Abstract
Tris(2,3,5,6-tetrathiaaryl)methyl cations, which were generated from the corresponding triarylmethanols in the presence of strong acids, underwent reaction with nucleophiles to give trityl radicals, as the product of a one-electron reduction of the carbocation. Depending on the nature of the nucleophile, the only byproducts were either diamagnetic quinone methides or asymmetrical monosubstituted trityl radicals. Herein, we report a protocol for the large-scale synthesis of the Finland trityl, which has the advantage of high overall yield and reproducibility.
Keywords: Radicals, Carbocations, Quinones, Reaction mechanisms, Synthesis design
Introduction
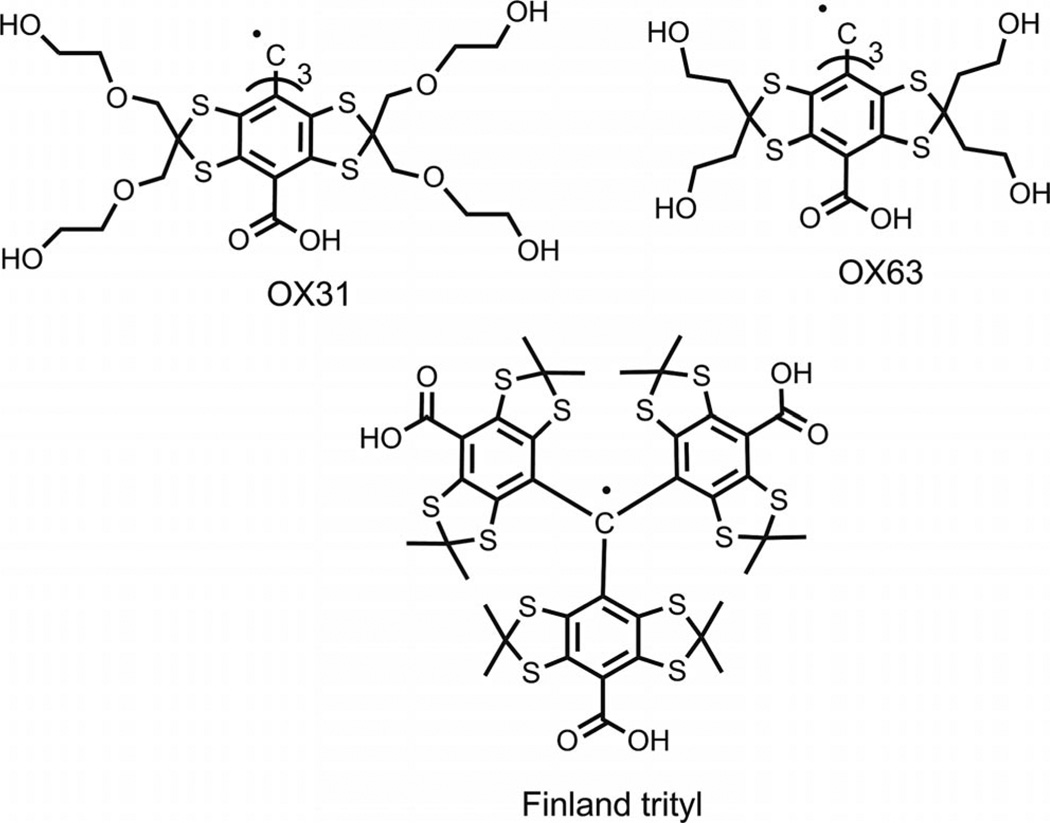
The family of tris(2,3,5,6-tetrathiaaryl)methyl radicals (trityls, TAMs), which was first designed by Nycomed Innovations[1] (see Figure 1), has recently been used for a number of magnetic resonance applications because of their favorable relaxation and spectral properties. These trityl compounds demonstrate high chemical stability and excellent solubility in aqueous solutions, which make them attractive spin probes in numerous applications of low-frequency electron paramagnetic resonance (EPR) for in vivo detection of free radicals,[2] 3D EPR imaging,[3] and other studies such as for the simultaneous measurement of redox status and oxygen concentration using dual function nitroxide-trityl biradicals,[4] intracellular oxymetry,[5] the measurement of concentration of a superoxide anion[6] and pH in a biologically relevant range of acidity,[7] and studies of molecular diffusion in microheterogeneous systems.[8] Trityl radicals also have a single, narrow electron paramagnetic resonance line, even at high fields, and have long relaxation times in liquid solutions, which make them useful electron spin reagents for studies of dynamic nuclear polarization.[9]
Figure 1.
Molecular structures of representative TAMs.
The strong interest in trityls has stimulated numerous efforts towards optimization of synthetic strategies and searches for efficient approaches to a large-scale synthesis of these challenging compounds. The major part of these studies has focused on the simplest representative in the series of highly persistent trityls – tris(8-carboxy-2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiol-4-yl)methyl (alias Finland trityl, see Figure 1). Although the preparation of the Finland trityl has been reported both in patent and academic literature,[10–12] we have found that these synthetic procedures allow sufficient room for further improvement. Herein, we describe a practical procedure for the large-scale synthesis of the Finland trityl radical. The unexpected effect of the formation of TAMs as a result of the nucleophilic quenching of tris(2,3,5,6-tetrathiaaryl)methyl cations is also reported.
