Abstract
Objectives
Pancreatic stump leak (PL) after elective distal pancreatic resection significantly impacts cost and increases subsequent health care resource utilization. We sought to provide an economic framework for potential interventions aimed at reducing its occurrence.
Design
Retrospective case series and economic evaluation.
Setting
University-affiliated, tertiary care referral center.
Patients
Sixty-six patients undergoing elective distal, pancreatectomy.
Main Outcome Measures
Postoperative complications; hospital and professional costs.
Results
Overall postoperative morbidity occurred in 34 patients (52%) with no deaths. The total number of patients with complications directly related to PL was 22 (33%). The mean ± SD number of total hospital days for the no-PL group was 5.2 ± 1.7 days (range, 3–12 days) vs 16.6 ± 14.6 days (range, 4–49 days) for the PL group (P = .001). The average patient with PL-related problems incurred a total cost that was 2.01 times greater than the average patient in the no-PL group. A decision analytic model developed to evaluate threshold costs showed that a hypothetical intervention designed to reduce the complication rate of distal pancreatectomy by one third would be financially justifiable up to a cost of $1418 per patient.
Conclusions
Complications derived from PL following distal pancreatectomy double the cost and dramatically increase health care resource utilization. There is an urgent need to develop strategies that reduce the incidence of this common complication. Interventions aimed at decreasing the incidence of PL should take into account this cost differential. We provide an economic model to serve as a guide for developing these technologies.
Despite overall improvements in morbidity following pancreatic resection, leakage from the pancreatic stump (PL) following distal pancreatectomy remains problematic.1 Its reported incidence varies markedly in the surgical literature from 0% to 64%.2 This large variability is in part attributed to the lack of standardized definitions applied to PL and to whether fistula is combined with sterile and infected collections or other wound problems that are also a consequence of postoperative PL.3,4
Sequelae from PL following distal resection have a wide spectrum. In the best-case scenario, a patient who develops a PL following distal pancreatectomy is identified early after surgery and will have a controlled fistula through a closed-suction drain placed intraoperatively. Typically, this is managed as an outpatient without need for further intervention. Other patients, however, will develop collections or wound complications related to a PL. These often require emergency department visits, hospital readmissions, radiology-guided percutaneous drainage, prolonged parenteral antibiotic therapy, radiological surveillance, and multiple postoperative office visits. The goals of this study were to quantify the impact of PL in terms of cost and hospital resource utilization, and to provide an economic framework for potential interventions aimed at reducing this event. We hypothesize that PL after elective distal pancreatic resection significantly impacts cost and increases subsequent health care resource utilization.
METHODS
The study was approved by the Massachusetts General Hospital Institutional Review Board. Hospital records of 109 patients who underwent distal pancreatic resections at Massachusetts General Hospital from January 1, 2002, through August 31, 2004, were identified from a prospectively entered pancreatic surgery database. To obtain a homogeneous group, 43 patients who underwent additional organ resection or nonelective resection were excluded. This resulted in a study cohort of 66 patients who underwent purely elective distal pancreatic resections.
PATIENT CHARACTERISTICS
The cohort included 25 men (38%) and 41 women (62%) with a mean age of 59 years (range, 21–85 years). Twenty-eight patients (42%) had at least 1 comorbid factor at the time of surgery. Elective distal pancreatic resections were performed for malignant pathology in 16 patients (24%) and for benign conditions in 50 (76%). Diagnoses arc described in the Table.
Table.
Diagnoses in a Cohort of 66 Patients Undergoing Elective Distal Pancreatectomy
| Diagnosis | No. (%) of Patients* |
|---|---|
| Mucinous cystic neoplasm | 19 (29) |
| With cancer | 1 (2) |
| Pancreatic neuroendocrine tumor | 14 (21) |
| Pancreatic ductal adenocarcinoma | 10 (15) |
| Intraductal papillary mucinous neoplasm | 5 (8) |
| With cancer | 1 (2) |
| Chronic pancreatitis/pseudocyst | 5 (8) |
| Serous cystadenoma | 4 (6) |
| Solid pseudopapillary tumor | 2 (3) |
| Other† | 5 (8) |
| Total | 66 (100) |
Because of rounding percentages may not total 100.
Metastatic renal and adrenal cancer, sacroma, and dermoid cyst.
