Abstract
Males of some strains of mice retain their mammary epithelium even in the absence of nipples. Here, we have characterized the mammary gland in male CD-1 mice both in whole mounts and histological sections. We also examined the effects of bisphenol A (BPA), an estrogen mimic that alters development of the female mouse mammary gland. BPA was administered at a range of environmentally relevant doses (0.25 – 250 μg/kg/day) to pregnant and lactating mice and then the mammary glands of male offspring were examined at several periods in adulthood. We observed age- and dose-specific effects on mammary gland morphology, indicating that perinatal BPA exposures alter the male mammary gland in adulthood. These results may provide insight into gynecomastia, the most common male breast disease in humans, where proliferation of the mammary epithelium leads to breast enlargement.
Keywords: endocrine disruptor, apoptosis, proliferation, non-monotonic dose response, morphometric analysis, histology
1. Introduction
In both males and females, hormones are responsible for the development of the sex organs. The mammary gland is strongly influenced by estrogens, progesterone, testosterone, and growth hormone [1]. In humans, the breast is an example of a sexually dimorphic structure both in overall appearance as well as in tissue organization characteristics. For example, in contrast to the female breast which is predominantly comprised of ducts, glandular epithelium and non-adipose stroma, the male breast is mostly adipose tissue with sparse ducts and periductal stroma [2].
The female mammary gland has received considerable attention in the fields of developmental biology, endocrinology and cancer biology. In fact, the mammary gland has been demonstrated to be one of the more sensitive organs following perinatal exposures to endocrine disrupting chemicals [3, 4]. In mice and rats, perinatal exposure to low doses of the xenoestrogen bisphenol A (BPA) alter the morphology of the mammary gland, induce preneoplastic lesions, alter the response of the gland to natural hormones, and sensitize the gland to chemical carcinogens [5-18].
In contrast to the well characterized female mammary gland, the male mammary gland and its associated diseases remains largely unstudied. In humans, conditions of the male mammary gland include gynecomastia, the benign enlargement of the breast due to proliferation of the ductal epithelium; pseudogynecomastia, the benign enlargement of the breast due to growth of the adipose tissue; and male breast cancer [2]. Gynecomastia has been reported to occur in domestic animals either spontaneously, after exposure to environmental estrogens, or due to the presence of testicular tumors that alter hormone production [19]. However, there is currently no well-established animal model to study these conditions.
In mice, mammary gland development proceeds similarly in male and female fetuses through embryonic day (E)13. On E14, due to the testosterone surge produced by the male testes, the mammary mesenchyme condenses around the epithelial stalk causing a detachment of the gland from the epidermis [20-23]; thus, males lack nipples. Because of this, some researchers have concluded that they do not retain any epithelial or glandular tissue [3]. However, studies that examined the effects of pre-pubertal exposures to hormones and pharmaceuticals on growth parameters of the male mouse mammary gland [24-27] provided evidence that the male mouse could serve as a model to understand male breast diseases.
In some strains of rats, histological analyses suggest that there are significant differences between male and female mammary epithelium; adult females have glands comprised mostly of ducts and alveolar structures, both of which are composed of a single layer of cuboidal epithelium with a distinct lumen and underlying myoepithelial cells. In contrast, the male epithelium is typically lacking organization, with large contiguous groups of alveoli [28]. The male ductal epithelium is squamous-type with abundant vacuoles, highly eosinophilic cytoplasm, and indistinct lumena that occasionally contain secretory substances. Some traditional toxicology studies have examined histological changes in the male rat mammary gland following exposures to environmental estrogens [29, 30]. These studies indicate that male mammary glands in some rat strains develop characteristics that are more similar to control female glands following estrogen treatment. However, because the rat mammary gland has distinct sexual dimorphisms, it may not be the best model for assessing endocrine disruptors
Herein, we report findings from male CD-1 mice, which do not have nipples yet have rudimentary epithelial trees present in the mammary fat pad. We observed these epithelial trees at all ages examined from E18 – 15 months of age. We also compared histological aspects of the male mouse epithelium with the known characteristics of the female mouse mammary epithelium, and in contrast to the findings reported in the rat found them to be largely indistinguishable. Finally, we examined whether exposure to low doses of BPA during perinatal development altered morphological or histological aspects of the male mammary gland. We observed that BPA altered the development of the male mammary gland at every age examined, with similar effects to those reported previously in the female [6-8, 18].
2. Materials and Methods
2.1 Animals
Sexually mature, female CD-1 mice (8 weeks of age; Charles River, MA) were maintained in temperature and light controlled (14 hour light, 10 hour dark) conditions at the Tufts Medical Center Animal Facility in accordance with the Guide for Care and Use of Laboratory Animals. Animals were housed in polysulfone cages with corncob bedding; both released only negligible amounts of estrogen as determined using the E-SCREEN assay [31]. Food (Harlan Teklad 2018) was supplied ad libitum; estrogenicity of feed was measured at ≤20 femtomoles of estrogen equivalents per gram, a negligible amount [31]. Water was supplied in glass bottles only.
For the generation of animals exposed to BPA, the morning on which a vaginal plug was observed was designated day 1 of pregnancy. On the evening of day 8 of pregnancy, dams were weighed and implanted subcutaneously with Alzet osmotic pumps (Alza Corp, Palo Alto, CA) designed to deliver either 50% dimethyl sulfoxide (DMSO; vehicle control) or BPA (Sigma, St. Louis, MO) dissolved in 50% DMSO. Tested doses included 0.25 μg BPA/kg BW/day (0.25BPA), 2.5 μg BPA/kg BW/day (2.5BPA), 25 μg BPA/kg BW/day (25BPA) and 250 μg BPA/kg BW/day (250BPA). Dams were allowed to deliver naturally, and the litters were culled to eight pups per mother 1 day after birth. The pumps continued to release through day 16 of lactation, the age when pups begin to eat solid food. Litters were weaned on postnatal day (PND) 20-22.