Results and Discussion
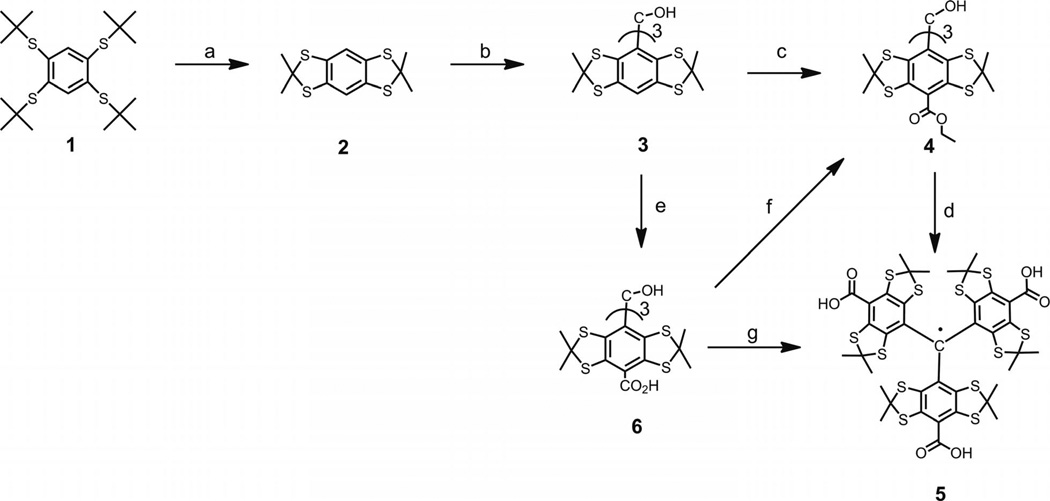
The general concept for the synthesis of the Finland trityl was similar to that described in the literature,[1a,8,10–12] but improvements were implemented at each step (see Scheme 1). Tetra-tert-butylthiobenzene (1) was obtained by analogy to a literature protocol[10] through the treatment of tetrachlorobenzene with sodium tert-butylthiolate in anhydrous N,N-dimethylformamide (DMF) followed by heating the reaction mixture at reflux for 4 h and stirring at ambient temperature overnight. The reaction time was increased in comparison to the prototype, which resulted in a slight increase of the product yield (69–71% vs. 63%).
Scheme 1.
Reagents and conditions: (a) acetone (10 equiv.), BF3•Et2O solution (3 equiv.), d-10-camphorsulfonic acid (0.2 equiv.) in CHCl3, 93%; (b) nBuLi (2.5 m in hexane, 1.1 equiv.), ether as a solvent, diethyl carbonate (0.32 equiv.), 72%; (c) nBuLi (2.5 M in hexane, 10 equiv.), hexane/N,N,N′,N′-tetramethyl-1,2-ethylenediamine (TMEDA) solution, diethyl carbonate (40 equiv.), 32%; (d) CF3SO3H (15 equiv.) in dichloromethane, SnCl2 (1 equiv.), hydrolysis with aqueous KOH (10 equiv.), aqueous HCl, 92%; (e) nBuLi (2.5 m in hexane, 10 equiv.), hexane/TMEDA solution, solid CO2, 62%; (f) Thionyl chloride (30 equiv.) in CHCl3/NEt3, ethanol in presence of pyridine, 98%; (g) tri-fluoroacetic acid (TFA), SnCl2 (0.5 equiv.), 96%.
Compound 1 was further converted into intermediate thioacetonide 2 by heating at reflux with acetone. Boron trifluoride and chloroform were used as the catalyst and solvent, respectively, instead of HBF4 and toluene, which was recommended by the literature sources. After the crude material was heated at reflux with methanol, the product was isolated in high yield (86–93% vs. 51–61%[10,11]). The revised procedure was simple and high yielding and was especially relevant to synthesis of the deuterated form of 2 (and all the further products) if [D6]acetone was used as the ketone component.
Triarylmethyl alcohol 3 was prepared by treatment of arene 2 with nBuLi and the subsequent addition of 0.32 equiv. of diethyl carbonate. Purification of the crude product did not require lengthy and tedious column chromatography. Instead, we used the simple and fast procedure of heating the crude material at reflux with 1:1 mixture of hexane and carbon tetrachloride, which readily afforded the highly pure product 3 in a good yield of 66–72% based on arene 2 (56–69%[10,11]).
Potentially, converting triarylmethanol 3 into the triple ester 4 may be performed by lithiation of 3 with an excess amount of nBuLi-TMEDA complex in benzene solution followed by pouring the intermediate tris(lithium) derivative into a large excess amount of diethyl carbonate.[1a,10] Unfortunately, the direct application of the literature procedure did not provide satisfactory results, and the yield of 4 never reached 12%. Our attempt to improve the result by replacing diethyl carbonate with sterically hindered di-tert-butyl dicarbonate (DIBOC), which was recommended in the literature,[11] did not provide any notable effect.
Initially, a somewhat respectable yield was only obtained when benzene was replaced with hexane.[8] Under these modified conditions, ester 4 was isolated in 16–36% yield (44–45%[10,11]) after a lengthy and solvent-consuming chromatographic purification (see Exp. Section, Method A). A possible rationale for the observed improvement is that hexane is a very weak C–H acid, contrary to benzene, and, therefore, this solvent is inert towards the nBuLi-TMEDA complex and does not compete with 3 in the conversion to an aryllithium derivative.[13]
Trityl 5 was generated by following an earlier method,[8,10] that is, the treatment of alcohol 4 with trifluoromethanesulfonic acid in dichloromethane (DCM) followed by reduction of the obtained cation with 1 equiv. of SnCl2. Hydrolysis of ester functions of the intermediate trityl radical with aqueous KOH and addition of aqueous HCl converted the tris(carboxylate) into the acidic form of the Finland trityl. The latter was isolated in 92% yield based on initial trityl alcohol 4 (see Exp. Section, Method C).
On the basis of trityl alcohol 3, the overall yield of Finland trityl (5) was low (15–33%). In addition, the synthesis of tris(ester) 4 showed low reproducibility and required laborious chromatographic purification. These factors substantially limit the utility of any reaction pathway that relies on the participation of intermediates such as 4, especially in the case of the large-scale production and synthesis of the extra narrow-line form of the Finland trityl – the deuterated analogue of 5.
This explains our search for alternative methods for the carboxylation of triarylmethanol 3. First, we turned to the direct insertion of carboxy functions into the para positions of the aryl moieties of the substrate. We found that a slurry of the tris(lithium) derivative, which was obtained by treating 3 with nBuLi in TMEDA/hexane solution, readily underwent reaction with solid carbon dioxide to afford triacid 6 in a good isolated yield (52–62%). Purification of the triacid was easy and fast, that is, the addition of brine to a homogeneous aqueous solution of the sodium salt of crude 6 led to the immediate precipitation of the contaminants as insoluble salts (i.e., the dicarboxylic and monocarboxylic acids). Filtration of this mixture followed by addition of aqueous HCl to the filtrate resulted in pure 6. This present procedure not only is higher yielding than the reported methods but also avoids the use of purification by column chromatography.
