1. Introduction
1.1 Neural basis of abstract semantic knowledge
Concreteness is a critical organizing factor in semantic memory and recognition of the dichotomy between abstract and concrete concepts has a long history in psychology and philosophy (Locke, 1685). An extensive empirical literature supports this dichotomy. The “concreteness effect” - concrete concepts are easier to learn, use, recall and recognize – has been shown in a wide variety of tasks, in both patient groups and healthy populations (Bleasdale, 1987; Day, 1977; de Groot, 1989; Howell & Bryden, 1987; James, 1975; Kroll & Merves, 1986; Rubin, 1980; Whaley, 1978).
However attempts to localize neural differences between abstract and concrete concepts has proven difficult. Patients with specific cortical lesions who have acquired language deficits most commonly experience greater impairments with abstract words than concrete words, but the lesions associated with this deficit do not have a consistent localization (Coltheart, 1980; Goodglass, Hyde, & Blumstein, 1969; Katz & Goodglass, 1990; Martin & Saffran, 1992; Saffran & Martin, 1990). In contrast, only a small number of cases exist in which a reversal of the concreteness effect – a specific deficit for concrete words leaving abstract words intact – have been reported (reviewed by Bonner et al., 2009). Bonner and colleagues (2009) identified 11 patients with semantic dementia who exhibited the reversal of the concreteness effect, specifically with visual-based stimuli, and located their peak neurological degeneration to a portion of the ventral surface of the anterior temporal lobes. This region is commonly affected in semantic dementia, however the reversal of the concreteness effect is quite rare in these patients (Hoffman & Lambon Ralph, 2011).
Neuroimaging studies have also yielded inconsistent findings. For example, some neuroimaging studies have shown stark distinctions in regional activity throughout the brain for concrete and abstract concepts, with almost no overlap (Binder, Westbury, Mckiernan, Possing, & Medler, 2005; D’Esposito et al., 1997; Perani et al., 1999; Wise et al., 2000) while others have failed to identify any regional differences, with all activations completely overlapping (Beauregard et al., 1997; Fiebach & Friederici, 2003; Friederici, Opitz, & von Cramon, 2000; Kiehl et al., 1999; Noppeney & Price, 2004; Sabsevitz, Medler, Seidenberg, & Binder, 2005). In a recent meta-analysis of the neuroimaging literature Binder and colleagues (2009) reported that the left inferior frontal gyrus (IFG) and the anterior-most portion of the left superior temporal sulcus (STS) were consistently activated in processing or retrieving abstract knowledge (see also Wang, Conder, Blitzer, & Shinkareva, 2010). Although this finding is promising, it is important to note that there are large inconsistencies between individual studies thus it is difficult to identify cortical regions specific to processing abstract conceptual knowledge. One explanation for these disparate findings is that important stimulus dimensions were not controlled in the tested abstract concepts, an issue that is discussed in the next section.
1.2 Concreteness, Imageability and Emotional Valence
An important advancement in studying the representational foundations of abstract concepts is the idea that abstract concepts may be grounded, or embodied, in affective meaning. The affective embodiment account suggests that while concrete words are learned and understood through sensory-motor referents, abstract words are learned and understood through emotional referents, and that emotional valence is a key component of abstract conceptualization (Vigliocco, Meteyard, Andrews, & Kousta, 2009).
Proponents of the AEA insist that this prior research has overlooked a key confounding variable: imageability. Most research in this field covaries imageability with concreteness because these two variables are tightly linked, but it is important to note their distinctions. Imageability is typically defined as the ease to which a word can evoke a visual image, while concreteness typically refers to whether the concept itself is situated in time and space (see for example, Paivio, 1967). These variables are conceptually related and tightly correlated with each other (e.g. imageability can account for 72% of the variability in concreteness (Kousta et al., 2011), but nevertheless, distinct. Kousta and colleagues demonstrated that when imageability is controlled between abstract and concrete words, the concreteness effect disappears and in fact, abstract words are processed more quickly than concrete words (Kousta et al., 2011).
On this evidence, the AEA is formed. This account suggests that three kinds of information contribute to semantic knowledge: sensorimotor, affective and linguistic (Vigliocco et al., 2009). What ultimately divides abstract words from concrete words is that abstract words are more dependent on affective and emotional information, and concrete words are more dependent upon sensorimotor information, and both rely on linguistic information to some degree. According to this model, imageability is related, but ultimately independent, and failure to control for imageability in studies of concreteness have led to inaccurate findings. Emotional valence, in this model, works as a function of abstractness and cannot be controlled without losing some essence of abstract meaning. The decision to control one variable, and not the other, has obvious implications for behavioral research, as demonstrated by the absence and so-called reversal of the concreteness effect found by Kousta and colleagues (2011). It also has implications for studying the neural representation of abstract concepts, described below.
1.3 Neuroimaging Concreteness and Valence in the Anterior Cingulate
In a recent study, subjects were asked to carry out a lexical decision task on abstract and concrete words while undergoing an fMRI scan (Vigliocco, Kousta, Della Rosa, Vinson, Tettamanti, Devlin & Cappa, 2013). The abstract and concrete words were tightly controlled on an impressive range of lexical and sublexical variables, including imageability. However, the abstract words were significantly more valenced than the concrete words, using a measure of hedonic valence that does not differentiate negativity from positivity. The results of a subtraction analysis indicated that recognition of abstract concepts was associated with activations in one region: the rostral anterior cingulate cortex (rACC). Within the rACC alone, BOLD activity was modulated by hedonic valence. The authors argue that this evinces that abstract concepts are grounded in affective experience while concrete concepts are grounded in sensory-motor experience and that this has a neurological basis.
An alternative explanation for this finding is that the rACC was responding to emotional valence rather than abstract concepts per se. There is sound evidence behind this explanation. The rostral and ventral aspects of the ACC are part of paralimbic cortex (Bush, Luu, & Posner, 2000), playing a specific role in social and emotional processes, such as monitoring behavioral expectations (Apps, Balsters, & Ramnani, 2012). Non-human primates with experimentally created lesions to this region show decreased social interaction and decreased preference for social information (Hadland, Rushworth, Gaffan, & Passingham, 2003; Rudebeck, Bannerman, & Rushworth, 2008; Rudebeck, Buckley, Walton, & Rushworth, 2006). Neuroimaging studies have reported activations in this region to a wide variety of social-emotional tasks and manipulations such as watching emotional films (Lane, Reiman, & Schwartz, 1998) or social cartoons (Castelli, Frith, Happe, & Frith, 2002; Castelli, Happe, Frith, & Frith, 2000). This region has been implicated in social anxiety and processing of emotional faces (Klumpp, Post, Angstadt, Fitzgerald, & Phan, 2013). Perhaps most tellingly, activations are even observed with more subtle manipulations of emotion, such as the contrast between emotional and neutral words. Whalen and colleagues scanned subjects while they performed an emotional Stroop task and reported that emotion words, but not neutral words, activated the ventral ACC (Whalen et al., 1998).