Operations were performed with a uniform technique. The pancreas was routinely transected with electrocautery. The pancreatic duct was ligated if identified (35 cases [53%]), and the stump was closed with silk sutures. In only 7 cases (11%), a reticulating stapler (TA-55; United States Surgical Corp, Norwalk, Conn) was used to divide the pancreas. A closed-suction drain (Jackson-Pratt) was left in the vicinity of the pancreatic stump. Splenic preservation, achieved by ligation of the splenic artery and vein and relying on collateral circulation from the short gastric vessels, was performed whenever possible in benign lesions (24 patients [36%]).5 There were no laparoscopic resections in this cohort.
DEFINITIONS
The patients’ clinical courses were reviewed in hospital and clinic charts. Complications identified as being directly or indirectly related to PL were categorized according to the following criteria: (1) Pancreatic fistula was defined as a daily output of at least 30 ml of amylase-rich fluid (3 times the scrum concentration) from the surgically placed drain after postoperative day 5. Typically, these patients were discharged home with die drain in place; the drain was gradually withdrawn and removed when the output decreased. Somatostatin was not administered. (2) A sterile collection was defined as a 3×3-cm or greater accumulation of fluid identified radiologically and prompting interventional radiology drainage that yielded amylase-rich fluid. These patients generally presented with pain and occasionally fever during or after their initial hospitalization. (3) An abscess was defined as a collection of fluid that, on aspiration and culture, grew bacteria. Although the amylase concentration was not measured in all cases of abscess, they were considered results of PL. These patients usually presented with fever, pain, and leukocytosis after discharge. (4) A wound disruption was considered an indirect complication of a PL when ongoing drainage of thick fluid through the incision was present and the patient also had a documented pancreatic fistula, collection, or abscess. Other complications not related to PL were also recorded.
COSTS
Hospital and professional costs were analyzed separately. Total net hospital costs were determined using an accounting database (Eclipsys Corporation, Boca Raton, Fla), which is institutional software that generates direct and indirect costs for the hospital. Institutional policy precludes disclosure of specific costs; therefore, only relative differences or ratios are presented. Costs included those of readmissions and emergency department visits. Professional costs were calculated by averaging reimbursements across all insurers. These included radiologist and anesthesiologist as well as surgeon reimbursements, since these constituted the bulk of the professional costs. Other consultant costs were not calculated. Once average per-patient costs were calculated for both the PL and no-PL groups, a cost differential was obtained. A decision analytic model was then developed to evaluate threshold costs of hypothetical distal pancreatectomy interventions that would result in an absolute reduction in the proportion of patients with PL.