Next, tricarboxylic acid 6 was converted into tris(ester) 4 in a very good yield (96–98%, see Exp. Section, Method B) and then into the title product. This two-step sequence (see Scheme 1, steps f and d) could potentially complete an efficient protocol that is capable of affording trityl 5 in good overall yield with high reproducibility by using simple and highly scalable procedures.
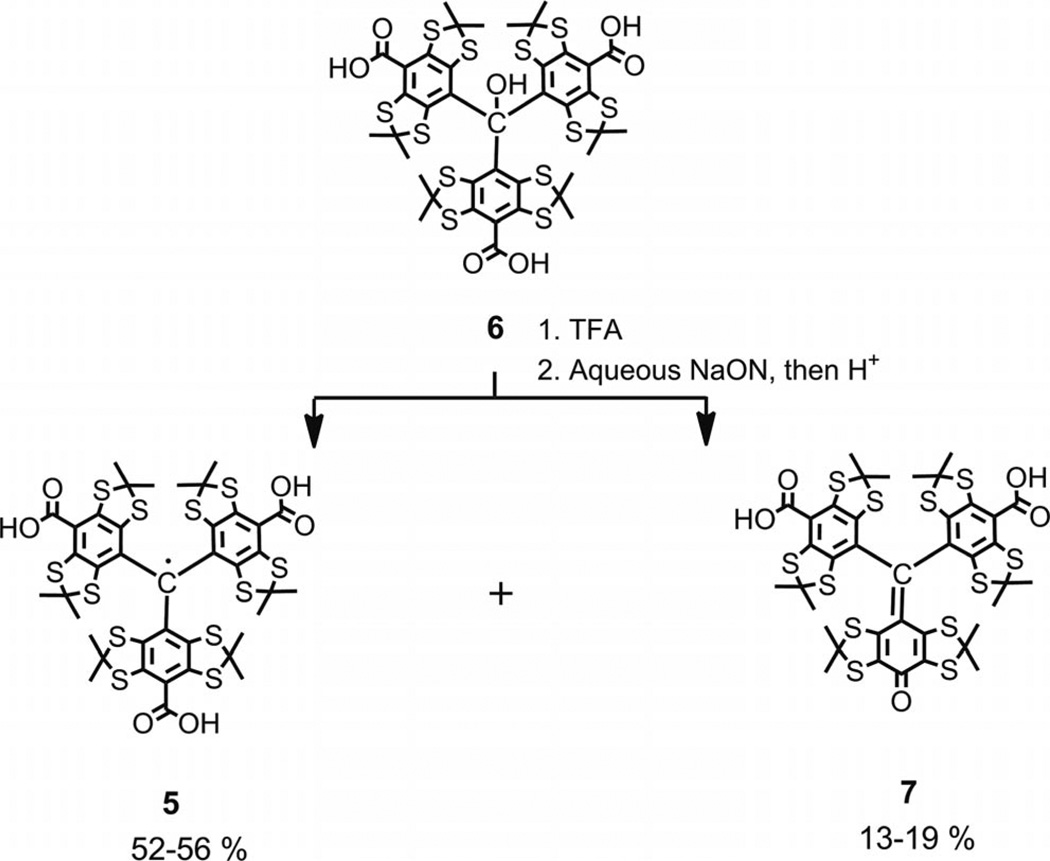
However, a shorter synthetic procedure that gave the Finland trityl directly from triacid 6 through a one-pot operation[14] seemed reasonable and eventually practical. Literature searches revealed only one method suitable for these purposes. It involved the treatment of various bulky tris-(tetrathiaaryl)methanols with trifluoroacetic acid, and the corresponding trityl radicals were isolated quantitatively after a standard water workup procedure.[5,11,15] Nothing certain is known about the mechanistic details of this reaction, apart from the statement that “this formal one-electron reduction of the central carbon was quite surprising”.[11] This conclusion is still more convincing if one takes into account the absence of evident and indubitable reductants for the initial reagents. Again, a priori, it seemed unreasonable to predict that the reaction would generate an intermediate that could play the part of a reducing agent.
To gain better insight into mechanistic details of this process, we attempted a series of reactions between triarylmethanol 6 and TFA. Some reaction conditions were strictly consistent with the original protocols, whereas others involved modifications of the reaction conditions, for example, the presence or absence of atmospheric oxygen in the reaction vessel and the variation of the reaction time in the range of 6–36 h. Regardless of reaction conditions, the crude product was never a single component, but instead was two major components easily observable on TLC plates (see Supporting Information). The products were identified as trityl radical 5 and diamagnetic quinone methide 7 (see Scheme 2), which were isolated in 52–56% and 13–19% yield, respectively (see Exp. Section).
Scheme 2.
Synthesis of trityl 5 and quinoide 7 by treatment of triarylmethanol 6 with TFA followed by water workup.