1.4 Goals of this study
This study had two goals. First, we examined whether abstract verbal stimuli activate cortical regions overlapping with those activated by emotional stimuli, while carefully controlling emotional valence across the abstract and concrete stimuli. Using a 2 × 2 (concreteness × emotional valence) design, we asked participants to think deeply about word meanings, and to answer semantically meaningful questions, while undergoing an MRI scan. We then used subtraction analyses to explore whether any shared cortical regions responded to abstract concepts and to emotionally valenced concepts. Second, we specifically tested whether the rACC, cited in previous research as being selectively sensitive to abstract concepts and modulated by valence, would respond to abstract words more than concrete, when valence is controlled. In other words, we tested whether the rACC responded to valence regardless of concreteness, which would refute the specific hypothesis that the rACC plays a role in abstract conceptual knowledge in addition to responding to emotional valence. Unlike previous neuroimaging studies cited in the two major reviews discussed in the introduction (Binder et al., 2009; Wang et al., 2010), our two independent factors of emotional context and concreteness allowed us to explore areas of neural overlap between abstract concepts and emotional concepts. Furthermore, our stimuli were matched on a much larger range of lexical and sublexical variables, as compared to neuroimaging studies that pre-date studies by Vigliocco and colleagues (2013).
2. Materials and Methods
2.1 Participants
Nineteen young adults were recruited to participate through Temple University (11 female, mean age = 23 years). All participants were neurologically and psychologically healthy, native English speakers, and right handed.
2.2 Stimuli
Stimuli consist of 164 nouns collected from the MRC psycholinguistic database (Wilson, 1988). The words are either very abstract (concreteness <350, n=82) or very concrete (concreteness < 550, n=82) and also vary along the dimension of imageability, such that abstract words had low imageability scores and concrete words had high imageability scores. Imageability was intentionally differed between the abstract and concrete condition, although it was controlled in the study we are attempting to replicate (Vigliocco et al., 2013). We chose to not control imageability, because imageability and concreteness are tightly correlated with each other (Paivio, 1968), and because visual images are easy to generate for most concrete concepts, but not for most abstract concepts (at least not without resorting to metaphors, see for example: Barsalou & Wiemer-Hastings, 2005). The issue of choosing which variables to control in a psycholinguistic experiment are crucial, and there is often a trade-off of purity versus naturalistic stimulus sampling (Cutler, 1981). Vigliocco and colleagues assert that controlling imageability is critical when trying to isolate the effects of concreteness (Kousta et al., 2011; Vigliocco et al., 2013). However, we assert that imageability is so closely related to concreteness, that controlling for imageability leaves researchers with a highly unusual sample of abstract words that are highly imageable, and that such a sample is not representative of abstract words as a whole. Since concreteness and imageability are so tightly linked, we chose to vary imageability along with concreteness, but this decision and the tradeoff is discussed more in the general discussion.
Word stimuli were either highly emotionally valenced (n=82) or neutral (n=82). Valence and arousal measures were collected by testing an independent sample of 15 students at Temple University. The words were rated on a 7-point likert scale for arousal (1=low arousal, 7=high arousal) and valence (1=negative, 4=neutral, 7=positive). The responses on the valence scale were then transformed into a measure of hedonic valence, which indicates distance from neutral regardless of whether a word is positive or negative. Since abstract-valenced words tend to be more social in nature, all words in the emotionally valenced conditions were explicitly social, and typically person-labels. This allowed for a qualitatively better controlled set of stimuli, in that words like guilt (abstract) and thief (concrete) are better matched than words like guilt and snake. A large database of valence and arousal ratings has recently been published (Warriner, Kuperman, & Brysbaert, 2013), to which we compared our ratings. The new database did not have valence or arousal scores for 18 of our 160 words, but in general our ratings corresponded well. Table 1 shows a complete list of the verbal stimuli used in this experiment.
Table 1.
Verbal stimuli used in the fMRI experiment. Stimuli were rated for arousal and valence.
| Abstract | Concrete | ||
|---|---|---|---|
| Neutral | Valenced | Neutral | Valenced |
| ABUNDANCE | ACCUSER | ARC | ACROBAT |
| ADDITION | ADVICE | BEE | ADULT |
| AMENDMENT | AGREEMENT | BUTTERFLY | AVIATOR |
| BENEFIT | ALLEGATION | CATERPILLAR | BANDIT |
| CAPACITY | APTITUDE | CENT | BROTHER |
| CHAOS | ATTITUDE | CIRCUIT | CHILD |
| CHRONOLOGY | BELIEF | COAST | CORPSE |
| CIRCUMSTANCE | BENEFACTOR | CONSTRUCTION | DAMSEL |
| CITATION | BEQUEST | CONTINENT | DOCTOR |
| CONDITION | CRITICISM | CRANBERRY | EMBRACE |
| CONTENTS | DEBATE | DANDELION | EXAMINATION |
| DIMENSION | DECEIT | DAYLIGHT | FATHER |
| DISPARITY | DEITY | FOUNDATION | FISHERMAN |
| ESSENCE | DESPERATION | HONEYCOMB | FOREHEAD |
| EXCEPTION | DISCUSSION | HURRICANE | GENTLEMAN |
| EXCISE | DISGRACE | INSECT | GRANDFATHER |
| EXTERIOR | DISHONESTY | INTERIOR | GRANDMOTHER |
| EXTRA | DISPOSITION | LETTUCE | HENCHMAN |
| FORMER | EXCUSE | LINK | INSTRUCTOR |
| GAIN | FEAR | MICROSCOPE | KISS |
| HEREDITY | FORFEIT | MOUNTAIN | LADY |
| INCREMENT | FOUNDER | NIGHTFALL | LAUGH |
| INFERIOR | GUESS | PROJECTOR | MINISTER |
| INFINITY | GUILT | PROPELLER | MONASTERY |
| INSTANCE | HERESY | PROVISION | MUSICIAN |
| ITEM | HONESTY | RECTANGLE | NURSERY |
| LACK | HUMILIATION | REGISTER | OFFICER |
| MAGNITUDE | INNOCENCE | SHORE | PHYSICIAN |
| NECESSITY | INTEGRITY | SPINACH | PIANIST |
| ORIGINAL | INTELLECT | STRAWBERRY | POLICEMAN |
| PERCEPTION | MOTIVE | SUNSHINE | POLITICIAN |
| PRETENSE | NICETY | TABLESPOON | PROFESSOR |
| PRIOR | PACT | TERRITORY | PROSECUTOR |
| PROPORTION | PROMISE | THERMOMETER | SISTER |
| QUANTITY | REMARK | THORN | SMILE |
| RARITY | RIDICULE | TRIANGLE | SOPRANO |
| REPLACEMENT | SCHEME | TWILIGHT | STUDENT |
| SELECTION | SCORN | VEGETABLE | SULTAN |
| SUPERIOR | UNBELIEVER | VOLCANO | TEACHER |
| SURPLUS | VOW | WEED | THIEF |
All conditions were also matched on frequency per million words in the SUBTLEX database, with the exception of the concrete-valenced words which had a higher frequency than the other three conditions; although the mean differences were very small (mean=2.9 words per million for concrete-valenced, mean=2.3 words per million for the concrete-neutral words for example) they were significant. Concrete and abstract words differed on age of acquisition, such that abstract words were learned at a younger age than concrete words, using the recent Kuperman norms (Kuperman, Stadthagen-Gonzalez, & Brysbaert, 2012). However, valenced and neutral words did not vary on age of acquisition, for either the concrete or the abstract words. Stimuli were matched for word length, as measured by both number of letters and number of phonemes, and familiarity as collected from the MRC psycholinguistic database (Coltheart, 1981). All conditions were controlled for orthographic neighborhood, sum bigram frequency, number of morphemes, number of phonemes and number of letters, all collected from the English Lexicon Project (Balota et al., 2007). Table 2 shows the mean scores on psycholinguistic variables for each condition.
