinant murine IL-10. As shown in Fig. 5C, T-cells produced less IFN-γ in response to cognate antigen presented by LPS-treated macrophages when recombinant IL-10 was added. (Figure 5C, LPS alone, black bar versus gray bar). Similarly, the enhanced APC function displayed by macrophages treated with 12.5 nM of LAQ824+LPS (Fig. 5C, LAQ+LPS, gray bar) was abrogated when recombinant IL-10 was added back to the cultures (Fig. 5C, LAQ+LPS, black bar). This result was not surprising given the well known ability of IL-10 to inhibit TH1-type responses(33). Given that the inhibition of IL-10 induced by LAQ824 at the dose of 12.5 nM is incomplete (Fig, 1C), we asked next whether neutralization of the remaining secreted IL-10 with anti-IL-10 antibodies could further augment the APC function of LAQ-treated macrophages. As shown in Figure 5D, IL-10 blockade was insufficient to enhance the APC function of LPS-treated macrophages to augment IFN-γ production by CD4+ T-cells (Fig. 5D, LPS alone, black bar versus gray bar), nor was it able to significantly enhance the effect of LAQ824 treatment (Fig. 5D, LAQ+LPS, black bar versus gray bar). Taken together, these data point to a contributory role of IL-10 inhibition in the enhanced APC function displayed by LAQ824-treated macrophages since this effect was reversed when recombinant IL-10 was added-back to the cultures. However, neutralizing the remaining IL-10 protein that could have been still produced by LAQ-treated macrophages did not result in further enhancement of their APC function.
IL-10 mRNA expression and histone acetylation of the IL-10 gene promoter in PEMs treated with LAQ824
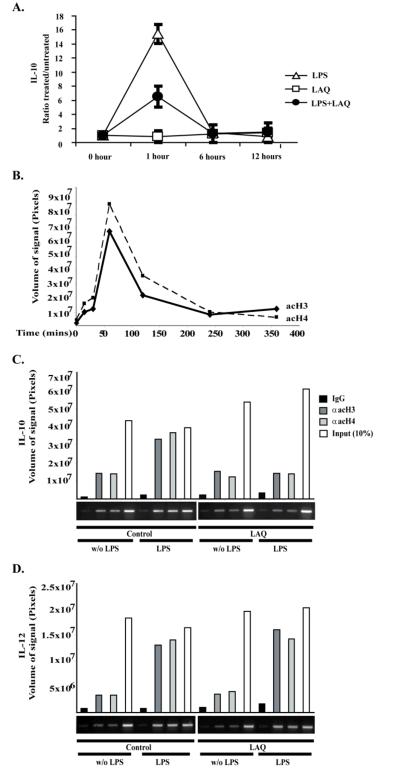
IL-10 is an anti-inflammatory cytokine that plays an important role in the establishment and maintenance of T-cell anergy(34, 35). One of the most striking and consistent effects of pan-HDIs was their ability to inhibit IL-10 production (Fig. 1C and 3-4). To better understand the mechanism(s) underlying this inhibitory effect, we evaluated next the kinetics of IL-10 mRNA expression in macrophages treated with LPS, LAQ824 or LPS plus LAQ824. In response to LPS stimulation, a rapid increase in IL-10 mRNA was observed at one hour, followed by a rapid decline and, after 6 hours, the IL-10 mRNA levels were back to baseline (Figure 6A-open triangles). In PEMs treated with LPS plus LAQ824 a decreased induction of IL-10 mRNA was observed at one hour (Figure 6A-closed circles). This initial response was followed by a progressive decline and, by 6 hours, IL-10 mRNA levels were back to baseline levels. No changes in IL-10 mRNA levels were observed in PEMs treated with LAQ824 alone (Figure 6A-open squares). Therefore, treatment of PEMs with LAQ824 resulted in decreased IL-10 gene transcriptional activity in response to LPS stimulation.
Figure 6. Changes in IL-10 mRNA expression and histone acetylation of the IL-10 gene promoter in PEMs treated with LAQ824.