Recently, quinoide 7 was reported as the only product to result from the oxidative decarboxylation of trityl 5 with nicotinamide adenine dinucleotide phosphate hydride (NADPH)/O2, which was catalyzed by rat, pig, and human liver microsomes,[16] and the reaction of 5 with superoxide, which was generated by a xanthine/xanthine oxide system.[16,17] The rationale for this reaction involves the attack of the O2·− at the para carbon of the TAM aryl ring followed by the loss of CO2 from the resulting diamagnetic intermediate and a proton-catalyzed heterolytic cleavage of the O–O bond of the hydroperoxide group.[16,17]
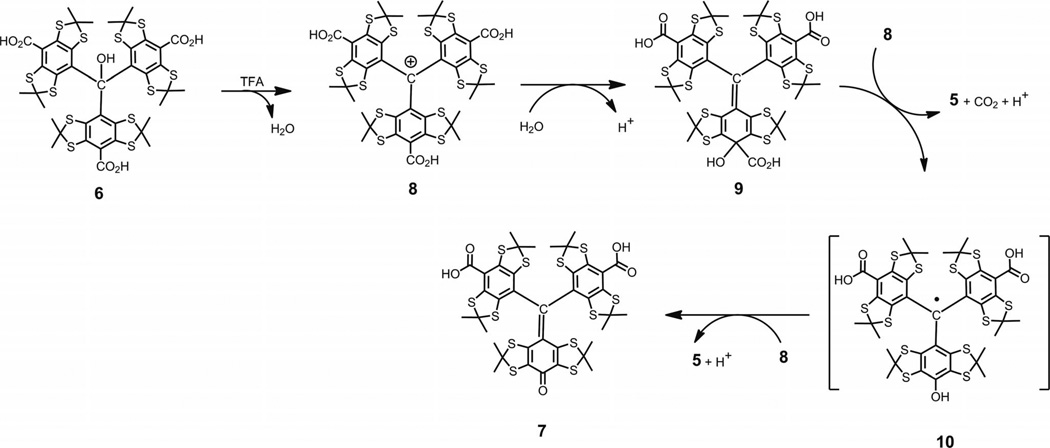
The absence of superoxide or the source of any other peroxide species means that the generation of quinoide 7 by the mechanism described in literature, and above, is highly improbable in our case. A plausible explanation for the simultaneous formation of trityl 5 and diamagnetic quinoide 7 might follow from what is known about the ready reaction of sterically hindered trityl cations with nucleophiles.[18] Typically, they attack aryl rings at the para position to give 4-methylenecyclohexa-2,5-diene intermediates analogous to 9 (see Scheme 3). Very recently C. Decroos et al. reported the formation of trityl radicals through an electron transfer (ET) reaction between intermediate methylenecyclohexa-2,5-dienes and trityl cations, which were generated in situ by oxidation of trityl 5 either by potassium hexachloroiridate(IV)[19] or hydrogen peroxide in the presence of peroxidases (horse radish peroxidase, lactoperoxidase, prostaglandin synthase, and other hemeproteins).[20]
Scheme 3.
Proposed mechanism for formation of quinone methide 7 and TAM 5 through a two-step sequence of ET reactions.
This fruitful concept of ET reactions with trityl cations participating as an oxidant provides the missing link to interpret our results as shown in Scheme 3. The explanation involves the reaction of cation 8 with water to yield intermediate cyclohexadiene 9. The decarboxylation of 9 followed by oxidation with cation 8 (or vice versa) gives trityl 5 and transient trityl 10. The latter should be readily oxidized by cation 8 along with the eventual formation of quinoide 7 and the next crop of trityl 5. The overall balanced reaction follows Equation (1).
| (1) |
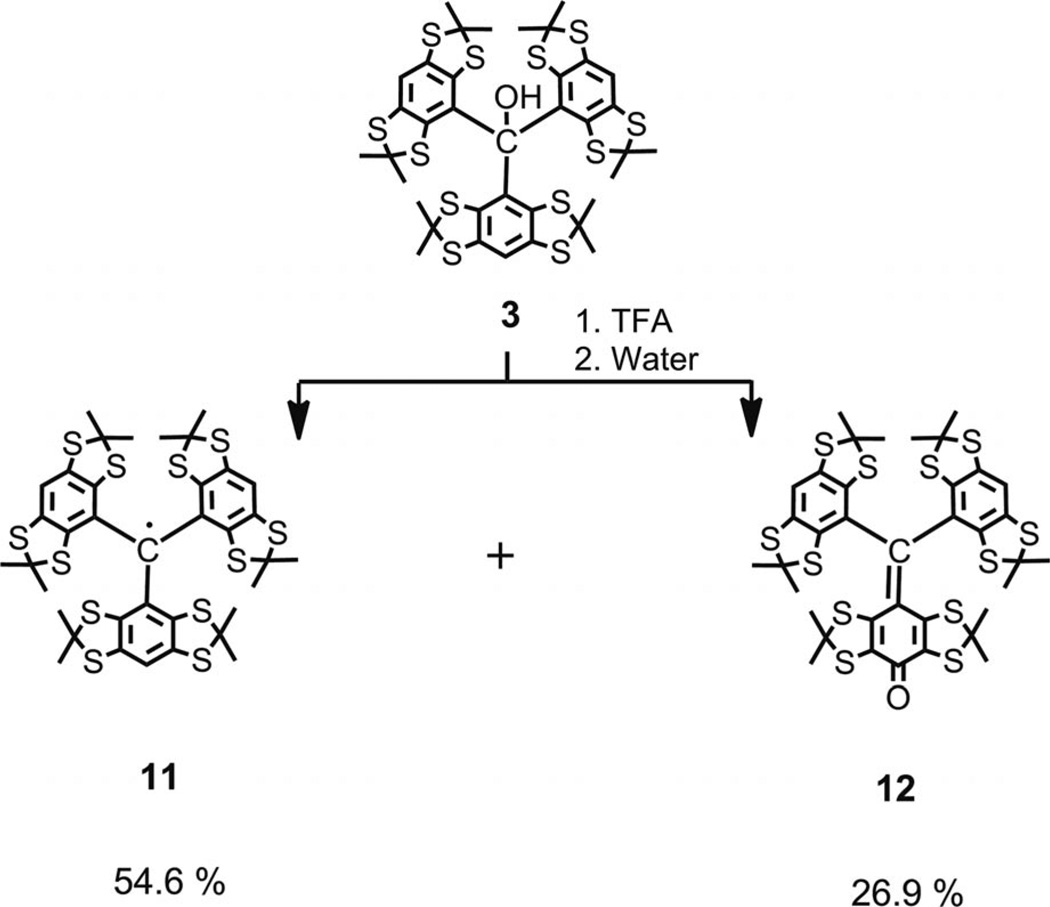
This proposed mechanism predicts the yield of trityl 5 to be below 66.7% and the ratio of trityl to quinoide to be strictly equal to 2:1. The first part of this prediction is in good agreement with our experimental data. The second one is notably in disagreement with the experiment. It is obvious that the high polarity of both products presents a severe problem for their quantitative isolation through standard preparative means (e.g., column chromatography), and, thus, for an accurate evaluation of the product distribution. Searching for a more suitable model, we examined less polar triarylmethanol 3. Analogously, the treatment of 3 with TFA and the subsequent quenching of the hypothetical carbocation with water afforded a mixture of two major products (see Scheme 4), both of which were isolated by column chromatography with minimal loss.[21] Trityl 11 and quinoide 12 were obtained in ratio of 2.17:1, which thus confirmed the plausibility of Scheme 3. Replacing TFA with alternative strong acids such as CF3SO3H and HBF4 etherate gave similar results of 2.13:1 and 2.07:1, respectively.
