Background
Healthcare-associated infections (HAIs) are among the most common adverse events in healthcare settings (Leape 1991). Approximately 1.7 to 2 million HAIs occur in U.S. hospitals each year, with the greatest burden due to catheter-associated urinary tract infections (36%), surgical site infections (20%) and central line associated bloodstream infections (11%) (Jarvis 2007; Klevens 2007). Morbidity and mortality associated with HAIs are significant, resulting in lower in quality of life, longer hospitalizations, and approximately 99,000 excess deaths each year (Anderson 2007; Klevens; Warren 2006). Nationally, the economic burden of HAIs is estimated at $17 to $29 billion dollars annually (Jarvis 2007; Stone 2005). For hospitals, the costs of HAIs are also real and significant, making the prevention of HAIs a management imperative. One study estimated these average costs at over $15,000 per HAI (Roberts et al. 2003), while another showed a range of between $500 to $40,000 depending on the type of infection (Jarvis 1996).
In 2008, the Centers for Medicare and Medicaid Services (CMS) implemented the use of a new financial mechanism—no added payment for preventable complications—which is a “stick” rather than a “carrot” form of pay-for-performance (P4P). Under this mechanism, CMS no longer pays hospitals for treating certain healthcare associated infections (HAIs) if they are not present on admission, including catheter-associated urinary tract infections (CAUTIs), central line-associated bloodstream infections (CLABSIs), and mediastinitis after coronary artery bypass graft (CABG) surgery (Centers for Medicare and Medicaid Services 2007).
Recently, a conceptual framework has been identified for pursuing important research questions around how the CMS policy might help produce changes in various quality and cost outcomes (see Stone et al. 2010). This framework is applicable to examining the role of organizational context in shaping the extent to which the CMS policy is recognized, accepted and acted upon by hospital personnel. Notable about the framework is that it places several different organizational factors front-and-center as mediating variables that shape how CMS changes in reimbursement for HAIs (i.e. the substance of the policy) may ultimately facilitate quality improvement with respect to infections at the organizational level. These organizational factors, categorized generally in the framework as leader behavior, organizational culture, and staff behavior help to enable the CMS policy in promoting quality improvement (QI) in hospitals.
The importance of organizational factors in shaping QI processes and outcomes has been stressed for some time (see Flood 1994; Ferlie and Shortell 2001). Similar to findings from other industries, extant research supports the contribution of factors such as work culture, organizational learning leadership behaviors, teamwork, infrastructure readiness, technology, staff preparation and training to improved quality within health care organizations (cf. Alexander et al. 2006; Nembhard et al. 2009; Shortell et al. 1995; Shortell, Bennett, and Byck 1998; Weiner, Shortell, and Alexander 1997). Consistent with these findings, we view various features of the organization as potentially important vehicles through which pay-for-performance policies such as the CMS policy are implemented. In this study, we pursued the following research questions:
What types of organizational factors facilitate the recognition, acceptance, and significance of the CMS policy as a QI driver within hospital settings?
Are there additional contextual features within hospitals that moderate how certain organizational factors facilitate the relationship between the CMS policy and its recognition, acceptance, and significance within hospital settings?
Data and Methods
The present study was part of a larger multi-year examination of how hospitals are adapting to the new CMS policy. A qualitative approach using interviews was chosen to explore the two research questions comprising this part of the overall project. This method was chosen for two main reasons: (a) qualitative methods are ideally suited to examining the roles of organizational factors and context in facilitating policy implementation, as Stone et al. (2010) and others (Shortell 1999) reinforce; and (b) the study is exploratory in nature, seeking to identify preliminary relationships rather than validating those relationships on a generalizable scale (Sofaer 1999). We conducted semi-structured, in-depth telephone interviews with lead infection preventionists from 36 non-federal, acute care hospitals in the United States. Infection preventionists are defined as staff working within hospital settings that are specially trained in infection prevention, control, and surveillance. They are responsible for surveillance of HAIs, conducting outbreak investigations, educating clinical staff regarding infection prevention measures, and monitoring appropriate use of infection prevention practices in the hospital.
Data Collection and Sample
Hospitals were purposively sampled from the American Hospital Association (AHA) database. The hospitals were stratified based on key characteristics, including bed size, geographic location, and nurse staffing levels (see Table 1). These stratifying characteristics were used for two main reasons: (a) to assure a comparative case analytic framework, in line with the qualitative approach used that contained adequate numbers of preventionists working in several different hospital and infection control work settings; and (b) to assure a diverse array of organizational factors that could be explored through the interviews.
Table 1.
Facility characteristics for hospitals in study
| Facility Characteristica | N/Mean/% | Range |
|---|---|---|
| Region | ||
| Northeast | 9 | |
| Midwest | 8 | |
| South | 12 | |
| West | 7 | |
| Total hospital beds | 388.7 | 40–1501 |
| Adult ICU bedsb | 44.2 | 6–222 |
| Mean length of stay (days) | 4.9 | 2.5–7.4 |
| Hospital discharges paid by Medicare (%) | 43% | 24%–70% |
| RN hour per patient day (hours)c | 14.8 | 7.9–26.8 |
From AHA database
Does not include burn care, Neonatal ICU or Pediatric ICU
Defined by AHA variables: (FTERN*2080)/IPDTOT
Recruitment was conducted between September 2009 and February 2010. A total of 36 infection preventionists working at 36 different hospitals participated in the interviews. These hospitals were located in 24 states. Thirty-two of the 36 hospitals (89 percent) were located in states that had some form of mandatory reporting requirement for healthcare-associated infections. Ten of the 36 (28 percent) hospitals were classified as teaching hospitals. Most preventionists stated that there were between one and four full-time employed staff working in infection prevention in their hospitals. Most preventionists also reported that they had worked for their current employer ten years or more. Interviews lasted approximately forty-five minutes. Interview questions and associated probes are included in Appendix A.