STATISTICAL ANALYSIS
Results are presented as mean ± SD, unless otherwise specified. The χ2 test was used to compare categorical variables, and the 2-sample t test, continuous variables. P < .05 was considered statistically significant. Analysis was performed with SPSS statistical software (SPSS Inc, Chicago, Ill).
RESULTS
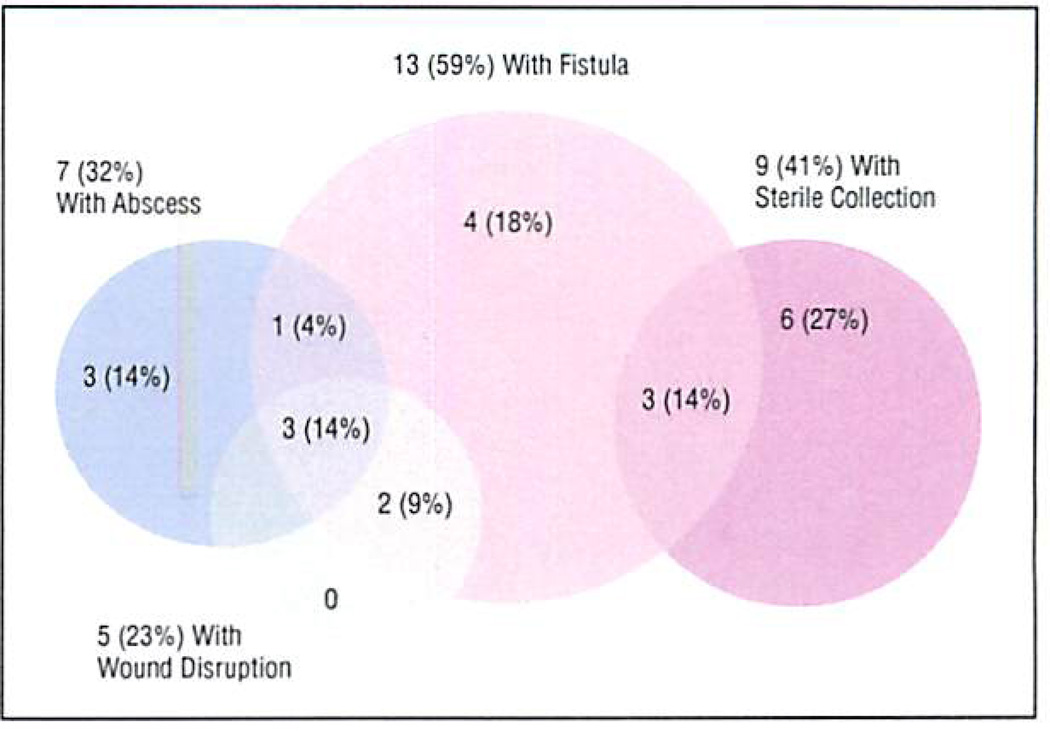
Overall, morbidity occurred in 34 patients (52%) Following distal pancreatectomy. Of these, 22 (33% of the cohort) had complications attributed to PL. The distribution of PL-related complications is further illustrated in Figure 1. The most common presentation of PL was a fistula, which occurred in 13 (59%) of the 22 patients. A sterile collection was documented in 9 patients (41%) and an abscess in 7 (32%). There was considerable overlap, with many of the PLs manifesting as both a fistula and a sterile collection, or a fistula and an abscess. Five patients (23%) had wound disruption attributed to PL, and all of these had either an abscess or a fistula as well. Other complications not considered directly related to a PL are listed in the following tabulation:
| Complication | No. (%) |
|---|---|
| Urinary tract infection | 7 (11) |
| Pleural effusion | 5 (8) |
| Pneumonia | 5 (8) |
| Ileus | 3 (5) |
| Atelectasis | 2 (3) |
| Other* | 6 (9) |
New-onset diabetes mellitus, pancreatic exocrine insufficiency, myocardial infarction, delirium, and cardiac arrhythmia.
Figure 1.
Distribution of pancreatic stump leak complications in 22 patients following distal pancreatectomy.
Three patients (5%) required a second surgical procedure. In the PL group, a patient who underwent concomitant splenectomy was reexplored at 90 days for persistent intra-abdominal abscess. Another, who had splenic preservation, underwent reexploration at 3.5 months for splenic infarct and persistent collection in the splenic hilum. A third patient, in the no-PL group, who had undergone splenic preservation, underwent reexploration for presumed splenic infarct but required no further intervention. No patient required operative repair for pancreatic fistula. There were no deaths.
HEALTH CARE RESOURCE UTILIZATION
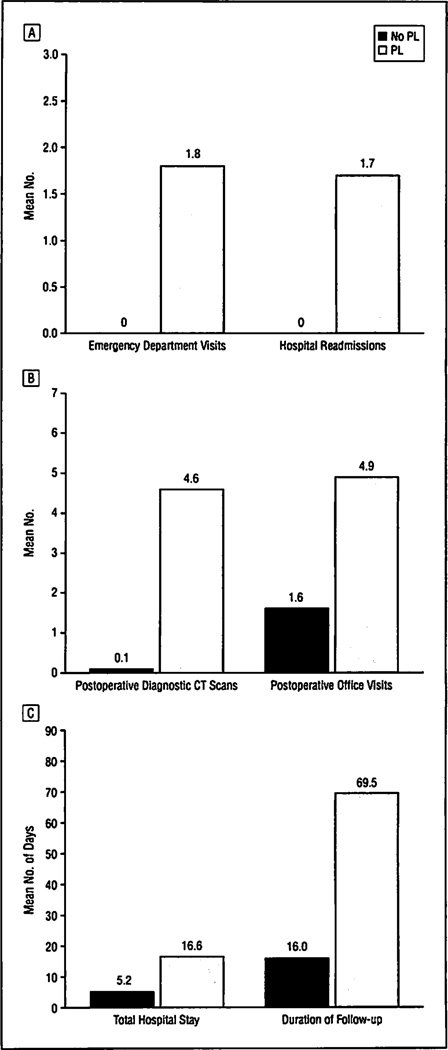
Sixteen of the 22 patients in the PL group (73%) required readmission for a mean of 13.8 hospital days (range, 1–38 days). The average number of readmissions in this group was 1.7 (range, 1–5 readmissions). The mean number of total hospital days (including readmissions) for the no-PL group was 5.2 ± 1.7 days (range, 3–12 days) vs 16.6± 14.6 days (range, 4–49 days) for the PL group (P = .001). Of the patients with PL, 13 (59%) visited the emergency department after discharge an average of 1.8 times (range, 1–6 times). Twelve patients in the PL group (55%) required interventional radiology drainage (mean, 1.1 procedures [range, 0–5 procedures]). They also had an average of 4.6 computed tomographic scans per patient following resection (range, 0–15 scans). Fifteen (68%) of the patients in the PL group received at least 1 course of broad-spectrum antibiotics for an average of 12.6 days (range, 7–24 days).