Table 2.
Mean scores on psycholinguistic variables for each condition. Abstract and concrete words differed significantly on measures of concreteness and imageability. Neutral and valenced words differed significantly on the measures of arousal and hedonic valence. Log SUBTLEX WF refers to the log of the frequency count per million in the SUBTLEX database.
| Abstract | Concrete | |||
|---|---|---|---|---|
| neutral | valenced | neutral | valenced | |
| Concreteness | 310.91 | 302.97 | 544.53 | 543.63 |
| Imageability | 333.63 | 342.30 | 563.68 | 571.00 |
| Hedonc Valence | 0.01 | 1.69 | 0.01 | 1.45 |
| Arousal | 3.01 | 4.60 | 2.61 | 4.55 |
| Age of Acquisition | 9.93 | 9.51 | 7.25 | 7.45 |
| log SUBTLEX WF | 2.24 | 2.36 | 2.31 | 2.90 |
| Orthographic Neighbors | 1.10 | 1.45 | 1.95 | 0.75 |
| Sum Bigram Frequency | 15252.05 | 13275.18 | 15216.61 | 13010.87 |
| Number of Morphemes | 1.82 | 1.64 | 1.64 | 1.67 |
| Number of Phonemes | 7.03 | 6.43 | 6.43 | 6.35 |
| Number of Letters | 7.83 | 7.33 | 7.85 | 7.35 |
The stimuli also include a set of pronounceable nonwords, matched for length with the real word stimuli (selected from the set used by Binder, Westbury, McKiernan, Possing, & Medler, 2005).
2.3 Task
All subjects were given a practice version of the task prior to entering the MRI scanner. The practice task was a shorter version of a single run in the scanner, and utilized words and nonwords not used in the main MRI task.
This experiment was carried out in a block design to maximize power. Each block lasted for 12 s, followed by a 4 s question screen. Each block began with the presentation of a single word in black sans serif font the center of the screen, which remained on the screen for 3500 ms. Participants were instructed to think deeply about the concept that the word describes during this time. The word is then removed, and a fixation appeared for 500 ms, followed by another word, again presented for 3500 ms. In total, three words and three fixations appeared consecutively within a single block, totaling to 12 s. Following the third and final fixation, a question screen appeared and remained on the screen for 4000 ms . The participants’ task was to respond with a “yes” or “no” to the question in reference to the three words in that block. For example, a question may be “Is one a member of a family?” or “Is one found in a store?” For the nonword blocks, the question was about spelling, for example “Did one begin with P?” The questions were not controlled on any psycholinguistic variables, because the question screen was not included in the imaging analyses; only the 12 s block was modeled. However, if questions were answered incorrectly, that block was removed from analysis. A schematic depiction of an example trial block is shown in Figure 1.
Figure 1.
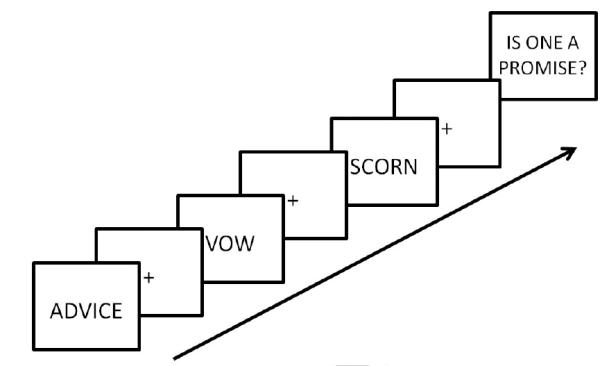
Schematic illustration of a single block during the MRI scan. All three words were either abstract or concrete, as well as being either emotionally valenced or neutral. On the final screen, the participant reads and responds to a question pertaining to the three words in the block. Only the time during the word reading and fixation were included when modeling the hemodynamic response.
2.4 Imaging Parameters
Neuroimaging sessions were conducted at the Temple University Hospital on a 3.0 T Siemens Verio scanner (Erlangen, Germany) using a twelve-channel Siemens head coil.
Functional T2*-weighted images sensitive to blood oxygenation level-dependent contrasts were acquired using a gradient-echo echo-planar pulse sequence (repetition time (TR), 2s; echo time (TE), 19 ms; FOV= 240 × 240; voxel size, 3 × 3 × 3 mm; matrix size, 80 × 80; flip angle = 90°) and automatic shimming. This pulse sequence has been optimized for anterior temporal lobe (ATL) coverage and sensitivity based on pilot scans performed for this purpose, details of which are reported in Ross and Olson (Ross & Olson, 2010). Participants underwent five functional runs, each consisting of 165 TRs, including the introduction and closing slides. Forty slices were collected. Temporal lobe function was of particular interest to this study and for other analyses that were conducted but not reported here, and the design presented here optimizes coverage of the entire temporal lobe, sometimes at the cost of losing portions of the most superior slices covering frontal and parietal lobes.
The five functional runs were preceded by a high-resolution structural scan. The scanning procedure began with an approximately 10 min long high- resolution anatomical scan. The anatomical image was used to fit the volume of covered brain tissue acquired in the functional scan. The T1-weighted images were acquired using a three-dimensional magnetization-prepared rapid acquisition gradient echo pulse sequence (TR, 3 s; TE, 3 ms; FOV=201 × 230 mm; inversion time, 900 ms; voxel size, 1 × 0.9000 × 0.9000 mm; matrix size, 256 × 256 × 256; flip angle=15°, 160 contiguous slices of 0.9 mm thickness). Visual stimuli were shown through a projection system, and participants can view the screen through mirrors mounted on the headcoil. The stimulus delivery was controlled by E-Prime software (Psychology Software Tools Inc., Pittsburgh, PA) on a windows laptop located in the scanner control room.
2.5 Image Preprocessing
fMRI data was preprocessed and analyzed using FSL software (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). The preprocessing of the functional data includes a correction for head motion (trilinear/sinc interpolation), the removal of linear trends and high-pass temporal filtering. The resulting volumetric time course data were then smoothed using a 6mm FWHM Gaussian kernel.
For all blocks, a canonical hemodynamic response function (HRF) was modeled spanning the 12s for each block. Predictors were built by convolving the boxcar waveform for each condition with a double-gamma hemodynamic response function. Motion parameters were not included as covariates in the regression, because motion was corrected for in preprocessing. Including motion covariates in the regression has been shown to have a deleterious effect on the mean contrast estimates in block design studies (Johnstone, Ores Walsh, Greischar, Alexander, Fox, Davidson & Oakes, 2006).