Appendix A.
Sample Interview Questions for Infection Preventionists
| Question |
|---|
| Could you give a brief overview of the surveillance activities that take place in your hospital around HAIs? |
| Medicare has a new policy to stop paying for care associated with certain HAIs. What is your opinion of this policy? Sample Probes: Gauging what the CMS policy is adding that is new or different; assessing awareness levels among physicians, nursing staff, and administration about new CMS policy |
| Can you describe aspects of your hospital that you have felt over time are worthy of noting or unique with respect to prevention of HAIs? Sample Probes: extent of guideline/best practice adherence in hospital; preventionist knowledge of adherence across hospital units |
| Describe the different ways you know about how your hospital is responding or may respond to the new CMS policy? Sample Probes: gauging the extent of interactions between Infection Control and Quality Improvement departments in hospital; assessing organizational factors that impact success/failure of CMS policy |
| Do you have any worries or concerns about how the new CMS policy might play out within your hospital? Sample Probes: identifying other approaches to HAI prevention and control facilitated or undermined by the new CMS policy; assessing the impact of the CMS policy on guideline or best practice adherence |
| Do you believe that the new CMS policy has, through the organizational interventions it has spurred (or is spurring) affected (or will affect) infection-related outcomes in any way? |
Data Analysis
Data were analyzed with Atlas.ti qualitative analysis software using a content analytic approach with elements of grounded theory built into the design. The content analytic approach involved digitally taping interviews, transcribing them into text documents, and then coding them using Atlas.ti software. The coding process identified interpretive schemes within the data that captured the important descriptive and explanatory elements illuminating the new CMS policy and its interaction with organizational features of the participating hospitals. Coding was characterized by several best practices associated with systematic qualitative research (Miles and Huberman 1994; Patton 2002): (a) validation of the data categories identified using multiple coders that reviewed the same interviews and coding categories; preliminary coding of batches of interviews, which then were validated through additional analysis of later interview batches; and regular meetings of the research team to review and critique the coding categories. In addition, the technique of theoretical sampling (see Strauss 1987) was employed during coding to assist in focusing data collection on data categories that, from early interview coding, appeared to reflect the most recurring and interesting phenomena embedded in the data. Two of the co-authors (TH and CH) were responsible for fully coding all the interviews and reviewing the others’ code lists. A third co-author (GL) reviewed a sample of the 36 interviews and their coding, providing a further validation check on the two coders’ analysis.
The analytic process yielded a total of 149 initial codes for the interview data as a whole. The coding strategy most often involved labeling paragraph sections of interviews with individual codes, which is an appropriate focus for “chunking” qualitative data (Strauss and Corbin 1998). Forty-seven of the 149 initial codes formed the bulk of the interpretive framework for the 36 interviews. A portion of the 47 codes were either combined or grouped together during a second level of analysis, in situations where one or more codes were defined similarly. Most of the codes remaining after this second level of analysis that supported various elements of the particular interpretation presented here were present in approximately 30 to 60 percent of the 36 unique interviews, providing some evidence of appropriate coding saturation across the data set as a whole. Once the coding process was complete, the research team focused on identifying the primary interpretive “story” that was supported by the primary codes.
Results
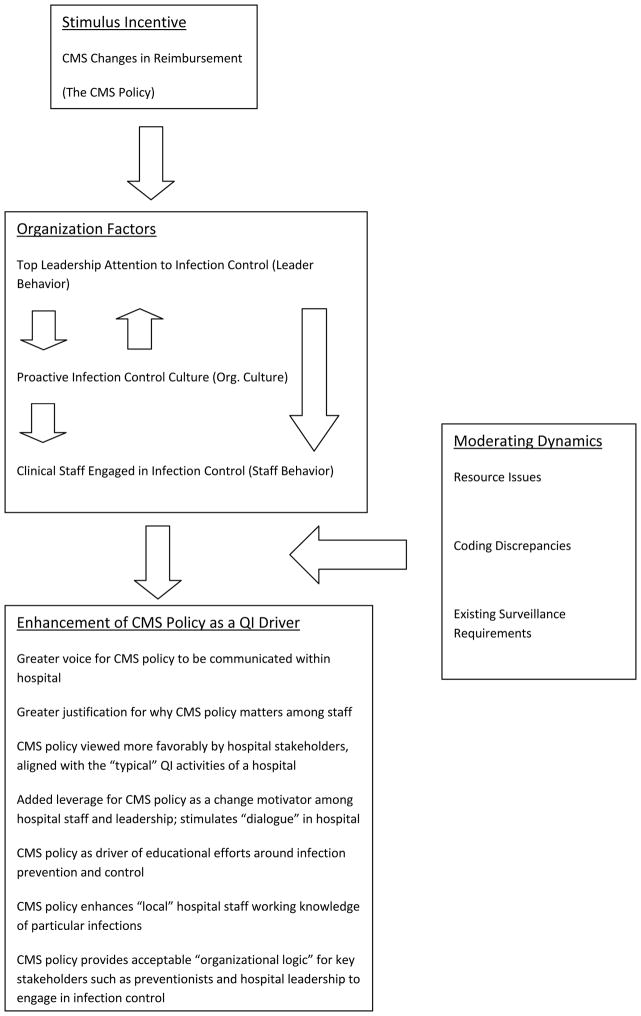
A model adapted from Stone et al. (2010) and derived from the interpretive analysis is seen in Figure 1. This diagram forms the basis for the remainder of the results discussion. The model suggests one way in which specific organizational factors may mediate between the existence of the CMS policy and its recognition, acceptance, and significance as a quality improvement (QI) tool within hospital settings.