Patients with PL required a mean of 4.9 postoperative office visits (range, 1–20 visits) and a median follow-up of 69.5 days (range, 14–347 days), and 18 patients (82%) required visiting nurse services for their postoperative care. By comparison, the no-PL group required 1.6 postoperative office visits (range, 1–6 visits) and a median follow-up of 16.0 days (range, 8–128 days), and only 3 patients (7%) required visiting nurse services (Figure 2).
Figure 2.
Health care resource utilization following distal pancreatectomy in 44 patients without and 22 with pancreatic stump leak (PL). All comparisons were significant (P<.001). A, Mean number of emergency department visits and hospital readmissions. B, Mean number of postoperative computed tomographic (CT) scans and office visits. C, Mean total number of hospital days and duration of follow-up.
COSTS
The average patient with PL-related problems incurred a total cost that was 2.01 times greater than the average patient in the no-PL group. This yielded a cost differential of $14179, which was largely accounted for by hospital-related costs. The difference in physician reimbursement between the PL and no-PL group was 24%; however, this represents less than 3% of the cost differential. The difference in hospital-related costs between the 2 groups was 122%.
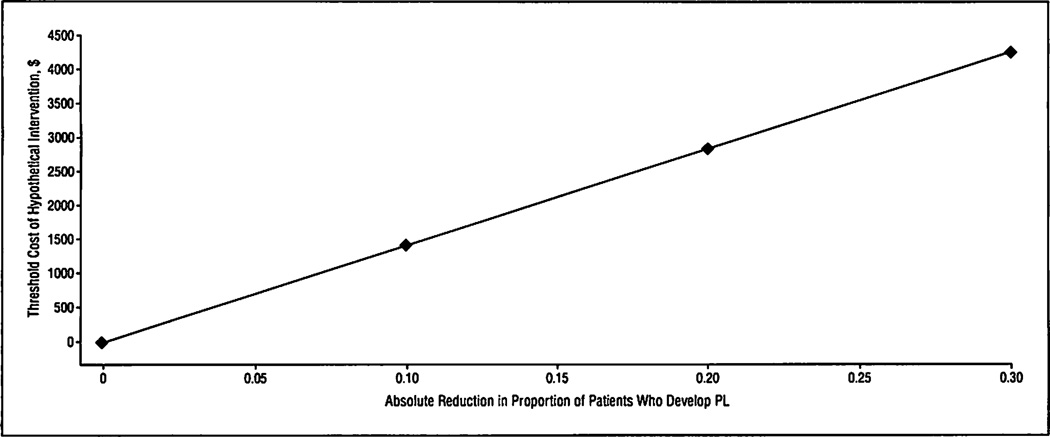
The decision analytic model was constructed from 2 surgical strategies: conventional surgery and surgery using a hypothetical new intervention intended to reduce the rate of PL. In the conventional surgery arm, it was assumed that 30% of patients would develop PL. In the hypothetical new intervention arm, it was assumed that PL would occur at a lower rate (with an absolute PL rate reduction equal to r) but that the intervention would incur an added cost (CI). The following equation was derived by using the previously mentioned cost differential ($14179), and its application is illustrated in Figure 3:
CI = r × 14 179
Figure 3.
Threshold cost of hypothetical distal pancreatectomy intervention as a function of the reduction in the proportion of patients with a pancreatic stump leak (PL).