2.2 Mammary gland whole mounts
For the control time-course experiment, male offspring exposed only to 50% DMSO (vehicle control) were killed on E18, PND1, PND5, PND10, 3-4 months, 7-9 months and 12-15 months of age. For the BPA experiments, a single male offspring from each litter was killed at 3-4, 7-9 and 12-15 months of age. One fourth inguinal mammary gland was dissected from the skin, spread on a glass slide and placed in phosphate buffered formalin overnight. Whole-mounted mammary glands were processed and stained with Carmine-alum using the methods described previously [7]. In a few cases, no visible epithelium was detected in the whole mount (<10% for all treatments, at all ages); we cannot determine whether this lack of epithelium was due either to technical errors or the biology of the gland. Whenever possible, if epithelium was not detected, another sample from a male littermate was collected. Sample sizes are indicated on graphs; these sample sizes indicate the number of males with detectable epithelium only.
2.3 Mammary gland morphometrics
Whole-mounted mammary glands were visualized using a Zeiss Stemi 2000-C dissection scope using a 0.8x objective and digital images were captured at 3600 dpi with a Zeiss AxioCam HRc digital camera (Carl Zeiss, Inc., Thornwood, NY). Quantitative analyses of mammary gland dimensions were performed using the Zeiss AxioVision program version 4.4. For each epithelial tree, ductal area, measured as the bound area from the outer edges of the tree, and the total number of branching points were measured.
2.4 Excision of ducts & histology
In some whole-mounts, epithelial ducts were excised using a scalpel with the aid of a dissection scope. The excised ducts were washed with xylene, infiltrated with paraffin under vacuum, and embedded. 5μm sections were cut on an RM2155 rotary microtome (Leica, Nusslock, Germany) and mounted on Superfrost (plus) slides (Fisher Scientific International Inc., Hampton, NH). Sections were deparaffinized with xylene, hydrated by a series of alcohols, and stained with Harris’ hematoxylin and eosin (H&E).
2.5 Immunohistochemistry & TUNEL
Paraffin sections were processed for immunohistochemistry as described previously [8]. Briefly, slides were treated with xylene to remove paraffin and rehydrated through a series of alcohols and PBS, then microwaved in 10mM citrate buffer (pH 6) for antigen retrieval. Non-specific binding was blocked for 1 hour with normal goat serum in 1.5% milk. Sections were incubated overnight at 4C in a humid chamber with primary antibodies against ERα (Novocastra), smooth muscle actin (Abcam) or Ki67 (Vector, VPK451). Biotinylated goat anti-rabbit IgG (Zymed) was applied to sections for 1 hour in a humidified chamber at room temperature. Slides were rinsed with PBS and detection of positive cells was accomplished using DAB (Vector).
For detection of apoptotic cells, a TUNEL kit (Chemicon, S7100) was used as per the manufacturer’s instructions. All samples were counterstained with Harris’ hematoxylin, dehydrated, mounted with a permanent mounting medium and imaged with a Zeiss Axioskop 2 plus light microscope at 40x magnification.
2.6 Statistics
PASW statistical software package 18.0 for Windows (SPSS Inc., Chicago, IL) was used for all statistical analyses. ANOVA followed by Bonferroni posthoc tests were used to compare morphometric and histochemical parameters between treatments at each age. To account for litter effects, only one animal was examined from each litter for each endpoint. For all statistical tests, results were considered significant at p < 0.05. All results are presented as mean ± SEM.
3. Results
3.1 Epithelium is present in the male mammary gland from E18 to 15 months of age
In the male mouse, a testosterone surge on approximately E14 causes the mammary mesenchyme to condense around the epithelial stalk, initiating detachment of the mammary epithelium from the overlying epidermis. For this reason, male mice lack nipples. In several mouse strains, apoptotic processes that follow detachment of the gland are thought to destroy any remaining epithelium [1, 32, 33]. To determine whether male CD-1 mice retain any portion of their epithelium, whole mount mammary glands from control male mice were examined at E18, PND1, PND5, PND10, 3-4 months of age, 7-9 months of age, and 12-15 months of age (Figure 1). Males with retained nipples were never observed. However, the majority of males (>90%) had visible epithelial ducts at all ages.
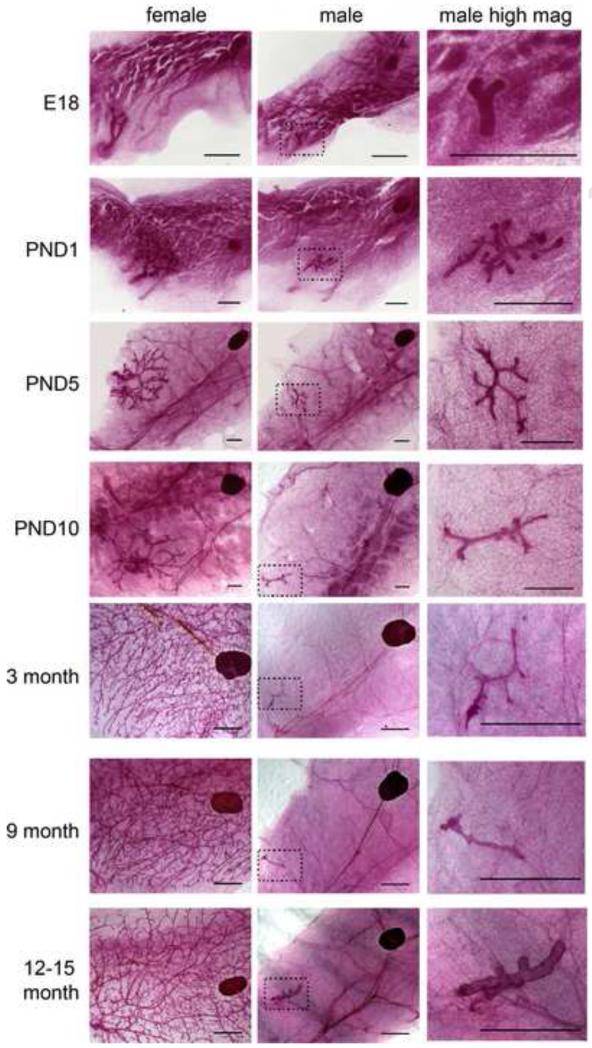
Figure 1. Morphology of the female and male mammary glands throughout life.