Figure 1.
Organization Factors Identified as Shaping How the CMS Policy Works in Hospital Settings (Adapted from Stone et al. 2010)
Organizational Factors That Matter for the CMS Policy
Table 2 presents the three organizational factors receiving strong support as mediators between the CMS policy and its role as a quality improvement driver within the hospital setting. Support was strongest for the presence of three interrelated organizational factors or dynamics which appeared to have relevance in making the CMS policy recognized and accepted as a QI driver within hospitals. These dynamics were: (1) the presence of a proactive infection control department; (2) top leadership attention to infection control; and (3) clinical provider participation in infection control activities. There was no evidence that these dynamics were unique to particular hospital settings of a certain size or resource availability. A proactive infection control department consisted of several dimensions: (a) a department in which staff “played the detective role” throughout the hospital where infection control and prevention was concerned; (b) meaningful levels of integration between the infection control department and formal QI activities occurring across the hospital; and (c) an infection control department that was “legitimated” within the hospital as a source for infection control and prevention expertise, initiatives, and solutions (see Table 3 for sample quotes from the data supporting this dynamic).
Table 2.
Summary of coding support for the three mediators identified in the study
| Mediator | Number of Supporting Codes in Data Set | Average Total Frequency of Supporting Codes Per Individual Interview * (n=36) | Supporting Codes for the Mediator Indentified |
|---|---|---|---|
| A Proactive Infection Control Department | 9 Supporting Codes | 6.5 Times per Interview | Playing the detective role Checking coder documentation Infection control (IC) as change driver IC—Quality improvement (QI) integration IC highly integrated into organization IC involved with QI IC as a driver of change IC function as legitimate in organization IC education of leadership |
| Top Leadership Attention to Infection Control | 6 Supporting Codes | 2.8 Times per Interview | Leadership involved in facilitating change CMS policy increases leadership attention to infection control CMS policy matters when it’s a big financial impact situation IC education of leadership “It’s all about the money” CMS policy creates high organizational awareness of infections |
| Clinical Staff Engaged in Infection Control | 4 Supporting Codes | 4.1 Times per Interview | Involving clinical line staff in QI Documentation issues and the physician’s role How to get clinicians to focus on infections CMS policy creates high organizational awareness of infections |
The “average total frequency” is a measure of coding saturation, i.e. the extent to which supporting codes appear collectively within and across interviews
Table 3.
Sample quotes gleaned from interview data supporting themes
| Factor Identified: | Sample Supporting Quotes Gleaned from Interview Data: |
|---|---|
| Mediators: | |
| Top Leadership Attention to Infection Control | They [hospital executives] like to look at data, but they’re also very supportive. When we have the medical staff meetings, there’s always someone from administration at those meetings. And so when quality talks about the CMS conditions for nonpayment, the administration supports that with their presence, with their words. Sometimes, they’re the ones that talk about it. They’ve talked about it to our staff. We have what we call town hall meetings that are quarterly with all employees. They’ve talked about it in there. They are very involved. They like to know what’s going on, not only in preventing the non-repayment, but also what are our processes to do that, and are we preventing infection. I think just being invited to this board retreat was something that was evidence of their interest. The whole board retreat was focused on patient safety and infection prevention. To be included and recognized as an important part of patient safety and to have that whole focus of the board retreat, I think, is really telling that it is such an important thing. Management is interested. Some of our management as far as at the administrative level, Chief Executive Officer, Chief Nursing Officer, and that level, are interested and they’re keeping well abreast of what are we doing, what we need to do, what we want. If I can’t get people to comply, then I go to my chief nursing officer and say, “Hey, I need some clout behind this.” And she will then send out an edict and get sometimes a better response than I will. |
| Proactive Infection Control Culture | Take central line insertion. We developed a monitoring tool that outlines every piece of that bundle. So for every central line that is inserted in our facility, the person who assists with that insertion fills out that form, sends it to our department and that gets entered into a database. Then we’re able to run reports and look at our compliance to the bundle. Say for instance we have a surgeon in the community, he does surgery here, but now the patient shows up at his office. If the surgeon sends us a sample, a specimen to the hospital and it’s positive, we get that on the line listing. And we will contact the physician and say, ‘Your patient has a positive culture.’ Each month, the physicians get a list of all the surgeries they have performed, and we ask them, ‘Have any of your patients come back to your office with an infection?’ And we include a data collection form. So it’s a form that they can fill out if the patient has an infection. What we do is work with various service lines such as our open heart service line and our orthopedic service line to make sure that they’re following the best practice for the surgical piece of it and we’re just making sure that they have good infection control pieces in their service line element, their literature and the patient education materials, et cetera. So, we’re part of those committees. And our orthopedic service line, at least the part for hips and knees, we actually collect that data for them. So they can see the antibiotic timing for any of the hips and knees that are done, like total hips, total knees [replacements]. Is it given within the right time frame before the surgery? Is it the right antibiotic and is it discontinued 24 hours after? We supply data to them. |