This equation demonstrates the intervention cost threshold below which cost savings are incurred at an absolute PL rate reduction of r. For example, if an intervention reduced the PL rate from 30% to 20%, then up to a $1418 per-patient expenditure (calculated from 0.1 × $14 179) would still favor the new intervention as a cost-saving strategy. Alternatively, an intervention that reduced PLs to 0% could cost up to $4254 (calculated from 0.3 × $14 179) and still be economically justified.
COMMENT
This contemporary series of patients undergoing open distal pancreatectomy shows that complications derived from PL are frequent (33%). This seemingly large number is in part a result of our zeal to be comprehensive by including (in addition to pancreatic fistula) amylase-rich sterile collections, abscesses, and wound disruptions. Most surgeons would agree that these complications are all manifestations of the same underlying problem.3 Had we included pancreatic fistula alone, we undoubtedly would have underestimated the magnitude of the problem. The PL rate in this study is consistent with those in other large series that use rigorous definitions when describing complications derived from PL. A well-designed, prospective, multicenter trial6 in the United States found that, of a subset of 52 patients who underwent distal pancreatectomy, 18 (34.5%) developed PL-related complications. In a more recent large European study, Balzano et al1 reported a fistula and abscess rate of 38.6% in a series of 123 distal pancreatic resections. The results are not limited to open surgery: another multicenter European study7 involving laparoscopic pancreatic resections found that, in a subset of 98 patients who underwent distal pancreatectomy (with or without splenic preservation), 32 (32.7%) developed PL-related complications.
The aim of our study was not to identify the risk factors for developing PL or to describe methods to prevent its occurrence but rather to highlight the impact of PL on cost and hospital resource utilization. To our knowledge, this is the first study that attempts to quantify this by using specific patient-derived data. We found that, in some cases, patients have virtually no consequence resulting from their PL other than the discomfort of maintaining a surgically placed drain for several days following surgery. However, many others endure a difficult and prolonged postoperative course. We have demonstrated that when these patients are considered in aggregate they have a significant impact on health care resource utilization and cost. Strikingly, nearly 75% of these patients required at least 1 hospital readmission, and the average hospital stay (including readmission days) for a patient with a complication resulting from PL increased more than 3-fold. These patients, on average, had more than 4 postoperative diagnostic computed tomographic scans per patient and required a mean follow-up with their surgeon of nearly 2½ months. When calculating these figures, we did not even broach the subject of emotional and psychological duress or the overall deterioration in quality of life these patients endure—the “intangible” consequences of postoperative morbidity.8
Not surprisingly, all this health care resource consumption resulted in a marked increase in cost. By using hospital software and average insurer reimbursement for physician services, we determined that a patient with PL more than doubled the cost of distal pancreatectomy, and that this increase was mostly from hospital-related costs rather than physician services. Our analysis did not include various indirect costs, such as home care, rehabilitation, loss of work, or frequent transportation to and from the hospital.
A recent publication1 appropriately describes PL following distal pancreatectomy as an “unsolved problem.” It is troubling that the reported incidence of PL has not changed for at least 15 years, despite progress in other areas of pancreatic surgery.9,10 Many studies2,11–15 (most of them retrospective and observational) have focused on technical aspects of the operation, addressing whether adhesives, staplers, suturing techniques, or combinations of these can be used to avoid PL; however, evidence supporting the benefit of any one technique over another is not conclusive.
Others6,10,16–23 have approached this problem from a pharmacologic perspective, using somatostatin or its analogues prophylactically with the intent of preventing PL-related complications. Their results are conflicting. An industry-sponsored cost-effectiveness analysis24 using data from those studies declared that the added expense of octreotide was justified in all pancreatic surgery. However, the central underlying assumption that octreotide is effective remains highly controversial.19 The effectiveness data in their analysis were derived exclusively from 4 positive randomized controlled trials10,21–23 and did not include 4 subsequent randomized controlled trials6,18–20 that found no statistically significant difference in the postoperative course between groups treated with somatostatin analogues and control subjects.