(A) PEMs were treated with 2mcg/ml of LPS (open triangles), 12.5 nM of LAQ824 (open squares) or the combination thereof (black circles). Cell aliquots were obtained at 1, 6 and 12 hours and total RNA was isolated. IL-10 mRNA relative to GAPHD mRNA was determined by quantitative RT-PCR. Data is depicted as the ratio of treated cells versus untreated cells. Shown is a representative experiment of three experiments with similar results. (B) Kinetics of H3 and H4 acetylation of the IL-10 gene promoter in response to LPS stimulation. PEMs were treated with LPS (1 mcg/ml) for different periods of time (minutes) as indicated. Nuclear extracts were then obtained and chromatin immunoprecipitacion (ChIP) using anti-hyperacetylated H3 or anti-hyperacetylated H4 antibodies was performed. Shown is a representative experiment of two experiments performed with similar results. (C and D) PEMs were treated –or not– with LAQ824 (20nM) for 24 hours. Then LPS (1 mcg/ml) was added –or not– to PEM cultures for an additional 60 minutes. Cells were then harvested, and nuclear extracts were obtained. ChIP, using anti-hyperacetylated H3 or anti-hyperacetylated H4 antibodies, was then performed and samples were subject to PCR analysis using primers specific for the IL-10 (C) or IL-12 (D) promoters. IgG and Input (10%) controls are also shown. Signal intensities obtained from ethidium bromide stained gels were quantified using the ImageQuant 5.2 software (Molecular Dynamics). Shown is a representative experiment of three performed with similar results.
Next, we determined the acetylation status of histones at the level of the IL-10 promoter. First, by using a chromatin immunoprecipitation (ChIP) assay we assessed the kinetics of acetylation of histones H3 and H4 in PEMs treated with LPS alone. As seen in Figure 6B, acetylation changes occurred within a particular time window with a peak acetylation occurring at 60 minutes. This was followed by a progressive decline and by four hours, H3 and H4 acetylation levels were almost back to baseline. Given that the peak acetylation was observed one hour after LPS stimulation, in the next set of experiments, PEMs were pretreated –or not– with LAQ824 for 24 hours and then stimulated –or not– with LPS plus LAQ824 replenished as appropriate to maintain pretreatment conditions for an additional one hour. By ChIP assay we found that in response to LPS, histones H3 and H4 display increased acetylation (Fig.6C, Control/LPS) relative to untreated PEM (Fig. 6C, Control/ w/o LPS). When PEMs were treated with HDI alone, no significant changes in acetylation of H3 and H4 were observed (Fig. 6C, LAQ/w/o LPS). However, treatment of PEMs with LPS in the presence of HDI resulted in decreased H3 and H4 acetylation (Fig. 6C, LAQ/LPS). This effect was specific for IL-10 since a similar analysis of H3 and H4 acetylation at the level of the IL-12 promoter, revealed no differences between PEMs treated with LPS alone and PEMs treated with LPS plus HDI (Fig.6D, Control/LPS versus LAQ/LPS). Taken together, treatment of PEMs with LAQ824 resulted in decreased IL-10 gene transcriptional activity and diminished H3 and H4 acetylation at the level of the IL-10 gene promoter.