| Clinical Staff Engaged in Infection Control | When we started surveillance for central lines, identifying issues, again it was a team that comes together, including staff nurses and doctors, to make sure that they get what they need and that they are happy with whatever initiative we’re implementing and were a part of it. I’ve fostered a good working relationship with my nursing staff and my physicians. They do not hesitate to pick up the phone and call me and say, “You know what? We’ve got five patients here with the same symptoms and we’re not really sure what’s going on.” They tell me that kind of stuff really quickly. I think that works well. I think that we really focus on team efforts and we try and get buy in from staff and I include medical staff, it involves them. We identify something that’s good on the OR, we’re trying to work with the surgeons, the leaders of the OR and have everybody’s input so we all come up with the recommendations. We all pilot the recommendations or implement them. If they’re working but need a little bit of tweaking, it becomes a team effort. Same thing with our central lines. When we started surveillance, identifying issues, again it was a team that comes together, including staff nurses and doctors, to make sure that they get what they needed and that they were happy with whatever initiative we’re implementing and were a part of it. |
| Moderators: | |
| Resource Issues | It’s heartbreaking to see the cuts have already happened knowing that if we were given another half FTE back, we could do more and we could help our patients more and really get out on the floors and impact change. I’m trying to convince our materials manager that we need to do this bundle, and use this central line bundle kit because it meets compliance. And it’s about the dollars for her, you know? So I think that’s a barrier, is as far as our mentality, we need the supplies to improve. Maybe it might be a catheter, it might be a kit, it might be something as small as a new form. But, the barriers are the money to pay for it and showing the clinical reasons why we need to have it. The CMS policy has added to my workload. In a sense, I’ve lost resources. It used to be that my position was actually a job share with another infection preventionist, and we have 1.1 FTEs between the two of us. And when she took a full-time position elsewhere, mine became a full time position, meaning that I lost that .1 FTE and then lost the other body because we had divvied up our responsibilities. So, I’m doing much more with no additional resources to do it. |
| Coding Discrepancies | What we’re finding is that sometimes, because I use the NHSN criteria, the criteria for calling a certain infection a particular way is not exactly the same as what a doctor would call it in order to treat that patient. And it’s certainly consistent with the medical management of the patient to call it such and treat it that way, but it doesn’t meet my criteria because I’m using NHSN criteria, which are very standardized. I just looked at one the other day. The patient had a skin flora in one blood culture and the physician actually documented that he considered it a contaminant. And yet, medical records coded it as line related blood stream infection (BSI). There was another one where the physician didn’t even mention line related. It was a MRSA and the patient was admitted with pneumonia. And it was likely, if I’d been doing surveillance, I would have said this is pneumonia, not the line. But the patient had a central line and medical records coded it as line related BSI. When I brought that to the attention of medical records, their supervisor reviewed it and said she thought they were coded correctly. What I’m saying is they don’t have the education and the training to be able to understand. The big problem here is that what goes to CMS, the data that goes to CMS comes from our coders. And our coders have to code from physician dictation. Physicians frequently don’t know what an infection really is. We’ve got the CDC definitions that we follow for infections, and frequently the physicians, whatever they dictate, gets reported as an infection when in fact it may not be. |
| Existing Surveillance Requirements | They’ve added more requirements for infection control. It’s nice that we’re moving up the food chain in attention, but it also puts more pressure because we didn’t exactly have a huge increasing in staffing to meet those new requirements. And then the CMS requirements add on top of that, but the real straw is when you have something like H1N1 that’s just absorbing so much of your attention. Then you realize how thin the stretch is. I think there are so many demands on the data, not only from internal reporting mechanisms like for unit boards, but the reporting demands from Leap Frog and IHI and Joint Commission and the state reporting mechanism and all that kind of stuff. We can’t do all the prevention activities we’d really like to do. Many hospitals are voluntarily engaged in implementing clinical bundles or guidelines and they have to monitor and report to accrediting agencies such as IHI and CMS. However, many infection preventionists have said that the emphasis on documenting and observing bundle use has taken away from IC’s ability to conduct risk assessment, environmental assessments, and interventions. They do not have time to implement interventions because they have to make sure there is compliance with bundle use. |
Preventionists described the detective role favorably. It consisted of task-oriented activities such as infection control staff regularly following up on laboratory findings through chart or electronic record review, interviewing clinical staff, conducting post-discharge surveillance for surgical patients, and auditing documents from the hospital floor for completeness, such as line lists used to assess compliance with guidelines for preventing select infections. This appeared to comprise what might be labeled the “grunt work” of infection control that interviewees felt was vital to achieving the implicit QI goals of a larger policy that would no longer reimburse hospitals for certain infection-related care. These types of activities were designed specifically to identify potential trouble spots in the hospital related to particular infections, and also to assess parts of the hospital where existing prevention processes could be strengthened.