Whole-mount mammary glands were collected from control male and female mice at E18, PND1, PND5, PND10, 3-4 months of age, 7-9 months of age and 12-15 months of age. Images were collected at 0.8x magnification for all glands to allow visual comparisons of the sexual dimorphism of the gland. At all ages examined, the male gland (middle panel) was significantly smaller than glands collected from female siblings (left panel). High magnification images of the male mammary gland (right panel) illustrate the changes in mammary gland morphology observed over time. In all panels, the scale bar represents 500μm for E18, PND1 and PND5 and 2mm for PND10, 3-4 months of age, 7-9 months of age, and 12-15 months of age.
One striking observation made when examining these whole-mount mammary glands was the age-dependent effects on the size of the mammary epithelium. The mammary gland undergoes a size increase between E18 and the first few days of life that largely resembles what is seen in females. By PND10, the gland has several branching points and is typically about 0.5 mm2. Yet by 3-4 months of age, the number of branching points decreases with relatively little change in area (Table 1). For the remainder of this study, we focused on males at 3-4, 7-9 and 12-15 months of age because these are ages when these changes have been previously characterized in female mice [8]. Although there were no significant differences in the number of branching points or ductal area in control males at these three ages, we noted that the oldest males had thicker, more distended ducts than 3-4 and 7-9 month old animals (Figure 1).
Table 1. Morphological parameters of the male mammary gland by age.
| Age | Ductal area (mm2) | Branching points |
|---|---|---|
| E18 | 0.19 ± 0. 01 | 0.29 ± 0.13 |
| PND5 | 0.52 ± 0.18 | 10.0 ± 1.9 |
| PND10 | 0.47 ± 0.17 | 8.7 ± 1.3 |
| 3-4 months | 0.64 ± 0.16 | 1.7 ± 0.40 |
| 7-9 months | 2.75 ± 1.50 | 3.0 ± 1.3 |
| 12-15 months | 0.67 ± 0.16 | 0.64 ± 0.28 |
3.2 Epithelium from male mammary glands has similar histological characteristics to epithelium from females
We next asked whether the male mammary epithelium histologically resembles the epithelium observed in female mice. To do this, we excised epithelial ducts, sectioned them, and performed H&E staining. We found that the male epithelium is indistinguishable in organization from what is typically observed in females (Figure 2A,B); epithelial ducts were hollow with a single layer of cuboidal epithelial cells surrounded by an eosinophilic periductal stroma (Figure 2B). The fat pad consisted of adipocytes and immune cells that were indistinguishable from fat pads examined in female mice. Additionally, as seen in the female gland, beneath the ductal epithelial cells was a layer of myoepithelial cells, visible with the H&E stain as well as by immunochemical detection of smooth muscle actin, a myoepithelial marker (Figure 2C, 2D).
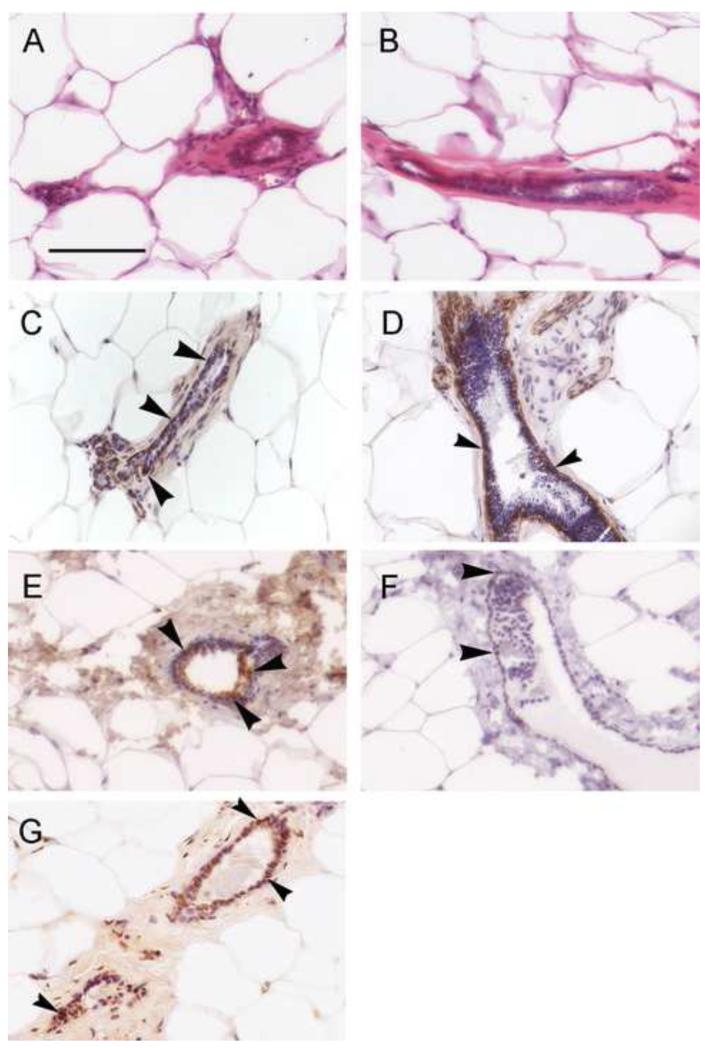
Figure 2. Histological and immunohistological analyses of the 7-9 month old male mammary epithelium reveal many similarities to the female gland.