Chromatin changes in the IL-10 gene promoter induced by the HDI LAQ824
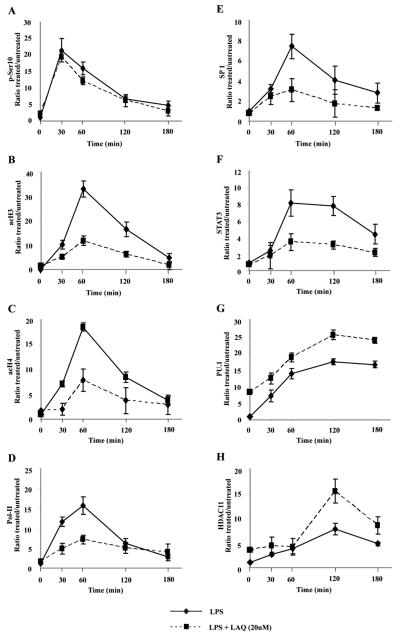
Previously, we have reported the sequence of chromatin modifications that occur at the level of the IL-10 gene promoter in macrophages stimulated with LPS(32). Briefly, phosphorylation of Ser-10 on H3 is an early event that occurs 30 minutes after LPS stimulation. This is followed by increased acetylation of H3 and H4 at 60 minutes, subsequent recruitment to the IL-10 gene promoter of the transcriptional activators Sp1 and STAT3 and, ultimately, IL-10 gene expression. This sequence of events was affected in RAW264.7 macrophage cells pretreated with LAQ824 for 24 hours and then stimulated with LPS (Fig. 7 A-H). First, phosphorylation of serine 10 on H3 at the IL10 promoter has been shown necessary for transcriptional activation(36). No differences in the kinetics of phosphorylation of H3 at the serine 10 position were observed among macrophages treated with LPS alone (Fig. 7A, solid lines) or cells treated with LAQ824 and LPS (Fig. 7A, dashed lines). Second, in macrophages treated with LPS, we observed a peak acetylation of H3 (Fig. 7B) and H4 (Fig. 7C) at one hour, which was followed by a progressive decline and, by three hours, H3 and H4 acetylation levels were back to baseline. In sharp contrast, a diminished H3 and H4 acetylation was observed at all evaluated time-points in cells treated with LAQ824 plus LPS (Fig. 7B-C, dashed lines). The effect of LAQ824 upon the global transcriptional activity of the IL-10 promoter was evaluated next by determining the binding of Pol-II to the IL-10 gene promoter. Unlike macrophages treated with LPS alone, only a minimal and transient increase in Pol-II binding was observed in macrophages treated with LAQ824 plus LPS (Fig. 7D).
Figure 7. Chromatin modifications in macrophages treated with LAQ824.
RAW264.7 cells were treated with LAQ824 (20 nM) for 24 hours. Cells were then washed and treated –or not– with LPS (1 mcg/mL) plus LAQ824 –or not– to maintain pretreatment conditions. Cells were collected at baseline (time 0), or at 30, 60, 120 or 180 minutes after LPS treatment and then subjected to ChIP analysis using: (A) an antibody to phosphorylated Ser 10 of H3, (B) anti-hyperacetylated H3, (C) anti-hyperacetylated H4, (D) anti-polymerase II, (E) anti-Sp1, (F) anti-STAT3, (G) anti-PU.1, or (H) anti-HDAC11. Quantitative RT-PCR analysis of the IL-10 gene promoter was performed. Values were obtained with the Pfaffl method and are presented relative to input before immunoprecipitation. Arbitrary units are presented as percentage relative to no-treatment condition. Error bars represent standard deviation from triplicates.
Sp1 and STAT3 are known IL-10 transcriptional activators that are recruited to the proximal region of the IL-10 gene promoter in macrophages stimulated with LPS(37-39). As shown in Fig. 7E, recruitment of Sp1 peaks at 1 hour after LPS stimulation and is then followed by a rapid decline at 2 hours. Similarly, STAT3 binding to the IL-10 promoter is evident within 60 minutes of LPS stimulation. This is followed by a progressive decline to basal levels by 3 hours (Fig. 7F). No such kinetics of recruitment of either Sp1 or STAT3 was observed in cells treated with LAQ824 and LPS (Fig. 7E-F, dashed lines).