Continuing efforts to find solutions to this far-too-common complication are clearly needed. In the present study we provide an economic framework to justify the investment in potential interventions designed to reduce the incidence of PL. While cost is not the only important criterion in making decisions concerning resource allocation, it is an important consideration when designing interventions to improve on existing practices.25 Our data, based on real direct and indirect hospital costs and physician reimbursement, demonstrate that a seemingly high additional expenditure of $1418 per patient would balance savings related to a mere 10% decrease in PL rate (from 30% to 20%) at our institution. A fully effective technique or application that would abolish PL could cost up to $4254 and still have a positive economic impact on the overall care of patients undergoing distal pancreatectomy. Needless to say, the ramifications of such a breakthrough would go well beyond economics by reducing the suffering of patients and their families, lessening the workload of health care providers, and opening hospital resources for patients with other health problems. We are hopeful these data will stimulate surgeons to come up with new strategies to address this common complication.
Acknowledgments
Funding/Support: Dr Rodríguez was supported by the Claude E. Welch, MD, Resident Research Fellowship, Massachusetts General Hospital, Boston.
DISCUSSION
Erwin Hirsch, MD, Boston, Mass: I was privileged to listen to Dr Rodriguez’s presentation at Tufts a few months ago, and he looked rather surprised when we announced he was the winner. There were a number of questions that we wanted to ask then that time did not allow. In the intervening months I was troubled and challenged by this excellent presentation bringing a subject from a very different point of view.
Right before his presentation, we had a patient with a distal pancreatectomy for trauma and, of course, he developed a leak and we all suffered through that. Since then we have had others. My question, months later, is that now you have a unique patient population in an institution that does a lot of pancreatic surgery.
If I remember correctly from the questions we asked the last time, all these cases were done by 2 surgeons. Have you considered a randomized prospective study in which patients will have ERCP [endoscopic retrograde cholangiopancreatography] prior to [surgery] and [that will] provide proximal drainage from the pancreas to minimize the possibility of a postoperative leak?
Dr Rodríguez: Actually, we have not talked about doing pre-operative ERCPs in an effort to decrease the incidence of post-operative leak after this operation, but it does seem like an interesting idea. Certainly, when considering the addition of a preoperative intervention to prevent a potential postoperative complication, it is important to evaluate the risks inherent to that procedure as well, and also the additional costs that patients would incur. Currently, what we are doing is using an autologous patch in our patients taken from the falciform ligament and securing it to the pancreatic remnant in an effort to reduce the incidence of leak. We haven’t published these results yet, but the preliminary results seem to be favorable. I hope to report on those soon.
Ronald Salem, MD, New Haven, Conn: I applaud your looking at the financial implications of our surgical complications in such a rigorous way. You’ve shown, as have others, that pancreatic fistula from distal pancreatectomy is higher than from pancreaticoduodenectomy. In evaluating those patients who did not get fistulas and comparing them with those who did, were you able to identify subsets in whom pancreatic fistulas were more common? In reviewing your experience, other than what you’ve just mentioned, were you able to identify any other techniques that allowed the incidence of fistula to be minimized?
Dr Rodriguez: Unfortunately, we did not make those comparisons in this particular study, as identifying risk factors for leak, including technical factors, was not our primary focus.
Footnotes
Previous Presentation: This paper was presented at the 86th Meeting of the New England Surgical Society; September 30th, 2005; Bretton Woods, NH; and is published after peer review and revision. The discussions that follow this article are based on the originally submitted manuscript and not the revised manuscript.