Whole brain analysis went through three steps. First, we explored for regions that increased activity in response to abstract words by carrying out a subtraction of abstract words versus nonwords. We also contrasted concrete words against nonwords. We chose to use a nonword baseline, rather than contrasting abstract words to concrete words directly, so that we could examine any overlapping regions for abstract and concrete words. However, we additionally contrasted abstract and concrete words directly in a whole brain subtraction. Second, we searched for regions that responded to emotional valence, by carrying out a subtraction of valenced words versus neutral words. We also contrasted valenced words to nonwords. Because hedonic valence was of primary interest, we did not compare negative and positive valenced words in this design. All subtractions were examined via mixed-effects analysis presented at an FDR corrected cluster threshold of p=.05.
In addition to this whole brain analysis, we carried out a region of interest analysis on the rostral anterior cingulate cortex (rACC), which in a prior study was reported to respond to both supported abstract concepts and hedonic valence (Vigliocco et al., 2013). Our ROI corresponded to the peak coordinates described in the conjunction analysis of that study. Our regions of interest had a diameter of 6mm and was centered around the MNI coordinates, x=12, y=45, z=12. This region fell with BA 32. Because we used a 2 × 2 (concreteness × valence) design, our abstract and concrete stimuli were similarly valenced, which allowed us to assess whether the rACC was involved both in abstractness and in valence. We then extracted the median parameter estimates (PE) for the four verbal conditions in our study (abstract-neutral, abstract-valenced, concrete-neutral and concrete-valenced) and performed a 2 × 2 ANOVA on these estimates to determine whether this region was primarily modulated by abstractness, valence, or both.
3. Results
3.1 Behavioral Results
A two-way ANOVA was performed on the accuracy data collected during the scans. There was a marginal main effect of concreteness, F(1,18)=4.10, p=.058, due to slightly higher accuracy rates for concrete trials (mean=.893) than for abstract trials (mean=.858). Accuracy was not affected by valence, F(1,18)=0.69, and the interaction was not significant, F(1,18)=3.94, p >.06. All blocks that were answered incorrectly were removed from the neuroimaging analysis.
The response time data, collected when participants were responding to the question screen while in the scanner, mimic the effects observed in the accuracy data. Again, there was a marginal main effect of concreteness, F(1,18)=4.28, p=.053, due to faster responses to concrete words (mean=1986 ms) compared to abstract words (mean=2069 ms). Response times were not affected by valence, F(1,18)=0.02, and there was no interaction, F(1,18)=1.56, p >.22. Table 3 shows the mean accuracy and response times for all conditions.
Table 3.
Average accuracy and response times on the question screen, for all condition. Standard deviation shown in parentheses.
| Accuracy | RT | |||
|---|---|---|---|---|
| neutral | valenced | neutral | valenced | |
| abstract | .879 (.079) | .837 (.121) | 2090.71 (256.88) | 2046.29 (305.81) |
| concrete | .884 (.114) | .902 (.084) | 1967.70 (296.59) | 2005.19 (254.34) |
| nonword | .792 (.104) | --- | 2127.99 (330.97) | --- |
3.2 Neuroimaging Results: Whole Brain Analysis
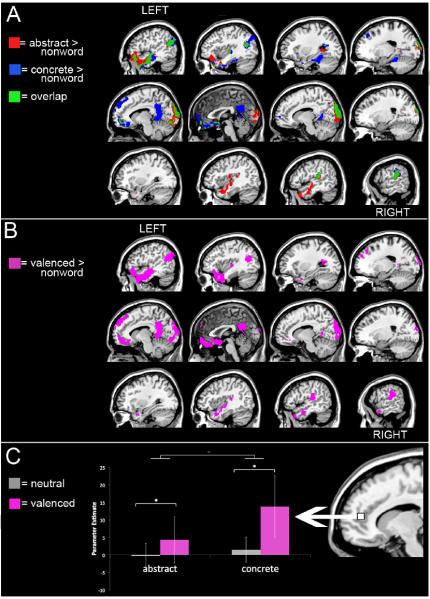
Regions responding to abstract concepts, defined by the contrast abstract words - nonwords (Figure 2A, red-yellow activations) included left lateral frontopolar cortex (BA 10), as well as swath of activation along the left and right superior temporal sulcus (STS) extending into the temporal pole (BA 38). Another cluster of activation was found in the left posterior middle temporal gyrus (MTG), just inferior to the angular gyrus. A large cluster of activation was also found in the medial orbitofrontal cortex (OFC) but did not extend into cingulate cortex. Activation in the right hemisphere was found on the most posterior portion of the STS, extending into the angular gyrus as well as a small cluster in the anterior MTG.
Figure 2.
(a) Map of regions activated in the abstract vs. nonword contrast (red-yellow) and the concrete vs. nonword contrast (blue). Overlapping activations are shown in green.
(b) Map of the regions activated in the valenced vs. nonword contrast.
(c) Region of interest using the peak coordinate from Vigliocco, Kousta, Della Rosa, Vinson, Tettamanti, Devlin & Cappa, 2013, and the mean parameter estimates extracted from this ROI across subjects. This graph shows that there were relatively higher activations to valenced words and concrete words.
Next, we examined activations to concrete concepts, defined by the contrast of concrete words - nonwords (Figure 2A, blue activations). A great deal of activation for this contrast overlapped with the results from the abstract subtraction. Overlapping activations (Figure 2b, green activations) were found in the left temporal pole and STS, as well as the left posterior MTG. Concrete activations also overlapped nearly perfectly with the abstract activations in the medial OFC and medial occipital cortex, and the right posterior STS. Areas that responded uniquely to concrete concepts were found in the left inferior surface of the anterior temporal lobe (ATL), posterior cingulate cortex and medial superior frontal cortex. Regions that remained exclusively responsive to abstract concepts were found only in the right STS and right temporal pole. Table 4 lists the MNI coordinates for peak activations to the abstract and concrete contrasts.
Table 4.
MNI coordinates of the peak activations to the abstract versus concrete, and concrete versus abstract, contrasts.