Preventionists described the second dimension of a proactive role as their own department’s ongoing awareness of and involvement in formal QI initiatives conducted within their hospitals. The key dynamic referred to in this regard was synergy, i.e. close interactions between themselves and various QI structures in the hospital (e.g. standing QI departments, ad hoc clinical quality improvement teams organized because of specific quality problems) that helped to improve the overall levels of effectiveness and acceptance of QI activities around infection control. This synergy was described in ways that implied a diverse and sometimes time-limited set of work relationships between preventionists and hospital QI personnel, opportunistic behavior exhibited by preventionists that placed them in ideal spots for participating in hospital QI efforts, and transparency in communication and information transfer related to infection rates between the IC department and hospital staff participating in QI activities.
These two dimensions related to a final aspect of a proactive IC department, i.e. the establishment of a “legitimate” infection control function within the hospital that was recognized by both hospital leadership and clinical provider staff as the appropriate source of information and analysis for infection control issues occurring in the institution. Preventionists believed that this legitimacy was reflected in the extent to which clinical provider staff and hospital executives sought out their input on different infection control situations; the subtle yet important behavioral changes seen in response to preventionists providing information or advice to hospital departments or staff; and the ability of preventionists to advocate for their own ideas both formally and informally to quality improvement personnel, hospital executives, and clinical providers.
The second organizational dynamic respondents identified as meaningful to facilitating the CMS policy’s relevance, acceptance, and significance a QI driver was increased top leadership attention to infection control. Triggered in part by the new policy, this attention was viewed favorably by preventionists who believed it could provide (and in some interview cases, did provide) their department with additional support and resources for the infection control endeavor (see Table 3 for representative quotes). In return, as these preventionists became more proactive, hospital leadership were often exposed to greater information and communications related to infection control. Top leadership attention involved chief executives, medical directors, and chief nursing officers taking notice of the importance of infection control departments and activities. This notice manifested itself through the provision of additional resources for infection control, greater management interest in looking at infection data, and top leadership support for infection control activities that were aimed at both surveillance and implementing proven interventions to reduce infection risks in the hospital.
Both of these dynamics fed into a third dynamic occurring within hospitals that interviewees identified as important for the new CMS policy. This was the presence of an involved group of clinical providers across different hospital departments that took a higher degree of interest in infection control and prevention activities. Preventionists in facilities where this involvement occurred talked about the important role played by physicians and nurses in making sure their “local” everyday patient care settings paid attention to infection control and prevention. To preventionists, who had limited resources at their disposal and who could not cover the entire hospital, a key way the CMS policy could be noticed, comprehended, and expanded upon hospital-wide was through clinical staff within each of the hospital departments that took personal accountability for controlling infections. Where clinical providers became particularly accountable, preventionists saw them as extensions of the IC department and reliable partners in infection control. Interviewees described provider involvement in terms of department or medical group-based activities such as clinical bundle implementation, monitoring, and compliance review; infection surveillance; participation on hospital committees and work groups addressing specific infection issues; and general support for the work of the infection control department.
Infection control staff discussed the three organizational dynamics identified as facilitating the recognition, acceptance, and significance of the CMS policy as a QI driver in several ways (see Figure 1). First and most important was the belief presented that that the dynamics served as consistent behavioral vehicles through which awareness was raised of the policy among stakeholders ranging from hospital executives to clinical provider staff such. In short, the policy found a strong everyday voice within the hospital through leadership, preventionists, and clinicians paying more attention to infection control generally. For example, as preventionists got more involved in hospital QI, and as hospital leadership grew supportive of and interested in infection control, some interviewees believed that details about the CMS policy were more likely to filter down into high-risk (for infections) areas of the hospital. This resulted from an increase in everyday opportunities for information transmission across organizational levels. In addition, there was added incentive on the part of staff to listen to this information given that it was transmitted with the support and knowledge of top leadership behind it.
In a related vein, some interviewees believed that the CMS policy gained increased credibility and significance within the hospital as it became associated closely with everyday infection control work and the efforts of credible stakeholders such as preventionists, prominent physicians and nurses, and executive leadership. Rather than a stand-alone policy that could be viewed negatively by hospital staff as an outside intrusion or unfair financial penalty, it could instead be linked through the organizational dynamics described here to hospital QI efforts whose processes and desired outcomes were tangible to staff. It also allowed preventionists to use the policy as an added logic for getting hospital personnel to accept the need for change and their own infection control activities. From the perspective of preventionists, the linkage of the CMS policy to sanctioned infection control activities provided a “safe haven” in which the policy gained credibility while stimulating enhanced dialogue among hospital staff regarding the pros and cons of such a policy for promoting change, and the appropriateness of including specific infections but not others in the policy.