Scheme 4.
Synthesis of trityl 11 and quinoide 12 by treatment of triarylmethanol 3 with TFA followed by water workup.
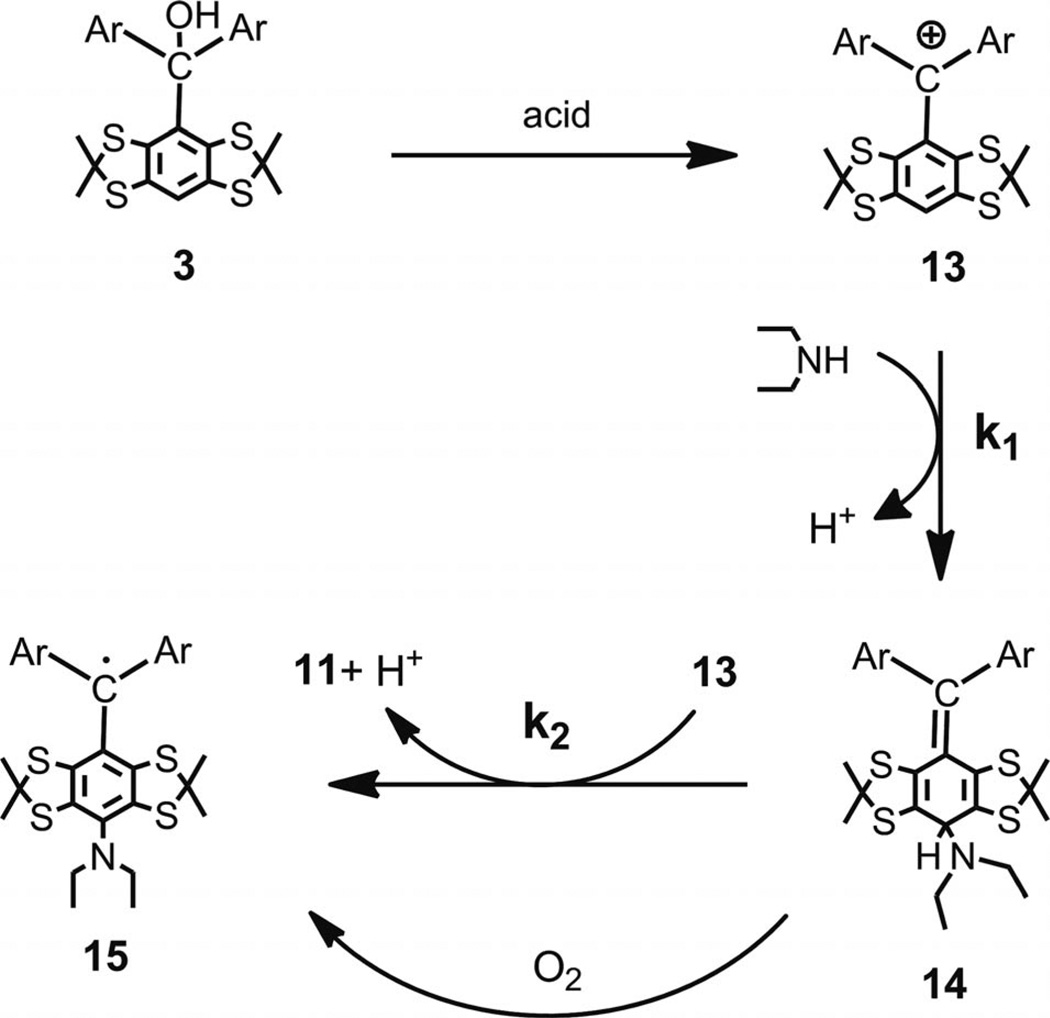
We may legitimately assume that TFA acts as a typical acidic reagent, which selectively generates trityl cations as other strong acids do. The assumption of the cationic nature of the primary product that results from the treatment of triarylmethanols 3 and 6 with TFA implies two important consequences. First, the reduction of intermediate cation 8 with sufficiently strong reducing reagent must result in trityl 5 as the only product and completely restrain the undesirable side reaction that leads to the quinoide. Indeed, as predicted, the addition of SnCl2 (0.5 equiv.) to a TFA solution of 6 (see Scheme 1) smoothly afforded the Finland trityl (5) as the only product isolated in 96% yield (see Exp. Section, Method D). Second, replacing water with alternative nucleophiles must result in the formation of asymmetrical monosubstituted trityls along with a symmetrical one. If the addition of the nucleophile to the para carbon atom of the cation is slower than the further oxidation of the intermediate cyclohehadiene, the reaction must give an equimolar mixture of the two forms of the trityl, however, that is, only if the asymmetrically substituted trityl is sufficiently stable towards further oxidation with the triaryl-methyl cation.
The support of this hypothesis followed from an experiment, in which the carbocation generated from 3 and TFA was quenched with diethylamine (5 equiv.). The crude product was composed of the known trityl 11 and asymmetrical monosubstituted trityl 15, which was easily detectable by the characteristic hyperfine splitting in the ESR spectrum, that is, a quartet and broad triplet, respectively (see Supporting Information). Trityls 11 and 15 were isolated in 47% and 42% yields, respectively, and their ratio was close to the predicted 1:1 ratio (see Scheme 5 for a summary of the arguments stated above and the literature data[19,20]).