PU.1 is a transcriptional repressor that interacts with the distal IL-10 gene promoter region(40, 41). Recently, we have demonstrated that HDAC11, by interacting with the distal segment of the IL-10 gene promoter, negatively regulates the expression of this cytokine in murine and human APCs(32). As shown in Figure 7G-H, ChIP analysis of the IL-10 gene distal promoter in macrophages treated with LAQ824 revealed some interesting findings. Recruitment of PU.1 to the IL-10 gene promoter in cells treated with LPS reached a peak within two hours and remained elevated for the duration of the analysis (Fig. 7G, solid line). In macrophages treated with LAQ824, the binding of PU.1 was already elevated at time zero, and increased further in response to LPS stimulation (Fig. 7G, dashed line). A similar pattern was observed when we evaluated the kinetics of HDAC11 recruitment to the IL-10 gene promoter. First, in macrophages treated with LPS alone, HDAC11 binding was detectable after 2 hours, the change was modest in magnitude and it remained slightly elevated at 3 hours (Fig. 7H, solid line). In macrophages treated with LAQ824, recruitment of HDAC11 reached a higher peak (relative to macrophages treated with LPS alone) within two hours and it was followed by a rapid return to baseline levels at 3 hours after LPS stimulation (Fig. 7H, dashed line).
Therefore, treatment of macrophages with LAQ824 is associated with several chromatin changes at the level of the IL-10 gene promoter, among them an enhanced recruitment of the transcriptional repressors PU.1 and HDAC11.
DISCUSSION
In this study we have demonstrated that the pan-HDI LAQ824, by inhibiting IL-10 and increasing the expression of B7.2 and the production of several pro-inflammatory mediators, induced inflammatory macrophages that effectively activate antigen-specific CD4+ T-cells and restore the responsiveness of anergic T-cells.
Among the above changes, the most striking effect of LAQ824 was its ability to inhibit the production of the immunosuppressive cytokine IL-10. Such an effect was also displayed by other members of the hydroxamic acid family like LBH589, TSA and SAHA, but not by the more specific HDI MS-275, which mainly target Class I HDACs. The central role of IL-10 in the establishment and maintenance of T-cell anergy (34, 35) (42-44), prompted us to further investigate the underlying mechanism(s) by which these particular HDIs inhibit IL-10 production in macrophages.
The dynamic production of pro- and anti-inflammatory mediators at the site of antigen encounter has been shown to shape the initiation, magnitude and duration of an immune response(45). IL-10 plays a key role in negatively regulating these dynamic changes to prevent self-tissue damage that might otherwise occur if an ongoing immune response is not kept in check(39). This protective property of IL-10 imposes however a significant barrier to our efforts to effectively harness antitumor immune responses. Indeed, production of this cytokine at the tumor site by tumor cells themselves or infiltrating cells, such as APCs or regulatory T-cells, creates a microenvironment that is conducive to T-cell anergy rather than T-cell activation(5). A better understanding of the genetic and/or epigenetic mechanisms regulating IL-10 production has therefore important implications to manipulate the inflammatory status of antigen-presenting cells and their intrinsic ability to prime, or not, antigen-specific T-cell responses.
Recent studies have shown that IL-10 production is regulated at the chromatin level by changes in the acetylation status of the gene promoter(46, 47). For instance, changes in the chromatin structure of the IL-10 promoter in T-cells differentiated into the TH1 or TH2 phenotype closely regulate IL-10 expression(48). In macrophages, increased acetylation of the IL-10 promoter has been associated with enhanced transcriptional activity(46). Conversely, we have recently demonstrated that decreased acetylation of the IL-10 promoter is associated with decreased IL-10 transcriptional activity in murine and human APCs(32). Given the above, we expected that treatment of macrophages with HDIs would result in increased histone acetylation and increased IL-10 production. Although, an increased global acetylation of histones H3 and H4 was observed in cells treated with LAQ824 (Fig. 1A), the opposite outcome was observed at the level of the IL-10 gene promoter of HDI-treated cells. To our surprise, diminished H3 and H4 acetylation at the IL-10 promoter was observed at all evaluated time-points in treated macrophages (Fig. 7B-C). This decrease in histone acetylation, which occurs early, might explain the decreased recruitment of the transcriptional activators STAT3 and Sp1 to the IL-10 gene promoter (Fig 7E-F). It is plausible that a more compacted chromatin due to diminished histone acetylation in the IL-10 promoter region might block the access of these transcriptional activators to the promoter region resulting in the decreased IL-10 gene transcriptional activity observed in LAQ824-treated cells.