| Abstract Words > Nonwords | ||||
|---|---|---|---|---|
| Region | BA | x | y | z |
| left anterior middle temporal gyrus | 21 | −56 | −6 | −20 |
| left temporal pole | 38 | −50 | 12 | −30 |
| left posterior middle temporal gyrus | 39 | −50 | −66 | 20 |
| left pars orbitalis | 11 | −46 | 30 | −16 |
| left inferior parietal gyrus | 7 | −38 | −60 | 18 |
| left parahippocampal gyrus | 28 | −36 | 6 | −32 |
| left calcarine sulcus | 26 | −32 | −50 | 2 |
| left middle occipital gyrus | 18 | −14 | −102 | 14 |
| left lingual gyrus | 18 | −10 | −84 | −10 |
| left medial orbitofrontal gyrus | 11 | −6 | 22 | −20 |
| left anterior cingulate | 11 | −6 | 50 | −12 |
| right putamen | 25 | 2 | 12 | −12 |
| right lingual gyrus | 18 | 2 | −90 | −10 |
| right anterior cingulate | 11 | 4 | 52 | −8 |
| right cuneus | 19 | 10 | −92 | 18 |
| right superior temporal pole | 38 | 42 | 8 | −28 |
| right anterior superior temporal sulcus | 22 | 42 | −2 | −20 |
| right middle superior temporal sulcus | 22 | 44 | −12 | −10 |
| right posterior superior temporal gyrus | 22 | 54 | −28 | 14 |
|
| ||||
| Concrete Words > Nonwords | ||||
| Region | BA | x | y | z |
|
| ||||
| left posterior superior temporal gyrus | 39 | −56 | −58 | 20 |
| left anterior middle temporal gyrus | 21 | −54 | −4 | −22 |
| left inferior parietal gyrus | 7 | −48 | −66 | 20 |
| left pars orbitalis | 11 | −48 | 32 | −14 |
| left insula | 52 | −40 | −18 | −2 |
| left superior occipital gyrus | 19 | −14 | −94 | 28 |
| left superior frontal gyrus | 9 | −8 | 54 | 34 |
| left posterior cingulate gyrus | 30 | −6 | −56 | 12 |
| left anterior cingulate gyrus | 32 | −6 | 30 | −14 |
| left medial orbitofrontal gyrus | 11 | −6 | 50 | −14 |
| right medial orbitofrontal cortex | 11 | 2 | 10 | −14 |
| right anterior cingulate | 11 | 2 | 54 | −8 |
| right superior occipital gyrus | 19 | 18 | −96 | 22 |
| right Heschl's gyrus | 41 | 56 | −28 | 20 |
In order to examine whether any overlapping regions might preferentially activate in response to abstract compared to concrete words, we carried out two additional analyses. In the first, we contrasted abstract trials against concrete trials, looking for areas that specifically preferred abstractness. The results revealed a subset of regions identified in the abstract versus nonword comparison. Specifically, areas responding to abstract greater than concrete words were the left inferior frontal cortex and bilateral occipital cortex. We also looked for regions responding to concrete, over abstract, words. The results again revealed only a subset of the activations from the concrete versus nonword comparison. Only one area responded to concrete greater than to abstract words: the inferior angular gyrus.
We performed a subtraction to identify regions that might be modulated by emotional valence. This test contrasted valenced words with neutral words, and was intended to look specifically at a potential role of emotional valence, while subtracting out semantic content. This contrast produced a single activation, in the posterior cingulate extending into the precuneus. This activation partially overlapped with an activation from the concrete contrast, but did not overlap with any abstract activations.
Finally, we performed an additional subtraction in the whole brain, comparing emotional word trials, collapsing across concreteness, to the nonword trials (See Figure 2B). The results of this analysis revealed activations in response to emotional words in the left anterior temporal lobe proceeding up the left STS, as well as bilateral activations in the posterior STS and angular gyrus, and bilateral anterior cingulate and left medial orbitofrontal cortex and left posterior cingulate. Table 5 lists the MNI coordinates for the peak activations in the two emotional valence contrasts.
Table 5.
MNI coordinates of the peak activations to the valenced words versus nonwords, and to the valenced words versus neutral words, contrasts.
| Valenced Words > Nonwords | ||||
|---|---|---|---|---|
| Region | BA | x | y | z |
| left posterior superior temporal sulcus | 22 | −56 | −56 | 18 |
| left anterior middle temporal gyrus | 21 | −56 | −6 | −22 |
| left anteior inferior frontal gyrus | 45 | −46 | 30 | −16 |
| left inferior parietal gyrus | 7 | −48 | −66 | 20 |
| left medial temporal pole | 38 | −32 | 6 | −32 |
| left posterior parahippocampal gyrus | 30 | −16 | −54 | 2 |
| left posterior cingulate gyrus | 23 | −16 | −42 | 42 |
| left superior frontal gyrus | 9 | −10 | 52 | 34 |
| left superior occipital gyrus | 19 | −10 | −100 | 16 |
| left inferior posterior cingulate | 31 | −6 | −56 | 12 |
| left anterior cingulate | 11 | −6 | 48 | −16 |
| right anterior cingulate | 11 | 6 | 52 | −10 |
| right inferior posterior cingulate | 31 | 6 | 54 | 4 |
| right middle occipital gyrus | 18 | 14 | −92 | 20 |
| right medial orbitofrontal cortex | 11 | 4 | 16 | −16 |
| right medial temporal pole | 38 | 38 | 8 | −30 |
| right middle superior temporal sulcus | 22 | 42 | −14 | −10 |
| right posterior superior temporal gyrus | 22 | 60 | −26 | 16 |
|
| ||||
| Valenced Words > Neutral Words | ||||
| Region | BA | x | y | z |
|
| ||||
| left posterior cingulate | 23 | −2 | −52 | 26 |
3.3 Neuroimaging Results: ROI analysis of the Rostral Anterior Cingulate
The 2 × 2 ANOVA revealed a main effect of concreteness in the rACC, F(1,19)=4.84, p<.05 due to higher parameter estimates for the concrete condition (M=7.63) as compared to the abstract condition (M =2.02). There was also a main effect of valence in the rACC, F(1,19)=4.77, p<.05 due to greater response in the valenced conditions (M = 9.08) as compared to the non-valenced condition (M =0.58). The interaction was not significant, F(1,19)=2.39, p=.13. The results of this analysis are shown in Figure 2C.
4. Discussion
This study had two goals. First, we examined whether abstract verbal stimuli activate cortical regions overlapping with those activated by emotional stimuli, while carefully controlling emotional valence across the abstract and concrete stimuli. Second, we tested the specific hypothesis that the rACC is particularly sensitive to abstract concept knowledge as reported by Vigliocco and colleagues (2013).
We began by conducting a whole brain analysis using traditional contrasts comparing the activations to abstract concepts, as well as concrete concepts, compared to nonwords. We identified several regions sensitive to both types of concepts, including the left temporal pole, STS, IFG and posterior MTG, as well as medial OFC and right angular gyrus. Processing abstract and concrete concepts also led to some unique activations. Abstract concepts uniquely activated the right temporal pole and right anterior MTG, while concrete concepts uniquely activated the left inferior temporal cortex and a large region in the posterior ACC. The two meta-analyses discussed previously (Binder, et al., 2009; Wang, et al., 2010) contrasted abstract words against concrete, instead of against a nonword baseline, so comparisons with these studies is difficult. However, these findings are somewhat in agreement with the meta-analyses. Both found abstract activations in the left temporal pole, STS and IFG, greater than concrete activations. Both also associated the left angular gyrus with concrete processes, which we also found. Finally, both meta-analyses identified several midline regions, including posterior ACC with perceptual knowledge, which we identified in our concrete condition as well.
A subtraction was also used to identify regions that responded to emotional valence of concepts, by contrasting words with high hedonic valence with neutral words. The only region identified was in the medial precuneus/ posterior ACC. This area overlapped with activations to concrete words, but not abstract words. This overlapping activation should not be taken to indicate that concrete words themselves are inherently valenced or rely on emotional valence. Simply finding similar activation in the brain may mean that some shared process is activated but should never be used as primary evidence that one task or stimuli is necessarily involved or inherent to another class of stimuli (refer to Mahon & Caramazza, 2008 for a thoughtful discussion). However, it is concerning that when abstract and concrete words are matched for emotional valence, there is no region that appears to be responding to both abstract words and to valenced words.
An additional subtraction was performed, comparing emotionally valenced words to nonwords. This subtraction used a weaker baseline, but captured semantic-word meaning and emotional valence regions. Activation in response to valenced words, collapsing across concreteness, in this analysis included bilateral temporal pole, STS, and angular gyrus, left IFG, and portions of the anterior and posterior cingulate cortex. These findings, particularly the medial activations, are highly consistent with other neuroimaging studies of single word reading of emotional words (Kuchinke, Jacobs, Grubich, Vo, Conrad & Herrmann, 2005; Nakic, Smith, Busis, Vythilingam, & Blair, 2006; Sass, Habel, Sachs, Huber, Gauggel & Kircher, 2012).