Preventionists also expressed views that in enhancing its recognition and acceptance, the organizational dynamics described here helped the CMS policy drive knowledge enhancement in hospital settings with respect to various infections. This enhancement was reported to occur among clinical providers, executive leadership, and preventionists. Interviewees talked about the increased levels of understanding these different stakeholders had in regards to issues such as the “preventability” of various hospital infections like urinary tract and central line bloodstream infections; the types of procedures and processes for preventing different infections; the actual and potential rates of infections across different parts of the hospital; and the kinds of resources required to maintain an appropriate infection control effort within hospital departments. This knowledge enhancement was achieved to a fuller extent when the organizational dynamics described here were in full force.
Other Contextual Dynamics that Moderate the Organizational Factors Identified
Preventionists identified several other factors occurring within their hospital settings that they believed affected the role played by the organizational dynamics described above in enhancing the recognition, acceptance, and significance of the CMS policy as a QI driver (see Figure 1). Some preventionists described resource issues involving shortages in money and supplies allocated to infection control within their hospitals. Examples given of supply shortages ranged from not having enough materials for implementing clinical bundles to prevent infections like central line bloodstream infections to the lack of computerized data mining systems that would allow quicker, more accurate infection surveillance. Resource issues were not limited to smaller hospitals. Rather, preventionists in both larger and smaller hospital settings expressed such perceptions.
Resource issues were also discussed in the context of “zero-sum” resource shifting, as in the case where a hospital reallocated preventionist time spent on surveillance, reporting, and risk reduction from higher to lower morbidity infections such as urinary tract infections. Interviewees identified this type of resource shift frequently. The most common resource issue identified related to infection control staffing. The vast majority of IC departments participating in the interviews were small, often employing only a few full-time equivalents with responsibility for an entire hospital. Interviewees felt that staffing size impacted the ability of infection control departments to be proactive, engage clinical line staff, and serve as a resource for hospital leadership. As a result, they perceived that recognition and acceptance of the CMS policy could be negatively impacted by infection control departments that were smaller and less resource rich.
A second moderating dynamic involved the potential for coding discrepancies related to different types of hospital infections that occurred within hospitals. Coding discrepancies referred to situations in hospitals where reduced consistency might exist in how healthcare-associated infections got identified and recorded between physicians, hospital coding staff, and infection control staff. Some preventionists either worried or actually believed that the results of their own infection surveillance in some cases did not coincide with the number of infections identified by physicians or hospital coding personnel. Preventionists stressed their use of the formal Centers for Disease Control and Prevention (CDC) and National Health Safety Network (NHSN) definitions for labeling something a healthcare-associated infection.
However, many preventionists felt that they were among the few within their hospital settings to know and apply the CDC/NHSN definitions consistently. Some felt that that many physicians did not know how to assess accurately the occurrence of a healthcare-associated infection (HAI), and that when physician documentation reflected an HAI incorrectly it was picked up by hospital coders incorrectly. What concerned preventionists who felt most strongly about the coding discrepancy problem was the potential misinformation from incorrect HAI coding that could find its way into surveillance reports sent to external organizations such as CMS, as well as to hospital leadership and staff. This misinformation or inconsistency in reporting could, according to them, reduce the overall impact of the CMS policy as a QI driver because it produced a much less accurate understanding of infection-related risks and problems across the hospital as a whole.
The final moderating dynamic suggested by interviewees was the requirements imposed on the hospital by existing surveillance requirements and activities. This dynamic was alluded to by preventionists across a range of hospital settings. In this respect, preventionists described the extent to which their hospitals were now engaged in multiple QI and surveillance efforts related to infection control. These added efforts were driven by: (a) increased mandatory reporting for HAIs and (b) voluntary hospital participation in state and national infection control efforts. For some participating hospitals, there was no shortage of external organizations now driving their infection control and surveillance efforts, including CMS, CDC, Institute for Healthcare Improvement, state health departments, and state legislatures. While interviewees acknowledged that some favorable economies of scale were created by having to respond to numerous external demands for HAI control and surveillance, often because the same HAIs were focused upon across different efforts, there were additional infections included in these other efforts that were not included in the CMS policy. Many preventionists felt that too much of their time was spent on documenting compliance, often for the main purpose of reporting “the numbers” to one external organization or the other, rather than on outcomes that could feed into more effective process improvement. For them, these existing surveillance burdens undermined the CMS policy because in cases where the burden required greater staff time and other resources, the policy simply became another external requirement with which “to comply”.
Discussion and Conclusion
The present study is exploratory. However, it offers important preliminary insight into the role played by organizational factors in helping to enhance the recognition, acceptance, and significance of the CMS policy as a QI driver within hospitals. These findings support the notion that organizational culture, leadership behaviors, and staff behavior matter as facilitators of CMS policy implementation, consistent with the preliminary conceptual model put forth in Stone et al. (2010). The study findings also support the idea that there are additional factors which moderate how these organizational factors work to give voice and credibility to the CMS policy on an everyday basis.
From a policy and management perspective, the model derived from the data (see Figure 1) suggests that key elements of the surrounding organizational context do matter as vehicles through which pay-for-performance (P4P) policies are interpreted and acted upon at the individual and group levels of organization. This should make such elements a focus for action by regulators, accrediting agencies, payers, and hospitals. In part, this means ensuring that the right kinds of everyday implementation environments exist within hospitals so that different P4P policies stand a better chance of working in the intended manner (Nembhard, Alexander, Hoff, and Ramanujam 2009).