However, we are left to explain how HDIs induce decreased histone acetylation at the level of the IL-10 gene promoter in the first place. Kinetic analysis of H3 and H4 acetylation provided some hints. In macrophages pre-treated with LAQ824 and then stimulated with LPS, we observed an initial acetylation of H3 and H4 that reaches its peak at one hour post-stimulation. However, the magnitude of these changes was significantly lower than in macrophages treated with LPS alone. Following this peak acetylation, we observed a rapid abrogation of such a response in cells treated with HDI, suggesting either a lack of stimuli to support H3 and H4 acetylation and/or the induction of counter-regulatory mechanism(s) that attenuated the degree of H3 and H4 acetylation observed in cells treated with LPS alone.
Of particular interest is the finding that following stimulation, enhanced recruitment of two transcriptional repressors to the distal promoter of IL-10 was observed, a process that occurs faster for PU.1 than for HDAC11 (Fig. 7G-H). It is plausible therefore that recruitment of PU.1 and HDAC11 to the IL-10 gene promoter might represent a counter-regulatory mechanism triggered by HDI to diminish H3 and H4 acetylation and block the sequence of events that lead to IL-10 gene transcriptional activation. Supporting the above, we have recently demonstrated that over-expression of HDAC11 in the macrophage cell line RAW264.7 resulted in decreased H3 and H4 acetylation of the IL-10 gene promoter and inhibition of IL-10 gene transcriptional activity(32). Interestingly, Bradbury et al. have found that treatment of myeloid leukemic blasts with TSA, a member of the hydroxamic acid family of HDIs, resulted in a 60- to 200-fold induction of HDAC11mRNA expression (49). Similarly, we have also found that RAW264.7 cells treated with either TSA or LAQ824 display increased HDAC11 mRNA and protein expression (data not shown). Such an effect of HDIs upon HDAC11 expression might explain –at least in part– the increased recruitment of this particular HDAC to the IL-10 gene promoter in cells treated with LAQ824. The mechanism(s) by which HDI increases the expression of HDAC11 in macrophages, however, remains to be elucidated.
An additional observation in macrophages treated with LAQ824 is their enhanced expression of B7.2 and increased production of several pro-inflammatory mediators, in particular IL-12. It is plausible that these effects are secondary to the inhibition of IL-10, a cytokine that has been shown to down-regulate the expression of co-stimulatory molecules and the production of IL-12 in APCs (50, 51). This switch from an IL-10 to an IL-12 producing and B7.2-expression state is critical in polarizing T-cells towards TH1, cell-mediated immunity. In our system, the demonstration that H3 and H4 acetylation in the IL-12 promoter was neither inhibited nor enhanced in cells treated with LAQ824 (Fig. 6D), suggests that the enhanced production of this pro-inflammatory mediator in treated macrophages might be secondary to the abrogation of IL-10 production by LAQ824. Our experiments using recombinant IL-10 also point to a contributory role of IL-10 inhibition (induced by LAQ824) in the enhanced APC function displayed by treated macrophages since this effect was reversed when recombinant IL-10 was added-back to the cultures. It remains to be demonstrated whether the upregulation of B7.2 is a direct or indirect effect of LAQ824. It is noteworthy to mention that B7.2 was preferentially upregulated in treated macrophages while no changes were observed in various other costimulatory molecules (data not shown). Though the exact mechanism for this selectivity has yet to be elucidated, our results are consistent with observations in human DCs whereby IL-10 mediated downregulation of B7.2, but not B7.1(50).