Finally, we created a region of interest within the rACC to match the region previously reported to respond uniquely to abstract words and to be modulated by the valence of these words (Vigliocco et al., 2013). While we confirmed that the region responded to emotional valence, we could not confirm that the region responded to a greater degree to abstract stimuli than to concrete. In fact, we found the reverse effect – that the rACC responded to a greater degree to concrete words than to abstract. Thus we can rule out the notion that this region is critically involved in emotionally grounding abstract conceptual knowledge. Rather, we suggest that the region is simply very sensitive to highly arousing, valenced stimuli.
This is hardly a novel idea, as many others have identified ACC activations in a range of tasks using valenced stimuli. For instance, fear conditioning, emotional conflict resolution, top-down control of emotion, and physical pain are consistently associated with ACC activations (for review, see Etkin, Egner, & Kalisch, 2011), and it is considered by some to be a limbic region (Bush et al., 2000). The ACC is a large region, and distinct functions are associated with particular subregions. The anterior ACC, including the rACC ROI used in this study, has been associated with directing attention based on emotional content of stimuli (Bush et al., 2000). In contrast, this region is rarely reported in studies of semantic memory. Indeed, two recent meta-analysis failed to find rACC activations in response to encyclopedic or abstract knowledge (Binder et al., 2009; Wang et al., 2010). Thus this region appears to be non-specifically sensitive to any valenced stimuli.
This logic can be applied to some other regions that we found responded exclusively to abstract conceptual processing. For example the right STS and temporal pole are modulated by a wide range of social and emotional stimuli (Olson, Plotzker, & Ezzyat, 2007; Ross & Olson, 2010; Skipper, Ross, & Olson, 2011; Zahn, Moll, Krueger, Huey, Garrido & Grafman, 2007). Several studies have reported increased activations in the superior anterior temporal lobe/temporal pole to social concepts (Zahn et al., 2007; Skipper & Olson, 2011) which tend to be emotionally valenced. In this study we found that the activations to abstract concepts in the temporal pole were part of the same region responsive to the valenced stimuli. The same can be said for some regions responding to concrete words, such as the limbic/parlimbic regions in the ACC and orbitofrontal cortex. These activations may be largely due to the valenced content of the stimuli.
4.1 The Affective Embodiment Account and the Representation of Emotional Content
Our findings differ significantly from those reported by Vigliocco and colleagues (2013), but our design differed in important ways as well. First, they controlled imageability in their abstract and concrete stimuli – such that their very abstract and very concrete words did not differ on imageability. We elected to allow imageability to vary with concreteness, because this allowed us to select a more naturalistic sample of English abstract and concrete words. Imageability and concreteness differ in important ways, but imageability can account for a huge amount of the variance in concreteness scores (Kousta et al., 2011). We believe that concreteness and imageability are tightly linked, and by matching abstract and concrete stimuli on imageability, you are left with an especially unusual set of (highly imageable) abstract words. However, we concede that the neurological effects of concreteness may be neurologically dissociated from the effects of imageability, and the confounding of these variables may be seen as a limitation in our study. Future research should follow the example set by Vigliocco and colleagues (2013), and seek to identify the different contributions of these two variables.
Second, we controlled for a variable that was not controlled by Vigliocco and colleagues (2013) - hedonic valence. In their stimuli, abstract words were significantly more valenced than the concrete words. They argue that this is not a confound because abstract words, when imageability is controlled, are inherently more emotional than concrete words, and that abstract words may be grounded in emotional content. However, the critical question in this analysis is whether abstract words are processed in the same cortical regions that are modulated by emotional valence. To test this very specific hypothesis, it is necessary to dissociate the effects of valence from the effects of abstractness. For this reason, we set up a 2×2 design, with concreteness and valence as factors, and then controlled for valence between the abstract and concrete conditions, and likewise controlled for concreteness across the valenced and nonvalenced conditions. Thus, the specific findings regarding the rACC as found by Vigliocco and colleagues were possibly due to a confounding of valence and concreteness, and that the effect of abstractness in this region may be influenced by other, uncontrolled semantic variables. When the factors of valence and concreteness were separated, we found that the rACC is primarily modulated by emotional valence, but that greater concreteness boosted the response to the valenced words.
We note that this study is not intended to test the overall Affective Embodiment Account. Instead, this experiment is meant to test whether abstract and emotional concepts share common nodes in the brain. If such overlapping activations were found, when valence is controlled across concreteness, then it would support the Affective Embodiment Account by providing neurological plausibility. Alternatively, the lack of overlapping activations would fail to support this account, although such a finding cannot refute the various behavioral findings and psycholinguistic correlates upon which the Affective Embodiment Account is founded.
For example, early research on emotion-words revealed that they were rated as more imageable, but less concrete, than their neutral abstract counterparts (Altarriba, Bauer, & Benvenuto, 1999; Altarriba & Bauer, 2004). There is also strong relationship between concreteness and valence across the span of English words, such that abstract words are both more negative and more positive compared to relatively neutral concrete words (Vigliocco et al., 2013). It has also been demonstrated that valence is a major predictor of word recognition times for abstract words when imageability is controlled (Kousta, et al., 2011). Furthermore, a recent study using hierarchical clustering analyses based on a large corpus of psycholinguistic ratings found that emotional valence was an important organizing variable for abstract words (Crutch, Troche, Reilly, & Ridgway, 2013; Troche, Crutch, & Reilly, under review). These findings have important implications regarding the semantic structure of abstract words, and support the Affective Embodiment Account, that abstract words are more reliant on emotional information than concrete words.
Furthermore, we did not control age of acquisition, because a large developmental literature has shown that abstract words are learned at a later age than concrete words (for review, see Maguire, Hirsh-Pasek, & Michnick Golinkoff, 2006). However, age of acquisition plays an important role in the Affective Embodiment Account. Although, abstract words in general are learned later, there is considerable evidence that children learn emotional labels at an early age, and have acquired basic labels (“happy” “sad”) by around 20 months (Bretherton & Beeghly, 1982; Ridgeway, Waters, & Kuczaj, 1985; Wellman, Harris, Banerjee, & Sinclair, 1995)Error! Bookmark not defined.. Kousta and colleagues (2011) have suggested that emotional valence may aid abstract word learning, giving certain abstract words an advantage both in adult performance and childhood acquisition, and that this occurs through a similar mechanism by which imageability can confer an advantage for concrete words. This is an interesting hypothesis, and supports the notion that emotionality is an organizing factor for abstract words, since key linguistic variables such as age of acquisition can vary so wildly between highly valenced abstract, and relatively neutral, abstract words. It remains unclear, however, whether how the emotional content that is presumed to permeate almost all abstract concepts relates to the majority of later-learned abstract words.