H&E staining of excised male mammary epithelial ducts reveal a simple cuboidal epithelium with an obvious lumen in both the females (A) and the males (B). The extracellular matrix is highly eosinophilic and the ducts are surrounded by lipid-filled adipocytes. B) Immunohistochemical detection of smooth muscle actin, a marker of myoepithelial cells, reveals the presence of these cells below the ductal epithelium in both females (C) and males (D). E) Immunohistochemical analysis reveals ERα expression throughout the epithelial compartment in the males. In total, approximately 15% of epithelial cells were strongly ERα-positive. F) Immunohistochemistry for Ki67, a marker of proliferation, indicates a low level of expression in male ductal epithelium. Approximately 2-3% of cells were strongly Ki67-positive. G) A TUNEL reaction marking apoptotic cells in the male mammary epithelium. Approximately 60% of cells were marked by TUNEL, indicating a high rate of apoptosis. In panels C-G, arrowheads indicate positive cells; hematoxylin was used as a counter stain. Scale bar = 50μm.
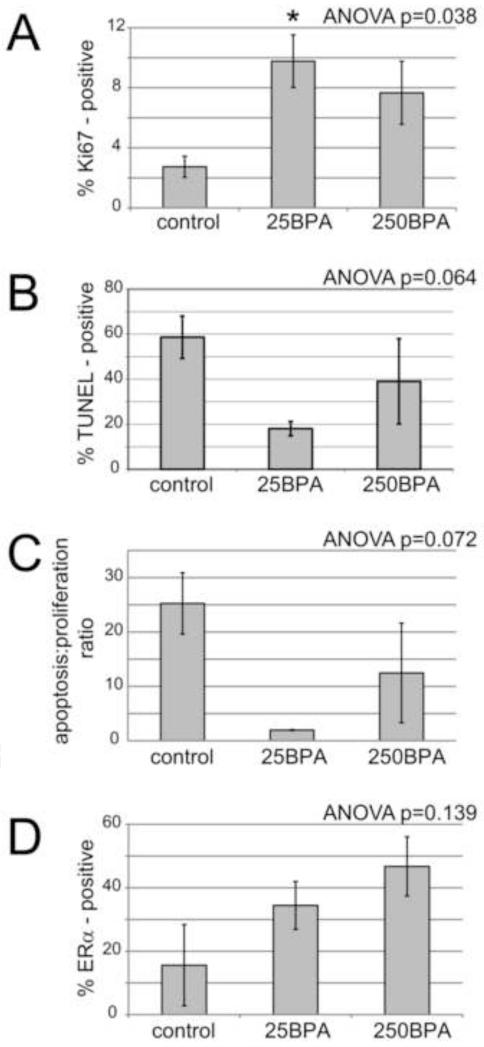
We also characterized the epithelial compartment using antibodies against ERα. Immunohistological data indicated that 10-20% of epithelial cells in the 7-9 month old gland were ERα positive (Figure 2E). In addition, because of the shape and size changes in the mammary epithelium collected from males at various ages, we examined the expression of Ki67, a marker of proliferation. At 7-9 months of age, only 1-4% of the epithelial cells in the male mammary gland were positive for Ki67 (Figure 2F). The TUNEL method was used to identify apoptotic cells. At this same age, 40-85% of epithelial cells in the male mammary gland were positively labeled by the TUNEL kit (Figure 2G). Collectively, these data suggest that the mammary epithelium in the male mammary gland is similar to the epithelium in the female, is likely to be responsive to estrogens, and undergoes significant remodeling.
3.3 BPA affects adult male mammary gland morphology
Because the male mammary gland expresses ERα, we next asked whether development of the gland would be altered by perinatal exposure to the xenoestrogen BPA. At 3-4 months of age, we observed a non-monotonic dose response to BPA, where animals exposed to 0.25BPA and 2.5BPA showed more advanced gland development than the controls, but 25BPA and 250BPA were statistically indistinguishable from controls (Figure 3A-B). In particular, glands from animals exposed to both 0.25BPA and 2.5BPA had significantly more branching points and males exposed to 2.5BPA had increased ductal area relative to controls (Figure 4). In the most severely affected group (2.5BPA), the mean number of branching points and ductal area represented 4.5- and 7.7-fold increases, respectively, compared to controls.
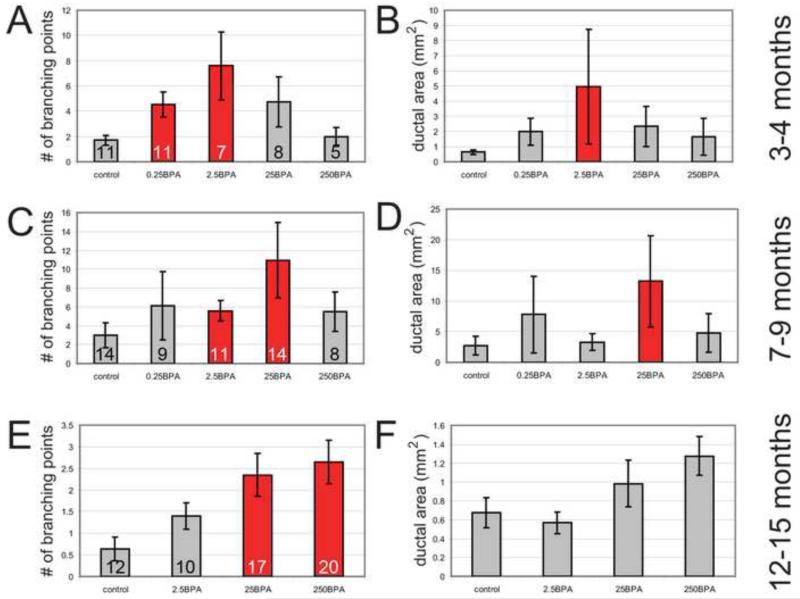
Figure 3. Analyses of control and BPA-exposed male mammary glands reveals age- and dose-specific effects on two morphological parameters, number of branching points and ductal area.