Our findings are at odds with the studies by Reddy et al, who recently found that the HDI SAHA actually attenuated inflammatory responses in DCs through IDO-dependent mechanisms(23). Injection of DCs treated ex vivo with SAHA also decreased the severity of graft-versus-host disease (GvHD) in their murine allogeneic bone marrow transplantation model. Several differences between Reddy’s study and ours may explain these seemingly conflicting data. First, in our in vitro system we used macrophages that were treated with HDI and LPS given at the same time. In their study, DCs primarily, but in one experiment macrophages, were pretreated with SAHA prior to stimulation with TLR agonist. Second, they found that SAHA treatment did not induce significant changes in the production of IL-10 by DCs. Supporting their observation, we have also found that among all the members of the hydroxamic family of HDI, SAHA is the weakest inhibitor of IL-10 production in macrophages (data not shown). It is plausible therefore that differences among members of the same family of HDI, with LAQ824 and LBH589 being more potent inhibitors of IL-10 production than SAHA, might explain at least in part the divergent effects of these HDI upon the inflammatory status of DCs and/or macrophages. Of note, the overall potencies of HDIs must also be reconciled with the relative potencies as pan-HDIs do not inhibit all members of the HDAC family at equimolar concentrations, which may explain the differing effects on a single target such as IL-10 (52). Our findings are however consistent with reports by others indicating that HDIs can potentiate inflammatory and anti-tumor responses in vitro and in vivo(25-27). Among the latter, Vo et al. has demonstrated an enhanced T-cell mediated immunity in tumor bearing mice treated with LAQ824. Whether the preferential expansion of adoptively transferred antigen-specific T-cells observed in this model is the result of T-cell interaction with inflammatory APCs induced by in vivo treatment with LAQ824 treatment remains to be explored.
Taken together, LAQ824-treated macrophages not only can more potently activate naïve T-cells, but also restore the reactivity of anergized T-cells from tumor bearing mice. LAQ824 by inducing inflammatory cells can potentially tip the balance towards T-cell activation rather than T-cell anergy, an effect that holds promise for the use of HDIs in the adjuvant setting to ultimately improve the efficacy of cancer immunotherapy.
Acknowledgments
This work was supported by PHS grants CA87583 and CA134807 (EMS) and by a grant from the Donald A. Adam Comprehensive Melanoma Research Center (CMRC).
ABBREVIATIONS
- HDAC
Histone Deacetylase
- HDI
HDAC Inhibitor
- APC
Antigen Presenting Cell
- PEM
Peritoneal Elicited Macrophage
- HA
hemagglutinin
Footnotes
Disclosures
The authors have no financial conflict of interest
References
- 1.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Staveley-O'Carroll K, Levitsky HI. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 4.Cuenca A, Cheng F, Wang H, Brayer J, Horna P, Gu L, Bien H, Borrello IM, Levitsky HI, Sotomayor EM. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003;63:9007–9015. [PubMed] [Google Scholar]
- 5.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogen B, Munthe L, Sollien A, Hofgaard P, Omholt H, Dagnaes F, Dembic Z, Lauritzsen GF. Naive CD4+ T cells confer idiotype-specific tumor resistance in the absence of antibodies. Eur J Immunol. 1995;25:3079–3086. doi: 10.1002/eji.1830251114. [DOI] [PubMed] [Google Scholar]
- 8.Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 9.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr., Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 10.Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, Kerr WG, Takeda K, Akira S, Schoenberger SP, Yu H, Jove R, Sotomayor EM. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19:425–436. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 11.Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells' ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horna P, Sotomayor EM. Cellular and molecular mechanisms of tumor-induced T-cell tolerance. Curr Cancer Drug Targets. 2007;7:41–53. doi: 10.2174/156800907780006940. [DOI] [PubMed] [Google Scholar]
- 13.Song XT, Evel-Kabler K, Shen L, Rollins L, Huang XF, Chen SY. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med. 2008;14:258–265. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.From immunity to tolerance through HDAC. Nat Immunol. 2009;10:13–14. doi: 10.1038/ni0109-13. [DOI] [PubMed] [Google Scholar]