In this sense, the first and perhaps most important aspect of “working in the intended manner” is that such policies are recognized and understood by hospital staff, and perceived as both credible and important to achieving quality improvement by different organizational stakeholders. The attention paid to assuring greater stakeholder familiarity with and early acceptance of incentive-based QI policies has been identified as important in P4P implementation (Institute of Medicine 2007). Practically speaking, a focus on macro-level policy design that seeks, for example, to identify the right types and mix of economic incentives and the appropriate inclusion criteria for P4P policies such as the CMS policy must be complemented by a micro-level focus that identifies the relevant organizational dynamics which can aid policy implementation, and then offers interventions and supports to ensure hospitals maintain those dynamics on an everyday basis.
From a research perspective, as stated the results provide a small yet important degree of support for the conceptual model put forth in Stone et al. (2010). This model requires further refinement and testing, but offers promise as a means of understanding CMS policy implementation and outcomes better. In fact, the model may be useful for thinking about P4P policy implementation generally. The model’s general focus is on articulating how different enabling and predisposing factors act on the CMS policy as it gets implemented within organizational settings, illuminating to some degree the “black box” of organizational context researchers increasingly assume play important roles in policy adoption and success. To take it further, however, requires researchers to move their empirical questions and explanations closer to the behavioral regularities of the everyday workplace. Through findings such as the ones in this study that identify specific manifestations of the general organizational factors put forth in Stone et al., greater understanding is gained of the particular theories and ideas that may be employed within the overall model to help identify the factors that matter more.
The present study is limited in several ways. First, the study is qualitative and relies upon the systematic interpretive process employed by the research team. While acceptable qualitative methods of analysis were used, the generalizability of the findings across a larger population of hospital settings cannot be gauged. Second, outcomes such as clinical quality within hospitals or cost reduction were not a focus of the study. Instead, the outcomes of interest involved the manner in which the CMS policy was accepted, recognized, or made more significant among hospital personnel. However, these types of outcomes may be realized without necessarily leading to the favorable gains in clinical quality or efficiency desired through the original intent of this P4P mechanism. Future studies should include these additional outcomes in their analysis. Finally, the study attempts to impose some order on the complexity of the everyday hospital setting, by teasing out specific contextual features of that setting that participants believe are most relevant to policy implementation. However, it is unclear whether there are other equally meaningful aspects of the hospital context not identified by interviewees that must be considered in this regard. As a result, this study represents only a first step in understanding the “black box” of implementation deemed important by health care researchers and practitioners.
Executive Summary.
Healthcare-associated infections are among the most common adverse events in hospitals, and the morbidity and mortality associated with them are significant. In 2008, the Centers for Medicare and Medicaid Services (CMS) implemented the use of a new financial policy that no longer provides payment to hospitals for services related to certain infections not present on admission and deemed preventable. At present, little is known about how this policy is being implemented in hospital settings. One key goal of the policy is to have it serve as a quality improvement driver within hospitals, providing the rationale and motivation for hospitals to engage in greater infection-related surveillance and prevention activities.
The present study examines the role organizational factors play in helping the CMS policy work effectively as a quality improvement driver within hospital settings. Organizational factors such as leadership and culture facilitate quality improvement activities generally across a variety of healthcare settings, including hospitals. Between late 2009 and early 2010, interviews were conducted with 36 infection preventionists working at a national sample of 36 hospitals. We found preliminary evidence for the favorable roles played by hospital executive behavior, a proactive infection control culture, and clinical staff engagement in enhancing the recognition, acceptance, and significance of the CMS policy as a QI driver within hospitals. We also found several other contextual factors which may impede the degree to which the above factors facilitate linkages between the CMS policy and hospital quality improvement activities.
Acknowledgments
The project described was supported by Award Number R21AI083888 (Lee) from the National Institute of Allergy and Infectious Diseases.
We gratefully acknowledge the infection preventionists who participated in this study and our colleagues at the Association for Professionals in Infection Control and Epidemiology, Denise Graham and Yolanda Tillery, for their support of this study.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the United States government.