REFERENCES
- 1.Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837–842. doi: 10.1016/j.gassur.2005.01.287. [DOI] [PubMed] [Google Scholar]
- 2.Knaebel HP, Diener MK, Wente MN, Buchler MW, Seiler CM. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–546. doi: 10.1002/bjs.5000. [DOI] [PubMed] [Google Scholar]
- 3.Bassi C, Dervenis C, Butturini G, et al. International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Bassi C, Butturini G, Molinari E, et al. Pancreatic fistula rate after pancreatic resection: the importance of definitions [published online ahead of print December 30, 2003] [Accessed February 2005];Dig Surg. 2004 21:54–59. doi: 10.1159/000075943. [DOI] [PubMed] [Google Scholar]
- 5.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. doi: 10.1001/archsurg.1988.01400290032004. [DOI] [PubMed] [Google Scholar]
- 6.Sarr MG Pancreatic Surgery Group. The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: a prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg. 2003;196:556–564. doi: 10.1016/S1072-7515(03)00104-2. discussion, 565–565. [DOI] [PubMed] [Google Scholar]
- 7.Mabrut JY, Fernandez-Cruz L, Azagra JS, et al. Hepatobiliary and Pancreatic Section (HBPS) of the Royal Belgian Society of Surgery: Belgian Group for Endoscopic Surgery (BGES): Club Coelio. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery. 2005;137:597–605. doi: 10.1016/j.surg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer MI. Introduction to health economics for physicians. Lancet. 2001;358:993–998. doi: 10.1016/S0140-6736(01)06107-4. [DOI] [PubMed] [Google Scholar]
- 9.Bonnichon P, Tong JZ, Ortega D, et al. Pancreatic fistula after left pancreatectomy: frequency and severity [in French] J Chir (Paris) 1988;125:321–326. [PubMed] [Google Scholar]
- 10.Buchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992;163:125–130. doi: 10.1016/0002-9610(92)90264-r. discussion, 130–131. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Kuroda Y, Morita A, et al. Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Arch Surg. 1995;130:952–955. doi: 10.1001/archsurg.1995.01430090038015. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan MK, Beck K, Creech S, Pickleman J, Aranha GV. Distal pancreatectomy: does the method of closure influence fistula formation? Am Surg. 2002;68:264–267. discussion, 267–268. [PubMed] [Google Scholar]
- 13.Ohwada S, Ogawa T, Tanahashi Y, et al. Fibrin glue sandwich prevents pancreatic fistula following distal pancreatectomy. World J Surg. 1998;22:494–498. doi: 10.1007/s002689900423. [DOI] [PubMed] [Google Scholar]
- 14.Fahy BN, Frey CF, Ho HS, Beckett L, Bold RJ. Morbidity, mortality, and technical factors of distal pancreatectomy. Am J Surg. 2002;183:237–241. doi: 10.1016/s0002-9610(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg. 2003;90:190–196. doi: 10.1002/bjs.4032. [DOI] [PubMed] [Google Scholar]
- 16.Petrin P, Antoniutti M, Zaramella D, et al. Effect of octreotide acetate on pancreatic exocrine and endocrine functions after pancreatoduodenal resection. Eur Surg Res. 1995;27:371–378. doi: 10.1159/000129423. [DOI] [PubMed] [Google Scholar]
- 17.Lowy AM, Lee JE, Pisters PW, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg. 1997;226:632–641. doi: 10.1097/00000658-199711000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo CJ. Does prophylactic octreotide benefit patients undergoing elective pancreatic resection [editorial]? J Gastrointest Surg. 1999;3:223–224. doi: 10.1016/s1091-255x(99)80063-8. [DOI] [PubMed] [Google Scholar]
- 19.Sue B, Msika S, Piccinini M, et al. French Associations for Surgical Research. Octreotide in the prevention of intra-abdominal complications following elective pancreatic resection: a prospective, multicenter randomized controlled trial. Arch Surg. 2004;139:288–294. doi: 10.1001/archsurg.139.3.288. discussion, 295. [DOI] [PubMed] [Google Scholar]
- 20.Barnett SP, Hodul PJ, Creech S, Pickleman J, Arahna GV. Octreotide does not prevent postoperative pancreatic fistula or mortality following pancreaticoduodenectomy. Am Surg. 2004;70:222–226. discussion, 227. [PubMed] [Google Scholar]
- 21.Pederzoli P, Bassi C, Falconi M, Camboni MG. Italian Study Group. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Br J Surg. 1994;81:265–269. doi: 10.1002/bjs.1800810237. [DOI] [PubMed] [Google Scholar]
- 22.Montorsi M, Zago M, Mosca F, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery. 1995;117:26–31. doi: 10.1016/s0039-6060(05)80225-9. [DOI] [PubMed] [Google Scholar]
- 23.Friess H, Beger HG, Sulkowski U, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis [published correction appears in Br J Surg. 1996;83:126] Br J Surg. 1995;82:1270–1273. doi: 10.1002/bjs.1800820938. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg L, MacNeil P, Turcotte L. Economic evaluation of the use of octreotide for prevention of complications following pancreatic resection. J Gastrointest Surg. 1999;3:225–232. doi: 10.1016/s1091-255x(99)80064-x. [DOI] [PubMed] [Google Scholar]
- 25.Gazelle GS, McMahon PM, Siebert U, Beinfeld MT. Cost-effectiveness analysis in the assessment of diagnostic imaging technologies. Radiology. 2005;235:361–370. doi: 10.1148/radiol.2352040330. [DOI] [PubMed] [Google Scholar]