- 15.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 16.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 17.Yang XJ, Seto E. Collaborative spirit of histone deacetylases in regulating chromatin structure and gene expression. Curr Opin Genet Dev. 2003;13:143–153. doi: 10.1016/s0959-437x(03)00015-7. [DOI] [PubMed] [Google Scholar]
- 18.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, Curly T, Moskowitz C, Portlock C, Horwitz S, Zelenetz AD, Frankel S, Richon V, Marks P, Kelly WK. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24:166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 20.A P. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 21.Camelo S, Iglesias AH, Hwang D, Due B, Ryu H, Smith K, Gray SG, Imitola J, Duran G, Assaf B, Langley B, Khoury SJ, Stephanopoulos G, De Girolami U, Ratan RR, Ferrante RJ, Dangond F. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, Mascagni P, Fantuzzi G, Dinarello CA, Siegmund B. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015–5022. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 23.Reddy P, Sun Y, Toubai T, Duran-Struuck R, Clouthier SG, Weisiger E, Maeda Y, Tawara I, Krijanovski O, Gatza E, Liu C, Malter C, Mascagni P, Dinarello CA, Ferrara JL. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA, Ferrara JL. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasi TB, Magner WJ, Khan AN. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–1184. doi: 10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan AN, Tomasi TB. Histone deacetylase regulation of immune gene expression in tumor cells. Immunol Res. 2008;40:164–178. doi: 10.1007/s12026-007-0085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ, Craft N, Economou JS, Marincola FM, Wang E, Ribas A. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suarez I, Takahashi Y, Cheng F, Horna P, Wang HW, Wang HG, Sotomayor EM. Identification of a novel negative role of flagellin in regulating IL-10 production. Eur J Immunol. 2007;37:3164–3175. doi: 10.1002/eji.200737306. [DOI] [PubMed] [Google Scholar]
- 30.Sotomayor EM, Fu YX, Lopez-Cepero M, Herbert L, Jimenez JJ, Albarracin C, Lopez DM. Role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. II. Down-regulation of macrophage-mediated cytotoxicity by tumor-derived granulocyte-macrophage colony-stimulating factor. J Immunol. 1991;147:2816–2823. [PubMed] [Google Scholar]
- 31.W M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, Nguyen D, Wright KL, Atadja PW, Bhalla K, Pinilla-Ibarz J, Seto E, Sotomayor EM. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339–359. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- 34.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells [see comments] J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.H R. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 36.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 37.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 38.Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- 39.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 40.Reuss E, Fimmers R, Kruger A, Becker C, Rittner C, Höhler T. Differential regulation of interleukin-10 production by genetic and environmental factors - a twin study. Genes and Immunity. 2002;3:407–413. doi: 10.1038/sj.gene.6363920. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki M, Yamada T, Kihara-Negishi F, Sakurai T, Oikawa T. Direct association between PU.1 and MeCP2 that recruits mSin3A-HDAC complex for PU.1-mediated transcriptional repression. Oncogene. 2003;22:8688–8698. doi: 10.1038/sj.onc.1207182. [DOI] [PubMed] [Google Scholar]
- 42.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 43.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Edwards JP, Mosser DM. Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription. J Immunol. 2006;177:1282–1288. doi: 10.4049/jimmunol.177.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–1903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Im SH, Hueber A, Monticelli S, Kang KH, Rao A. Chromatin-level regulation of the IL10 gene in T cells. J Biol Chem. 2004;279:46818–46825. doi: 10.1074/jbc.M401722200. [DOI] [PubMed] [Google Scholar]
- 49.Bradbury CA, Khanim FL, Hayden R, Bunce CM, White DA, Drayson MT, Craddock C, Turner BM. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia. 2005;19:1751–1759. doi: 10.1038/sj.leu.2403910. [DOI] [PubMed] [Google Scholar]
- 50.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–762. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 51.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. Am J Pathol. 2004;165:1853–1863. doi: 10.1016/S0002-9440(10)63238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]