Although abstract words are frequently imbued with emotional content, it does not follow that abstractness and valence are so intertwined as to be inseparable. This is intuitively straightforward, as it is very easy to generate both abstract and concrete words that are emotionally neutral. The Affective Embodiment Account has much explanatory power, but future studies must be cautious in carrying out analyses that examine the role of valence in abstract word processing, without confounding the two dimensions. Likewise, as Kousta and colleagues (2011) have pointed out, imageability and concreteness are clearly independent measures and, although imageability was not controlled in this study, the different ways that they affect processing needs to be clarified.
4.2 Shortcomings of this Study
On a few lexical and sublexical measures, our stimuli were not as well controlled as that used by Vigliocco and colleagues (2013). Specifically, our concrete words were acquired later in life than our abstract words, on average. However, typical vocabulary development is marked by early learning of concrete words, and only later learning of abstract words (Maguire et al., 2006). We were able to control word frequency using the Kuceris-Francis frequency measure, but not the SUBTLEX measure, which has been shown by some to be a better predictor of behavioral performance on lexical tasks (Brysbaert & New, 2009). The concrete-valenced condition had a higher frequency in the SUBTLEX measure than the other conditions, but the other three groups were all well matched, including the abstract-valenced and abstract-neutral conditions. Interestingly, the concrete-valenced condition was also numerically the best predictor of activity in the rostral ACC region of interest, but it did not differ significantly from the abstract-valenced condition, shown by the lack of interaction. Both of these measures, age of acquisition and word frequency, can significantly impact behavioral performance in lexical tasks, but it is unclear how they may have influenced our neuroimaging results.
Furthermore, it should be noted that we did not control for imageability between our abstract and concrete conditions. Recent research has shown that controlling or confounding concreteness and imageability can lead to strikingly different behavioral results in lexical tasks (Kousta et al., 2011). Thus, it is also likely that concreteness uses some neural circuitry that differs from those modulated by imageability specifically, and our results may have differed had we controlled imageability. However, we reasoned that imageability should confound with concreteness because the two measures are very closely related. Abstract words that can be matched on imageability with concrete words may be a unique set, and not representative of all abstract words. However, we suggest that future work attempt to differentiate the roles of imageability and concreteness on neural processing.
Finally, our failure to replicate Vigliocco and colleagues (2013) may be attributed to task differences. In their study, Vigliocco et al used a lexical decision task, and the imaging design was event-related so that each word could be analyzed independently. Our imaging, however, was block-design, which increases power but removes the ability to analyze independent items. Perhaps more importantly, our task was a relatively semantically-deep task, in which participants saw three words presented consecutively, were asked to think deeply about the meaning, and were required to answer a meaningful question at the end of each block. This difference is worth noting, as recent neuroimaging research has found that changes to task demands and context led to different activations during concrete and abstract multi-word reading (Sakreida, Scorolli, Menz, Heim, Borghi & Binkofski, 2013). We primarily used this design to increase power and BOLD signal, and because we reasoned that the psychological effects of emotionality and concreteness (both related to the actual meaning of the word) would be increased when participants thought meaningfully about each word. Although many studies supporting the Affective Embodiment Account have relied heavily on lexical decision tasks (Kousta et al., 2011; Kousta, Vinson, & Vigliocco, 2009; Vigliocco et al., 2013) there is nothing inherent to the model to suggest that the effects of concreteness and emotional valence should occur only at the level of word recognition, and not also at deeper levels of word understanding. However, it is possible that some activations, particularly in the word versus nonword comparisons, were driven by task demands such as working memory.
5. Conclusions
This fMRI experiment was intended to address the potential role of the rostral ACC in abstract vs. concrete, and emotional vs. neutral conceptual processing by using a 2 × 2 design with concreteness and valence as independent factors. By unconfounding these two variables, we were able to demonstrate a role for the rostral ACC in processing emotionally salient information, which is supported by the previous literature. However we did not find that this region was preferentially sensitive to abstract concepts. Careful consideration of which variables to control and which to allow to vary will be critical to the future research in this field, exploring how concreteness interacts with emotional valence, imageability and other lexical variables such as age of acquisition.
Highlights.
In an MRI, participants read abstract/concrete words that were emotional or neutral.
Valenced words activated both concrete and abstract regions throughout the brain.
The rostral anterior cingulate responded to emotional content but not abstract words.
Results failed to support the hypothesis that abstract words are inherently valenced.
Acknowledgements
We would like to thank Feroze Mohammed for assistance with imaging. This work was supported by a National Institute of Health grant to I. Olson [RO1 MH091113], as well as a National Science Foundation Graduate Research Fellowship to L.M. Skipper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altarriba JE, Bauer LM, Benvenuto C. Concreteness, context availability, and imageability ratings and word associations for abstract, concrete, and emotion words. Brain Research Methods, Instruments & Computers. 1999;31:578–602. doi: 10.3758/bf03200738. [DOI] [PubMed] [Google Scholar]
- Altarriba J, Bauer LM. The distinctiveness of emotion concepts: A comparison between emotion, abstract, and concrete words. The American Journal of Psychology. 2004;117:389–410. [PubMed] [Google Scholar]
- Apps MA, Balsters JH, Ramnani N. The anterior cingulate cortex: Monitoring the outcomes of others ' decisions. Social Neuroscience. 2012;7:424–435. doi: 10.1080/17470919.2011.638799. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Wiemer-Hastings K. Situating abstract concepts. In: Pecher D, Zwaan R, editors. Grounding cognition: The role of perception and action in memory, language, and thought. Cambridge University Press; New York: 2005. pp. 129–163. [Google Scholar]
- Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A. The neural substrate for concrete, abstract, and emotional word lexica: A positron emission tomography study. Journal of Cognitive Neuroscience. 1997;9:441–461. doi: 10.1162/jocn.1997.9.4.441. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, Mckiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Bleasdale FA. Concreteness-dependent associative priming: Separate lexical organization for concrete and abstract words. Journal of Experimental Psychology: Learning, Memory & Cognition. 1987;13:582–594. [Google Scholar]
- Bonner MF, Vesely L, Price C, Anderson C, Richmond L, Farag C, Avants B, Grossman M. Reversal of the concreteness effect in semantic dementia. Cognitive Neuropsychology. 2009;26:568–579. doi: 10.1080/02643290903512305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretherton I, Beeghly M. Talking about internal states: The acquisition of an explicit theory of mind. Developmental Psychology. 1982;18:906–921. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Coltheart M. Deep dyslexia: A right-hemisphere hypothesis. In: Coltheart M, Patterson K, Marshall JC, editors. Deep Dyslexia. Routledge & Kegan Paul; London: 1980. pp. 326–380. [Google Scholar]
- Crutch SJ, Troche J, Reilly J, Ridgway GR. Abstract conceptual feature ratings: The role of emotion, magnitude, and other cognitive domains in the organization of abstract conceptual knowledge. Frontiers in Human Neuroscience. 2013;7(186):1–14. doi: 10.3389/fnhum.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A. Making up materials is a confounded nuisance, or: Will we be able to run any psycholinguistic experiments at all in 1990? Cognition. 1981;10:65–70. doi: 10.1016/0010-0277(81)90026-3. [DOI] [PubMed] [Google Scholar]