Scheme 5.
Proposed mechanism for formation of trityl 11 along with monosubstituted trityl 15.
The proposed mechanism implies that a large excess amount of the nucleophile and/or its high reactivity could potentially channel the reaction to the preferential formation of intermediate 14 and, thus, hinder the pathway leading to trityl 11. Depending on stability of 14, it might be isolated as such or in the form of trityl 15. In an attempt to provide the above conditions for the slow oxidation of intermediate 14 with cation 13, that is, k1[HNEt2] ≫ k2[13] (see Scheme 5), a DCM solution of cation 13, which was generated from 3 and CF3SO3H, was added slowly through a syringe to large excess amount of diethylamine in DCM. The standard workup, which provided the exposure of the crude product to atmospheric oxygen, gave the expected product 15 in the high isolated yield of 82%.
In preliminary experiments, we have extended this approach to other nucleophiles and clearly showed the potential for the preparative synthesis of a wide variety of new diversely substituted persistent triarylmethyl radicals, which are useful for numerous applications in biology, spectroscopy, and material science. A detailed study of this intriguing chemistry is currently underway and will be reported in the next paper.
Conclusions
The reaction of nucleophiles with tris(2,3,5,6-tetra-thiaaryl)methyl cations, which were generated from the corresponding triarylmethanols in the presence of strong acids, resulted in trityl radicals, as the products of a one-electron reduction of carbocations. Depending on the nature of the nucleophile, the only byproducts were either diamagnetic quinone methides or asymmetrical monosubstituted trityl radicals. The latter is available on a preparative scale. A revised protocol for the large-scale synthesis of the Finland trityl is reported. The improved version of the synthesis has the advantage of simplicity, high reproducibility, and a notable increase in the overall yield.
Experimental Section
General Methods
The 1H and 13C NMR spectroscopic data were recorded with a Bruker AV-400 spectrometer (1H NMR, 400.134 MHz; 13C NMR, 100.624 MHz) and a Bruker AV-600 spectrometer (1H NMR, 600.302 MHz; 13C NMR, 150.964 MHz). Chemical shifts (δ scale) are given in ppm with reference to the residual signals of CDCl3 (1H NMR, 7.26 ppm; 13C NMR 77.16 ppm), [D6]DMSO (1H NMR 2.50 ppm; 13C NMR 39.52 ppm), or CD3OD (1H NMR, 3.31 ppm, 13C NMR, 49.00 ppm). IR spectra were recorded with a Bruker Tensor 27 and Bruker Vector 22 FTIR spectrometers, and KBr pellets were used. Wavenumber values are given in cm−1. UV/Vis absorption spectra were obtained with a Varian Cary 5000 UV/Vis/NIR spectrometer, and the data were collected using dilute solutions (0.1 mM) in quartz spectroscopy cells. The ESR spectra were recorded with a Bruker ELEXSYS E540 spectrometer (microwave power of 2 mW, modulation frequency of 100 KHz, and modulation amplitude of 0.003 mT). Electrospray ionization mass spectra (ESI-MS) were recorded with a hybrid quadrupole/time-of-flight Bruker micrOTOF-Q spectrometer. Methanol used as the solvent, and the spectra were scanned in the m/z range of 100–3000 in the positive and negative ionization modes. Nitrogen was used as the drying gas at 220 °C and at a flow rate of 4 Lmin−1. The nebulizer pressure was set to 1.0 bar. The capillary voltage was set at −4.0 kV. Sample solutions were infused into the ESI source by using a LC Agilent 1200 in the FIA mode (Flow Injection Analysis, 2–3 µL at a flow rate for CH3OH of 0.1 mLmin−1). MALDI-TOF mass spectra were recorded with an autoflex III MALDI-TOF mass spectrometer (Bruker Daltonics, Germany), which was equipped with a pulsed nitrogen laser (337 nm) in a positive reflectron mode. Ions formed by a laser beam were accelerated to 20 keV kinetic energy. The final spectra were obtained by the accumulation of a 1500 single laser shot spectrum. The solution of 2,5-dihydroxybenzoic acid (DHB) in acetonitrile (50 mg/mL) was used as a matrix. A sample solution in chloroform was mixed with the same volume of the matrix solution. Approximately 1 µL of the resulting solution was deposited on the 384 ground steel target plate and allowed to dry before being introduced into the mass spectrometer. External calibration in the positive mode was done by using Peptide Calibration Standard II (Part No. 222570, Bruker Daltonics, Germany). Mass accuracy of approximately 0.1% was usually achieved. Mass spectra were processed by flexAnalysis 2.4 software (Bruker Daltonik GmbH, Germany). Analytical HPLC analyses were carried out with an Agilent 1100 Series instrument, which was equipped with a ZORBAX Eclipse XDB C8 column [methanol and then methanol with the addition of 0.1% (v/v) trifluoroacetic acid]. Preparative column chromatography was performed using 60–200 µm silica gel, which was purchased from Acros. Chemicals were purchased from Ald-rich and Acros and were used without further purification.
1,2,4,5-Tetra-tert-butylthiobenzene (1)
Compound 1 was prepared by analogy to a known literature method.[10] Off-white powder (71% yield); m.p. 146–151 °C. C22H38S4 (430.78): calcd. C 61.34, H 8.89; found C 61.12, H 8.72. 1H NMR (400 MHz, CDCl3): δ = 1.38 (s, 36 H, CH3), 7.95 (s, 2 H, CH) ppm. 13C NMR (100 MHz, CDCl3): δ = 31.24 (CH3), 48.11 (CCH3), 139.24, 144.70 ppm.