A-B) Comparisons of morphological parameters in control and BPA-exposed animals at 3-4 months of age. C-D) Comparisons of morphological parameters in control and BPA-exposed animals at 7-9 months of age. E-F) Comparisons of morphological parameters in control and BPA-exposed animals at 12-15 months of age. For all panels, ANOVA p<0.05. Bonferroni posthoc analysis revealed differences between treated groups and controls, indicated by red bars (p<0.05). Of note, at 3-4 months of age, the lowest doses were most disruptive of mammary gland morphology. At 7-9 months of age, the moderate doses were most disruptive. And finally at 12-15 months of age, the highest doses of BPA tested had the greatest effects on mammary gland morphology. On all graphs, numbers indicate the sample size analyzed.
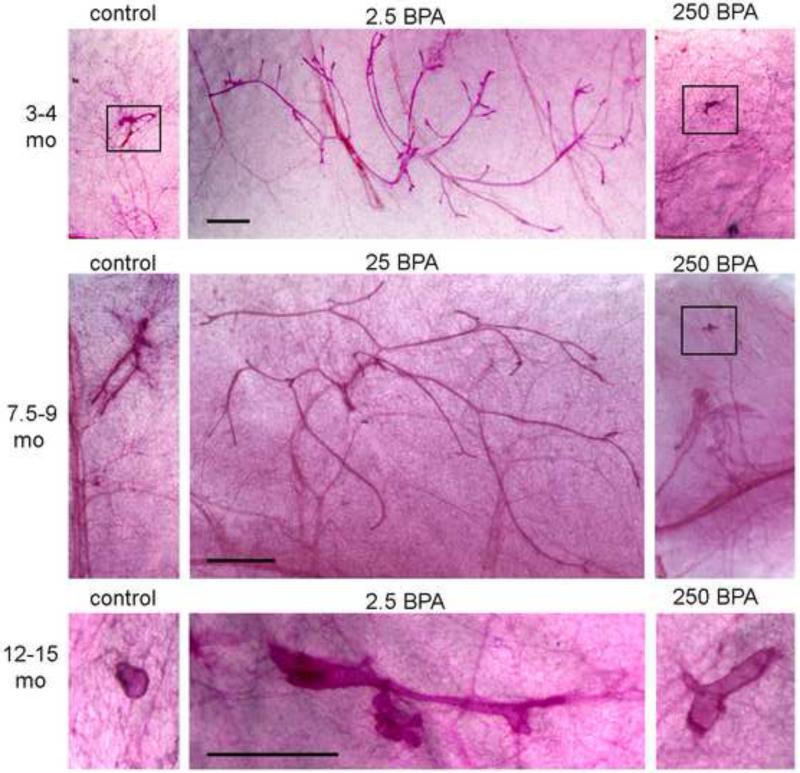
Figure 4. Whole mount mammary glands from control and BPA-exposed males.
At the three ages examined, mammary glands from a control and two BPA treatments groups are shown. The middle panel always includes a treatment that had significant differences from controls, and the right panel includes a treatment that was not statistically distinguishable from controls. In samples where the epithelium was small, it is indicated by a black box. All samples from the same age were taken at the same magnification. Scale bar = 1mm in all panels.
At 7-9 months of age, a non-monotonic relationship between dose and mammary gland morphology was still present, but had shifted such that animals in the 2.5BPA and 25BPA groups were significantly different from controls; however, lower doses (0.25BPA) and higher doses (250BPA) were statistically indistinguishable from controls (Figure 3C-D). Glands from 25BPA males had a 3.6-fold increase in the number of branching points and a 4.8-fold increase in the ductal area compared to controls (Figure 4).
At 12-15 months of age, the groups treated with the two highest doses had significantly more branching points compared to the controls, whereas the 2.5BPA group did not (Figure 3E). Although a similar trend was observed for the ductal area, there were no significant differences by treatment group (Figure 3F). For all four groups examined at this age, the epithelial ducts appeared to be thicker and more distended (Figure 4). There were no 0.25BPA animals available for analysis at this age.
3.4 Effects of BPA on proliferation, apoptosis and ERα expression in the male mammary gland
We next examined proliferation, apoptosis, and the ratio of these phenomena in the epithelium of 7-9 month old control males and compared these measures to 25BPA and 250BPA males of the same age. These two doses were selected because at this age, the 25BPA epithelium is significantly more developed than in the controls (more ductal area and number of branching points) whereas the epithelial characteristics in the higher dose group (250BPA) were statistically indistinguishable from the controls. For Ki67-positive cells, there was a significant increase in the 25BPA group relative to the controls; although there also appeared to be an increase in Ki67 expression in the 250BPA group, this increase was not statistically significant (Figure 5A). There were no significant differences in apoptotic cells as measured by TUNEL labeling, although there was a trend (ANOVA p=0.064; Figure 5B). Further, there was a trend for differences in the apoptosis/proliferation ratio by treatment (ANOVA p=0.072; Figure 5C).
Figure 5. Perinatal BPA exposure alters proliferation in adult male mammary glands.