Contributor Information
Timothy Hoff, Email: thoff@albany.edu, Department of Health Policy, Management, and Behavior, University at Albany, Room 181, GEC Building, 1 University Place, Rensselaer, NY 12144-3456, Ph: (518) 402-6512; Fax: (518) 402-0414
Christine W. Hartmann, Email: christine.hartmann@va.gov, Center for Health Quality, Outcomes, and Economic Research, Bedford VA Medical Center, 200 Springs Road (152), Bedford, MA 01730, Telephone number (781) 687-2738; Fax number (781) 687-3106
Peter Wroe, Email: Pwroe64@gmail.com, 5020 S. Lake Shore Drive, Apt. 3308, Chicago, IL 60615, Ph: 208-818-6835
Maya Dutta-Linn, Email: maya_dutta-linn@hphc.org, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, 133 Brookline Ave, 6th floor, Boston, MA 02215, Ph: 617-509-2417; Fax: 617-859-8112
Grace Lee, Email: grace_lee@hphc.org, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, 133 Brookline Ave, 6th floor, Boston, MA 02215, Ph: 617-509-9959; Fax: 617-859-8112
References
- Alexander JA, Lee, Lee SD. Does Governance Matter? Board Configuration and Performance in Not-for-Profit Hospitals. The Milbank Quarterly. 2006;84(4):733–758. doi: 10.1111/j.1468-0009.2006.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Kirkland KB, Kaye KS, Thacker PA, Kanafani ZA, Auten G, Sexton DJ. Underresourced Hospital Infection Control and Prevention Programs: Penny-Wise, Pound Foolish? Infection Control and Hospital Epidemiology. 2007;28(7):767–73. doi: 10.1086/518518. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. Hospital-Acquired Infections in Acute Inpatient Prospective Payment System Hospitals. 2007 Available online at: https://www.cms.gov/HospitalAcqCond/downloads/HACFactsheet.pdf/
- Ferlie EB, Shortell SM. Improving the Quality of Health Care in the United Kingdom and the United States: A Framework for Change. The Milbank Quarterly. 2001;79(2):281–315. doi: 10.1111/1468-0009.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood AB. The Impact of Organizational and Managerial Factors on the Quality of Care in Health Care Organizations. Medical Care Research and Review. 1994;51(4):381–428. doi: 10.1177/107755879405100402. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Rewarding Provider Performance: Aligning Incentives in Medicare. Washington, DC: National Academy of Sciences; 2007. [Google Scholar]
- Jarvis WR. The Lowbury Lecture. The United States Approach to Strategies in the Battle Against Healthcare-Associated Infections, 2006: Transitioning from Benchmarking to Zero Tolerance and Clinician Accountability. Journal of Hospital Infection. 2007;65(Suppl 2):3–9. doi: 10.1016/S0195-6701(07)60005-X. [DOI] [PubMed] [Google Scholar]
- Jarvis WR. Selected Aspects of the Socioeconomic Impact of Nosocomial Infections: Morbidity, Mortality, Cost, and Prevention. Infection Control and Hospital Epidemiology. 1996;17(8):552–57. doi: 10.1086/647371. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Edwards JR, Edwards CL, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, 2002. Public Health Reports. 2007;122(2):160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leape LL. The Nature of Adverse Events in Hospitalized Patients: Results of the Harvard Medical Practice Study II. New England Journal of Medicine. 1991;324(6):377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- Miles MB, Huberman AM. Qualitative Data Analysis. Thousand Oaks, CA: Sage Publications; 1994. [Google Scholar]
- Nembhard IM, Alexander JA, Hoff TJ, Ramanujam R. Why Does the Quality of Health Care Continue to Lag? Insights from Management Research. The Academy of Management Perspectives. 2009;23(1):24–42. [Google Scholar]
- Patton M. Qualitative Research and Evaluation Methods. 3. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Roberts RR, Scott RD, Cordell R, Solomon SL, Steele L, Kampe LM, Trick WE, Weinstein RA. The Use of Economic Modeling to Determine the Hospital Costs Associated with Nosocomial Infections. Clinical Infectious Disease. 2003;36(11):1424–32. doi: 10.1086/375061. [DOI] [PubMed] [Google Scholar]
- Shortell SM, O’Brien JL, Carman JM, Foster RW, Hughes EF, Boerstler H, O’Connor EJ. Assessing the Impact of Continuous Quality Improvement/Total Quality Management: Concept Versus Implementation. Health Services Research. 1995;30(2):377–41. [PMC free article] [PubMed] [Google Scholar]
- Shortell SM, Bennett CL, Byck GR. Assessing the Impact of Continuous Quality Improvement on Clinical Practice: What Will It Take to Accelerate Progress. The Milbank Quarterly. 1998;76(4):593–624. doi: 10.1111/1468-0009.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofaer S. Qualitative Methods: What are They and Why Use Them? Health Services Research. 1999;34(5 Pt 2):1101–1118. [PMC free article] [PubMed] [Google Scholar]
- Stone PW, Hedblom EC, Murphy DM, Miller SB. The Economic Impact of Infection Control: Making the Business Case for Increased Infection Control Resources. American Journal of Infection Control. 2005;33(9):524–27. doi: 10.1016/j.ajic.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Stone PW, Glied SA, McNair PD, Matthes N, Cohen B, Landers TF, Larson EL. CMS Changes in Reimbursement for HAIs: Setting a Research Agenda. Medical Care. 2010;48(5):1–7. doi: 10.1097/MLR.0b013e3181d5fb3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A. Qualitative Analysis for Social Scientists. New York: Cambridge University Press; 1987. [Google Scholar]
- Strauss A, Corbin J. Basics of Qualitative Research. 2. Thousand Oaks, CA: Sage Publications; [Google Scholar]
- Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable Cost of Catheter-Associated Bloodstream Infections Among Intensive Care Patients in a Non-Teaching Hospital. Critical Care Medicine. 2006;34(8):2084–89. doi: 10.1097/01.CCM.0000227648.15804.2D. [DOI] [PubMed] [Google Scholar]
- Weiner BJ, Shortell SM, Alexander J. Promoting Clinical Involvement in Hospital Quality Improvement Efforts: The Effects of Top Management, Board, and Physician Leadership. Health Services Research. 1995;32(4):491–510. [PMC free article] [PubMed] [Google Scholar]