2,2,6,6-Tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiole (2)
To a stirred suspension of 1 (10.78 g, 25 mmol) in chloroform (30 mL) were added acetone (17.5 mL, 240 mmol), D-(+)-10-camphor-sulfonic acid (1.16 g, 5 mmol), and BF3 (48 wt.-% BF3 in ether, 9.8 mL, 75 mmol). The flask was flushed with argon and connected to a reflux condenser that was equipped with a mineral oil bubbler. The mixture was then stirred at 75–80 °C for 24 h. The cooled mixture was poured into water (30 mL), and the resulting biphasic liquid was neutralized to pH = 7 by the portionwise addition of NaOH (2 n solution). The organic phase was separated, and the water phase was extracted with chloroform (3 × 10 mL). The combined organic layers were washed with brine, filtered through a short silica plug, and concentrated in vacuo. The resulting solid was heated at reflux in methanol (35 mL) for 30 min. The mixture was then filtered, washed with methanol/hexane (4:1 v/v, 3×5 mL), and dried in vacuo to give 2 (6.65 g, 93%) as a fine pale yellow precipitate; m.p. 145–147 °C. C12H14S4 (286.48): calcd. C 50.31, H 4.93, S 44.77; found C 51.13, H 4.96, S 44.36. IR (KBr): ṽ = 2990 (m), 2964 (s), 2928 (m), 1448 (s), 1423 (s), 1381 (m), 1364 (s), 1329 (s), 1258 (s), 1167(s), 1149(s), 1091 (s), 851 (s), 640 (m), 407 (m) cm−1. 1H NMR (400 MHz, CDCl3): δ = 1.88 (s, 12 H, CH3), 7.02 (s, 2 H, CH)ppm. 13C NMR (100 MHz, CDCl3): δ = 31.41 (CH3), 65.88 (CCH3), 116.96, 135.84 ppm.
Tris(2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiol-4-yl)methanol (3)
A suspension of 2 (10.00 g, 35 mmol) and sodium hydride (60 wt.-% paste in mineral oil, 0.140 g, 3.5 mmol) in anhydrous ether (100 mL) was stirred overnight at room temp. under argon. nBuLi (2.5 m in hexane, 15.4 mL, 38.5 mmol, 1.1 equiv.) was added dropwise over 1 h. The thick slurry was stirred for 24 h, and then a solution of freshly distilled diethyl carbonate (1.32 g, 11.2 mmol, 0.32 equiv.) in anhydrous hexane (4.5 mL) was added over 4 h. The resulting orange mixture was stirred at room temp. for 48 h and then quenched by the dropwise addition of ethanol (2 mL) followed by NH4Cl (2 m solution, 25 mL). Dichloromethane (20 mL) was added, and the organic layer was separated. The water phase was extracted with additional CH2Cl2 (2 × 10 mL). The combined organic layers were washed with HCl (0.2 m solution), filtered through a short plug of silica, and concentrated in vacuo. The resulting solid was heated at reflux in a mixed solvent of CCl4 (24 mL) and hexane (24 mL) for 15 min. After cooling to room temp., the solid powder was collected on a filter, washed with a CCl4/hexane (1:1 v/v, 3 × 5 mL), and dried in vacuo to give 3 (7.43 g, 72% based on arene 2) as a fine pale yellow precipitate; m.p. >280 °C (gradually turned black, decomposition). HPLC purity: >95%. C37H40OS12 (885.44): calcd. C 50.19, H 4.55, S 43.45; found C 49.88, H 4.54, S 42.94. IR (KBr): ṽ = 3366 (m), 2972 (m), 2955 (m), 2920 (m), 2910 (m), 1450 (s), 1377 (s), 1364 (s), 1344 (m), 1248 (m), 1167 (s), 1148 (s), 787 (s), 760 (s) cm−1. 1H NMR (400 MHz, CDCl3): δ = 1.67 (s, 9 H, CH3), 1.72 (s, 9 H, CH3), 1.80 (s, 9 H, CH3), 1.822 (s, 9 H, CH3), 6.22 (s, 1 H, OH), 7.17 (s, 3 H, CH) ppm. 13C NMR (100 MHz, CDCl3): δ = 27.74 (CH3), 29.29 (CH3), 32.38 (CH3), 34.93 (CH3), 63.49 (SCS), 64.18 (SCS), 83.79 (COH), 118.30 (CH), 131.99 (C), 137.39 (C), 137.98 (C), 138.46 (C), 139.36 (C) ppm.
Tris(8-ethoxycarbonyl-2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]-dithiol-4-yl)methanol (4)
Compound 4 was prepared by analogy to a recently published literature protocol.
Method A
[8] To a stirred suspension of 3 (0.886 g, 1 mmol) and freshly distilled TMEDA (1.16 g, 10 mmol) in n-hexane (2 mL) at 0 °C (bath temperature) was added dropwise nBuLi (2.5 m in hexane, 4 mL, 10 mmol) over 30 min under argon. After the mixture was stirred at room temp. for 3.5 h, anhydrous toluene (4 mL) was added. The resulting dark brown gel was stirred at room temp. for an additional 1 h and then poured into cooled (−15 °C, bath temperature) freshly distilled diethyl carbonate (4.75 g, 40 mmol) diluted with toluene (10 mL). The cooling bath was removed, and the stirring was continued overnight at room temp. Saturated aqueous NaH2PO4 (5 mL), water (10 mL), and ether (25 mL) were added. The organic phase was separated, filtered through a short plug of silica, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (dichloromethane/ hexane, from 1:6 to 1:1) followed by recrystallization from acetonitrile (15 mL) to afford 4 (0.352 g, 32%) as a lemon yellow powder [contained residual acetonitrile (6–8 mol-%)]; m.p. >270 °C (gradually decomposed, turned black). HPLC purity: >95 %. C46H52O7S12 (1101.63): calcd. C 50.15, H 4.76; found C 50.42, H 4.46. IR (KBr): ṽ = 3354 (m), 2974 (m), 2915 (w), 1707 (s), 1450 (m), 1368 (m), 1319 (m), 1246 (s), 1230 (s), 1101 (m), 1024 (m) cm−1. 1H NMR (400 MHz, CDCl3): δ = 1.43 (t, J = 7.1 Hz, 9 H, OCH2CH3), 1.63 (s, 18 H, CH3), 1.72 (s, 9 H, CH3), 1.74 (s, 9 H, CH3), 2.09 (s, approximately 0.21 H, acetonitrile), 4.41 (m, 6 H, OCH2),[22] 6.75 (s, 1 H, OH) ppm. 13C NMR (100 MHz, CDCl3): δ = 14.41 (CH2CH3), 28.80 (CH3), 29.36 (CH3), 32.01 (CH3), 33.98 (CH3), 61.01 (SCS), 61.09 (SCS), 62.49 (OCH2CH3), 84.46 (COH), 121.45 (C), 134.14 (C), 139.40 (C), 140.50 (C), 141.59 (C), 142.00 (C), 166.34 (CO2Et) ppm.