Immunohistological analyses were conducted in sections of 7-9 month old male mammary glands to quantify the expression of Ki67 (A), TUNEL-positive cells (B), and the ratio between these two factors, expressed as the apoptosis: proliferation ratio (C). Additionally, the percentage of ERα-positive cells was quantified (D). For all measures, ANOVA values are indicated on graphs. When p<0.05, Bonferroni posthoc tests were performed, *p<0.05 compared to controls. For all groups, n=4-5 were analyzed.
Finally, we examined the expression of ERα in epithelium from control, 25BPA and 250BPA males at 7-9 months of age. All males had visible expression of ERα with increasing expression as BPA dose increased (Figure 5D), although these differences were not statistically significant (ANOVA p=0.139).
4. Discussion
To our knowledge, there is no published characterization of the male mouse mammary gland. Herein we present a description of the male mouse mammary gland epithelium from just before birth until over 1 year of age, and detailed its response to perinatal EDC exposure. We focused on BPA, a xenoestrogen to which humans are commonly exposed [34-36], because this chemical has been shown to alter developmental parameters in the female mouse mammary gland [5-10, 18]. Our results indicate that perinatal BPA exposure influences morphological and histochemical endpoints in the male mouse mammary gland months after the period of exposure has ended.
One of the more surprising findings we observed was the effects of age and dose on ductal area and branching points, two parameters of mammary gland morphology and development. At different time periods, different doses induced significant increases in these parameters relative to controls. Namely, at the youngest age examined (3-4 months), the lowest doses were most effective; at the middle age examined (7-9 months), the moderate doses were most effective; and at the oldest age examined (12-15 months), the highest doses were most effective (Figures 3,4). These results may be indicative of an interaction between perinatal BPA exposure and the overall endocrine status of the male, which changes with age.
It is noteworthy that previous studies have reported non-monotonic responses of the mammary gland to BPA for several different endpoints [37]. In female mice exposed to BPA in utero, a low dose stimulated ductal extension whereas a higher dose inhibited it [6]. In female rats prenatally exposed to BPA, doses ranging from 2.5 – 1000 μg/kg/day induced hyperplastic ducts at postnatal day 50, but only animals exposed to the lowest dose retained these lesions at postnatal day 95 [38]. And finally, use of a transgenic mouse model that is prone to mammary tumors demonstrated that low dose exposures to BPA (2.5 – 25 μg/kg/day) in adulthood induced more and larger tumors, and more lung metastases than controls or higher doses of BPA (250 – 2500 μg/kg/day) [15]. Importantly, in this last study the authors proposed that the mechanism behind the observed non-monotonic response may be BPA-induced changes in the ratio of apoptotic and proliferating cells. Here, we have shown that perinatal exposure to BPA affects proliferation rates, and may influence apoptosis rates, in adulthood (Figure 5). Together, these factors may contribute to the inverted U-shaped responses observed in mammary gland morphology parameters (Figure 3). Additionally, studies from other hormone-sensitive organs have implicated ERs in the non-monotonic dose responses observed following BPA exposures [37]; roles for both ERα and membrane ER have been demonstrated in vitro and in vivo [39-41]. Because ERs have been implicated in other non-monotonic dose responses observed in the female mammary gland in response to estrogen treatment [42], this makes it a plausible mechanism for a similar outcome in the male mammary gland. Whether ERs mediate the non-monotonic dose responses we observed here remains unknown, and would require additional study at younger ages including during the period of BPA exposure. The results of this study also suggest that the male mouse may be an appropriate model to study gynecomastia (Figure 1), and the simplicity of the mouse gland compared to the rat gland may support its use as an in vivo tool to study chemical mixtures [26].
Gynecomastia is the most common male breast disease, affecting approximately 50% of boys at puberty [43]. It is thought to develop when there is an imbalance between circulating levels of testosterone and estrogen, and can occur in adult men following treatment with pharmaceuticals that alter the testosterone:estrogen ratio [44]. In rodents, testosterone and estrogen are essential for mammary gland development. In addition to its role in the destruction of the nipple in male mice, testosterone may have a role in postnatal development of the mammary gland. Female androgen receptor knock-out mice show impaired mammary ductal growth in postnatal life including altered ductal elongation [45]. Estrogen is equally important for the development of the mammary gland. Previous studies have shown that females exposed to BPA in utero have altered mammary gland responses to estrogen at puberty [7, 10] and prenatal BPA exposure may alter serum testosterone levels in adult mice [46]. Thus, the results we report here in male mice could be explained by either an altered sensitivity to circulating hormones, or altered production of hormones, in BPA-exposed males. Further studies are needed to address these hypotheses.
At this time, a link between human gynecomastia and EDCs is tenuous at best. Several individual case-studies suggest that therapeutic, accidental, occupational and dietary exposures to estrogen-mimics can contribute to the development of gynecomastia in adult men [47, 48]. A retrospective epidemiology study of adult men administered low doses of the potent pharmaceutical estrogen diethylstilbestrol (DES) during treatment of castrate-resistant prostate cancer showed that 59% of men developed gynecomastia [49]. Other reports of pubertal gynecomastia have been linked to exposures to health care products with estrogenic or anti-androgenic properties [50]. Additionally, a case-control study found that levels of two phthalates were higher in adolescent boys with gynecomastia compared to boys without this condition [51].
A small number of studies in rodents have also examined links between EDC exposures and male mammary gland development. For example, fetal exposures to large doses of flutamide, a pharmaceutical anti-androgen used to treat prostate cancer, caused persistence of nipples and mammary epithelium in a strain of rat that is not thought to retain these structures [52, 53]. Two other studies showed that high doses of genistein during early development alter the tissue architecture of the rat male mammary gland later in life [30, 54]. In contrast to these high dose studies, we have examined low doses of BPA, similar to the concentrations that humans likely encounter in the environment [35]. Our results clearly indicate that in the range of doses examined, there is no BPA exposure that is ineffective at altering male mammary gland development.