- Day J. Right-hemisphere language processing in normal right-handers. Journal of Experimental Psychology: Human Perception and Performance. 1977;3:518–528. doi: 10.1037//0096-1523.3.3.518. [DOI] [PubMed] [Google Scholar]
- de Groot AM. Representational aspects of word imageability and word frequency as assessed through word association. Journal of Experimental Psychology: Learning, Memory & Cognition. 1989;15:824–845. [Google Scholar]
- D’Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farrah M. A functional MRI study of mental image generation. Neuropsychologia. 1997;35:725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD. Processing concrete words: fMRI evidence against a specific right-hemisphere involvement. Neuropsychologia. 2003;42:62–70. doi: 10.1016/s0028-3932(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, von Cramon Y. Segregating semantic and syntactic aspects of processing in the human brain: and fMRI investigation of different word types. Cerebral Cortex. 2000;10:698–705. doi: 10.1093/cercor/10.7.698. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Hyde M, Blumstein S. Frequency, picturability and availability of nouns in aphasia. Cortex. 1969;5:104–119. doi: 10.1016/s0010-9452(69)80022-5. [DOI] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41:919–931. doi: 10.1016/s0028-3932(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Lambon Ralph MA. Reverse concreteness effects are not a typical feature of semantic dementia: Evidence for the hub-and-spoke model of conceptual representation. Cerebral Cortex. 2011;21:2103–2112. doi: 10.1093/cercor/bhq288. [DOI] [PubMed] [Google Scholar]
- Howell J, Bryden M. The effects of word orientation and imageability on visual half-field presentations with a lexical decision task. Neuropsychologia. 1987;25:527–538. doi: 10.1016/0028-3932(87)90077-7. [DOI] [PubMed] [Google Scholar]
- James CT. The role of semantic information in lexical decisions. Journal of Experimental Psychology: Human Perception and Performance. 1975;104:130–136. [Google Scholar]
- Jenkinson M, Beckmann C, Behrens T, Woolrich M, Smith S. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Human Brain Mapping. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RB, Goodglass H. Deep dysphasia: Analysis of a rare form of repetition disorder. Journal of Brain and Language. 1990;39:153–185. doi: 10.1016/0093-934x(90)90009-6. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD, et al. Neural pathways involved in the processing of concrete and abstract words. Human Brain Mapping. 1999;7:225–233. doi: 10.1002/(SICI)1097-0193(1999)7:4<225::AID-HBM1>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biology of Mood & Anxiety Disorders. 2013;3:1–9. doi: 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousta S, Vigliocco G, Vinson DP, Andrews M, Del Campo E. The representation of abstract words: Why emotion matters. Journal of Experimental Psychology: General. 2011;140:14–34. doi: 10.1037/a0021446. [DOI] [PubMed] [Google Scholar]
- Kousta S, Vinson DP, Vigliocco V. Emotion words, regardless of polarity, have a processing advantage over neutral words. Cognition. 2009;112:473–481. doi: 10.1016/j.cognition.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Merves JS. Lexical access for concrete and abstract words. Journal of Experimental Psychology: Learning, Memory & Cognition. 1986;12:92–107. [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. NeuroImage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Kuperman V, Stadthagen-Gonzalez H, Brysbaert M. Age-of-acquisition ratings for 30 thousand English words. Behavior Research Methods. 2012;44:978–990. doi: 10.3758/s13428-012-0210-4. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Schwartz GE. Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Locke J. Of abstract and concrete terms. In An essay on human understanding. 1685 retrieved April 15, 2012 from: http://oregonstate.edu/instruct/phl302/texts/locke/locke1/Essay_contents.html.
- Maguire MJ, Hirsh-Pasek K, Michnick Golinkoff R. A unified theory of word learning: Putting verb acquisition in context. In: Hirsh-Pasek K, Michnick Golinkoff R, editors. Action Meets World: How Children Learn Verbs. Oxford University Press; 2006. pp. 364–391. [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology - Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Martin N, Saffran EM. A computational account of deep dysphasia: Evidence from a single case study. Brain and Language. 1992;43:240–274. doi: 10.1016/0093-934x(92)90130-7. [DOI] [PubMed] [Google Scholar]
- Nakic M, Smith BW, Busis S, Vythilingam M, Blair RJ. The impact of affect and frequency on lexical decision: The role of the amygdala and inferior frontal cortex. NeuroImage. 2006;31:1752–1761. doi: 10.1016/j.neuroimage.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of abstract semantics. NeuroImage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Paivio A. Paired-associated learning and free recall of nouns as a function of concreteness, specificity, imagery and meaningfulness. Psychological Reports. 1967;20:239–245. doi: 10.2466/pr0.1967.20.1.239. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F. The neural correlates of verb and noun processing: A PET study. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- Ridgeway D, Waters E, Kuczaj S. Acquisition of emotion-descriptive language: Receptive and productive vocabulary norms for ages 18 months to 6 years. Developmental Psychology. 1985;21:901–908. [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. NeuroImage. 2010;49:3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC. 51 properties of 125 words: A unit analysis of verbal behavior. Verbal Learning and Verbal Behavior. 1980;19:736–755. [Google Scholar]
- Rudebeck P, Bannerman D, Rushworth M. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cognitive, Affective & Behavioral Neuroscience. 2008;8:485–497. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Rudebeck P, Buckley M, Walton M, Rushworth M. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. NeuroImage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Martin N. Neuropsychological evidence for lexical involvement in short-term memory. In: Vallar G, Shallice T, editors. Neuropsychological Impairments of Short-Term Memory. Cambridge University Press; Cambridge: 1990. pp. 145–166. [Google Scholar]
- Sass K, Habel U, Sachs O, Huber W, Gauggel S, Kircher T. The influence of emotional associations on the neural correlates of semantic priming. Human Brain Mapping. 2012;33:676–694. doi: 10.1002/hbm.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper LM, Ross LA, Olson IR. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. 2011;49:3419–3429. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche J, Crutch S, Reilly J. Heirarchical organization and the topography of English abstract and concrete nouns. doi: 10.3389/fpsyg.2014.00360. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigliocco G, Kousta S, Della Rosa AP, Vinson DP, Tettamanti M, Devlin JT, Cappa SF. The neural representation of abstract words: The role of emotion. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht025. doi: 10.1093/cercor/bht025. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Meteyard L, Andrews M, Kousta S. Toward a theory of semantic representation. Language and Cognition. 2009;1:219–247. [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: A meta-analysis of neuroimaging studies. Human Brain Mapping. 2010;21:1459–1468. doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warriner AB, Kuperman V, Brysbaert M. Norms of valence, arousal, and dominance for 13,915 English lemmas. Behavior Research Methods. 2013;45:1191–1207. doi: 10.3758/s13428-012-0314-x. [DOI] [PubMed] [Google Scholar]
- Wellman H, Harris P, Banerjee M, Sinclair A. Early understanding of emotion: Evidence from natural language. Cognition & Emotion. 1995;9:117–149. [Google Scholar]
- Whalen PJ, Bush G, Mcnally RJ, Wilhelm S, Mcinerney SC, Jenike MA, Rauch SL. The emotional counting stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Whaley C. Word-nonword classification times. Journal of Verbal Learning and Verbal Behavior. 1978;17:143–154. [Google Scholar]
- Wise RJ, Howard D, Mummery CJ, Fletcher P, Leff A, Büchel C, Scott SK. Noun imageability and the temporal lobes. Neuropsychologia. 2000;38:985–994. doi: 10.1016/s0028-3932(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. PNAS. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]