In conclusion, we have observed that the male CD-1 mouse retains its mammary epithelium throughout its lifetime, and that this gland is responsive to early life exposure to BPA, an EDC with widespread human exposure. We propose that the effects we observed in the adult male gland may be relevant to human gynecomastia, the most common disease of the male breast, and may further suggest that in some individuals this disease could originate during perinatal development. Although the male mouse mammary gland has a histological appearance that makes it indistinguishable from the female gland, other factors including circulating hormones are likely to affect the response of this gland to EDCs, including chemical mixtures. However, because the male mouse mammary gland has a very simple morphology in adulthood, it may be a useful tool to understand the effects of EDCs and EDC mixtures in vivo.
Highlights.
Male CD-1 mice lack nipples but retain mammary epithelium throughout their lifetime
The male mammary gland is histologically indistinguishable from the female gland
Adult male mammary epithelium expresses estrogen receptor α
Developmental exposure to low doses of BPA alters male mammary gland morphology
The morphological effects are seen in adulthood and are dose-dependent
Acknowledgements
Animals for these studies were generated via grants from the National Institute of Environmental Health Sciences (grants R21ES013884 and RC2ES018781). This work was also funded in part by NIEHS grant R01ES08314.
Abbreviations
- BPA
bisphenol A
- DES
diethylstilbestrol
- DMSO
dimethyl sulfoxide
- E
embryonic day
- ER
estrogen receptor
- EDCs
endocrine disrupting chemicals
- PND
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- [1].Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–55. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- [2].Iuanow E, Kettler M, Slanetz PJ. Spectrum of disease in the male breast. AJR Am J Roentgenol. 2011;196:W247–59. doi: 10.2214/AJR.09.3994. [DOI] [PubMed] [Google Scholar]
- [3].Rudel RA, Fenton SE, Ackerman JM, Euling SY, Makris SL. Environmental exposures and mammary gland development: state of the science, public health implications, and research recommendations. Environ Health Perspect. 2011;119:1053–61. doi: 10.1289/ehp.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C. Does breast cancer start in the womb? Basic and Clinical Pharmacology and Toxicology. 2008;102:125–33. doi: 10.1111/j.1742-7843.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evolution and Development. 2003;5:67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- [6].Markey CM, Luque EH, Munoz-de-Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biology of Reproduction. 2001;65:1215–23. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- [7].Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–47. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26:210–9. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–27. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect. 2007;115:592–8. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jones LP, Sampson A, Kang HJ, Kim HJ, Yi YW, Kwon SY, et al. Loss of BRCA1 leads to an increased sensitivity to Bisphenol A. Toxicol Lett. 2010;199:261–8. doi: 10.1016/j.toxlet.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ Health Perspect. 2010;118:1614–9. doi: 10.1289/ehp.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Betancourt AM, Mobley JA, Russo J, Lamartiniere CA. Proteomic analysis in mammary glands of rat offspring exposed in utero to bisphenol A. J Proteomics. 2010;73:1241–53. doi: 10.1016/j.jprot.2010.02.020. [DOI] [PubMed] [Google Scholar]
- [14].Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. Oral exposure to bisphenol A increases dimethylbenzanthracene-induced mammary cancer in rats. Environ Health Perspect. 2009;117:910–5. doi: 10.1289/ehp.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jenkins S, Wang J, Eltoum I, Desmond R, Lamartiniere CA. Chronic Oral Exposure to Bisphenol A Results in a Non-Monotonic Dose Response in Mammary Carcinogenesis and Metastasis in MMTV-erbB2 Mice. Environ Health Perspect. 2011;119:1604–9. doi: 10.1289/ehp.1103850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lamartiniere CA, Jenkins S, Betancourt AM, Wang J, Russo J. Exposure to the Endocrine Disruptor Bisphenol A Alters Susceptibility for Mammary Cancer. Horm Mol Biol Clin Investig. 2011;5:45–52. doi: 10.1515/HMBCI.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moral R, Wang R, Russo IH, Lamartiniere CA, Pereira J, Russo J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol. 2008;196:101–12. doi: 10.1677/JOE-07-0056. [DOI] [PubMed] [Google Scholar]
- [18].Ayyanan A, Laribi O, Schuepbach-Mallepell S, Schrick C, Gutierrez M, Tanos T, et al. Perinatal Exposure to Bisphenol A Increases Adult Mammary Gland Progesterone Response and Cell Number. Mol Endocrinol. 2011 doi: 10.1210/me.2011-1129. Epub 2011 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Basrur PK. Disrupted sex differentiation and feminization of man and domestic animals. Environ Res. 2006;100:18–38. doi: 10.1016/j.envres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- [20].Kratochwil K. Development and loss of androgen responsiveness in the embryonic rudiment of the mouse mammary gland. Dev Biol. 1977;61:358–65. doi: 10.1016/0012-1606(77)90305-0. [DOI] [PubMed] [Google Scholar]
- [21].Kratochwil K, Schwartz P. Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proc Natl Acad Sci U S A. 1976;73:4041–4. doi: 10.1073/pnas.73.11.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wasner G, Hennermann I, Kratochwil K. Ontogeny of mesenchymal androgen receptors in the embryonic mouse mammary gland. Endocrinology. 1983;113:1771–80. doi: 10.1210/endo-113-5-1771. [DOI] [PubMed] [Google Scholar]
- [23].Drews U. Regression of mouse mammary gland anlagen in recombinants of Tfm and wild-type tissues: testosterone acts via the mesenchyme. Cell. 1977;10:401–4. doi: 10.1016/0092-8674(77)90027-7. [DOI] [PubMed] [Google Scholar]
- [24].Kohlerova E, Skarda J. Mouse bioassay to assess oestrogenic and anti-oestrogenic compounds: hydroxytamoxifen, diethylstilbestrol and genistein. J Vet Med A Physiol Pathol Clin Med. 2004;51:209–17. doi: 10.1111/j.1439-0442.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- [25].Skarda J. Sensitivity and specificity of the bioassay of estrogenicity in mammary gland and seminal vesicles of male mice. Physiol Res. 2002;51:267–76. [PubMed] [Google Scholar]
- [26].Skarda J. Bioassay of steroid hormone agonist and antagonist activities of anti-androgens on mammary gland, seminal vesicles and spleen of male mice. J Vet Med A Physiol Pathol Clin Med. 2003;50:204–12. doi: 10.1046/j.1439-0442.2003.00526.x. [DOI] [PubMed] [Google Scholar]
- [27].Skarda J, Kohlerova E. Mouse bioassay for in vivo screening of oestrogen and progesterone antagonists. J Vet Med A Physiol Pathol Clin Med. 2006;53:145–53. doi: 10.1111/j.1439-0442.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- [28].Cardy RH. Sexual dimorphism of the normal rat mammary gland. Vet Pathol. 1991;28:139–45. doi: 10.1177/030098589102800206. [DOI] [PubMed] [Google Scholar]
- [29].Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, et al. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod Toxicol. 2009;28:342–53. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- [30].Delclos KB, Bucci TJ, Lomax LG, Latendresse JR, Warbritton A, Weis CC, et al. Effects of dietary genistein exposure during development on male and female CD (Sprague-Dawley) rats. Reprod Toxicol. 2001;15:647–63. doi: 10.1016/s0890-6238(01)00177-0. [DOI] [PubMed] [Google Scholar]
- [31].Soto AM, Lin T-M, Justicia H, Silvia RM, Sonnenschein C. An “in culture” bioassay to assess the estrogenicity of xenobiotics. In: Colborn T, Clement C, editors. Chemically induced alterations in sexual development: the wildlife/human connection. Princeton Scientific Publishing; Princeton: 1992. pp. 295–309. [Google Scholar]
- [32].Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Developmental Cell. 2001;1:467–75. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- [33].Robinson GW, Karpf ABC, Kratochwil K. Regulation of mammary gland development by tissue interaction. Journal of Mammary Gland Biology and Neoplasia. 1999;4:9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- [34].Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urine, serum and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–70. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- [36].vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, et al. Hormones and endocrine disrupting chemicals: low dose effects and non-monotonic dose responses. Endocrine Reviews. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–90. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- [41].Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha mediated Ca++ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–9. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vandenberg LN, Wadia PR, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. The mammary gland response to estradiol: monotonic at the cellular level, non-monotonic at the tissue-level of organization? Journal of Steroid Biochemistry and Molecular Biology. 2006;101:263–74. doi: 10.1016/j.jsbmb.2006.06.028. [DOI] [PubMed] [Google Scholar]
- [43].Maidment SL. Question 2. Which medications effectively reduce pubertal gynaecomastia? Arch Dis Child. 2010;95:237–9. doi: 10.1136/adc.2009.176768. [DOI] [PubMed] [Google Scholar]
- [44].Haynes BA, Mookadam F. Male gynecomastia. Mayo Clin Proc. 2009;84:672. doi: 10.4065/84.8.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yeh S, Hu YC, Wang PH, Xie C, Xu Q, Tsai MY, et al. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med. 2003;198:1899–908. doi: 10.1084/jem.20031233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kawai K, Nozaki T, Nishikata H, Aou S, Takii M, Kubo C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A. Environ Health Perspect. 2003;111:175–8. doi: 10.1289/ehp.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Braunstein GD. Gynecomastia. N Engl J Med. 1993;328:490–5. doi: 10.1056/NEJM199302183280708. [DOI] [PubMed] [Google Scholar]
- [48].Braunstein GD. Environmental gynecomastia. Endocr Pract. 2008;14:409–11. doi: 10.4158/EP.14.4.409. [DOI] [PubMed] [Google Scholar]
- [49].Clemons J, Glode LM, Gao D, Flaig TW. Low-dose diethylstilbestrol for the treatment of advanced prostate cancer. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nebesio TD, Eugster EA. Current concepts in normal and abnormal puberty. Curr Probl Pediatr Adolesc Health Care. 2007;37:50–72. doi: 10.1016/j.cppeds.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [51].Durmaz E, Ozmert EN, Erkekoglu P, Giray B, Derman O, Hincal F, et al. Plasma phthalate levels in pubertal gynecomastia. Pediatrics. 2010;125:e122–9. doi: 10.1542/peds.2009-0724. [DOI] [PubMed] [Google Scholar]
- [52].Tate-Ostroff BA, Bridges RS. Nipple development and pup-induced prolactin release in male rats treated prenatally with the antiandrogen flutamide. Psychoneuroendocrinology. 1988;13:309–16. doi: 10.1016/0306-4530(88)90055-8. [DOI] [PubMed] [Google Scholar]
- [53].Nation T, Balic A, Buraundi S, Farmer P, Newgreen D, Southwell B, et al. The antiandrogen flutamide perturbs inguinoscrotal testicular descent in the rat and suggests a link with mammary development. J Pediatr Surg. 2009;44:2330–4. doi: 10.1016/j.jpedsurg.2009.07.072. [DOI] [PubMed] [Google Scholar]
- [54].Wang XJ, Bartolucci-Page E, Fenton SE, You L. Altered mammary gland development in male rats exposed to genistein and methoxychlor. Toxicol Sci. 2006;91:93–103. doi: 10.1093/toxsci/kfj120. [DOI] [PubMed] [Google Scholar]