Method B
[12] A solution of triacid 6 (3.055 g, 3 mmol) and dry triethylamine (1.820 g, 18 mmol) in anhydrous chloroform (20 mL) was stirred at room temp. for 10 min. To the resulting homogeneous solution was added a solution of SOCl2 (3.580 g, 30 mmol) in chloroform (5 mL) dropwise over 20 min. The mixture was heated at reflux for 2.5 h, stirred overnight at room temp., and then concentrated to dryness to give an orange-yellow cake. Anhydrous ethanol (15 mL, 255 mmol), chloroform (5 mL), and pyridine (0.395 g, 5 mmol) were added. The resulting mixture was stirred at 60 °C for 4 h and then overnight at room temp. After removal of solvents in vacuo, chloroform (45 mL) and water (20 mL) were added to the solid residue. The organic phase was separated, washed with HCl (0.1 m aqueous solution, 15 mL) and water (3 × 10 mL), filtered through a short silica plug, and concentrated in vacuo to give product 4 (3.240 g, 98%) as a lemon yellow precipitate.
Tris(8-carboxy-2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiol-4-yl)methyl (5)
Compound 5 was prepared from trityl alcohol 4 by analogy to a literature procedure.[8]
Method C
To a stirred solution of 4 (0.551 g, 0.5 mmol) in dichloromethane (15 mL) was added a solution of CF3SO3H (1.125 g, 7.5 mmol) in anhydrous acetonitrile (3 mL) dropwise over 5 min under argon. After the mixture was stirred at room temp. for 10 min, a solution of SnCl2 (0.095 g, 0.5 mmol) in anhydrous tetrahydrofuran (THF, 8 mL) was added. The resulting dark brownish-green solution was stirred at room temp. under argon for 10 min and then was quenched with saturated aqueous NaH2PO4 (30 mL). The mixture was diluted with chloroform (15 mL), and the resulting solution was stirred vigorously for 2 min. The organic layer was separated, washed with brine (10 mL) and then water (2 × 10 mL), dried with MgSO4, filtered, and concentrated in vacuo to give the tris(ester) trityl as a black cake. Ethanol (5 mL), dioxane (5 mL), and a solution of KOH (0.280 g, 5 mmol) in water (4 mL) were added. The mixture was stirred at 50 °C under argon for 2 h, and then all of the solvents were removed in vacuo. Water (40 mL) was added. After stirring the resulting mixture overnight at room temp. under argon, the resulting homogeneous deep green solution was filtered through a paper filter, and the filtrate was acidified by the addition of HCl (2 m solution) to pH = 2.5–3. A viscous deep brown-colored gel resulted, which was left overnight at 5 °C under argon until the coagulation was complete. The resulting solid was collected on a filter, washed with HCl (0.1 m solution, 3 × 5mL) and then water, and dried in vacuo to give trityl 5 (0.506 g, 92%) as a brownish-black fine powder. MS (ESI): calcd. for C40H38O6S12 [M – H]− 997.932; found 997.942; calcd. for C40H37O6S12 [M – 2H]2− 498.462; found 498.464. IR (KBr): ṽ = 2958 (m), 2920 (m), 1691 (s), 1452 (m), 1385 (m), 1366 (m), 1232 (s), 1169 (m), 1149 (m), 1109 (m), 864 (m), 725 (m)cm−1. ESR: singlet; linewidth, 226 mG for solution in contact with atmospheric air; g = 2.0056 (0.5 mM in water, pH = 8.5). The paramagnetic purity for the radical was found to be >95% in experiment with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) used as a standard. HPLC purity >97%.
Method D
A suspension of 6 (2.040 g, 2.00 mmol) in freshly distilled TFA (12 mL) and DCM (12 mL) was stirred at room temp. under argon for 5 h. To the resulting greenish-brown solution was added slowly by syringe a solution of SnCl2 (0.190 g, 1.00 mmol) [23] in anhydrous THF (5 mL). The resulting thick brown slurry was stirred overnight at room temp. under argon. The mixture was poured into water (50 mL). The water phase was neutralized to pH = 3–3.5 by the slow addition of NaOH (2 m solution). After the addition of THF (80 mL), the organic phase was separated, and the water phase was extracted with THF (3 × 10 mL). The combined organic extracts were washed with brine (4 × 10 mL) and concentrated in vacuo. The solid residue was dissolved in a mixture of water (200 mL) and NaOH (2 m solution, 20 mL). Brine (100 mL) was added to resulting deep-green solution, which was left for 2 h at room temp. The solution was filtered through slow-filtering paper, and the filtrate was acidified to pH = 2–3 by the addition of HCl (2 m aqueous solution) to give a brown gel. After 5 h, the solid was collected on a filter, washed with HCl (0.1 m aqueous solution, 5 × 20 mL) and water (2 × 20 mL), and dried in vacuo to give trityl 5 (1.920 g, 96%) as a brownish-black fine powder. MS (ESI): calcd. for C40H38O6S12− [M – H]− 997.932; found 997.90. UV/Vis (methanol): λmax (ε, Lmol−1cm−1) = 267 (47000), 402 (17500), 483 (19400) nm. ESR: singlet; linewidth, 94 mG for deoxygenated water solution; g = 2.0056 (1.0 mM in water, pH = 8.0). MS (ESI) and ESR spectra are presented in the Supporting Information.
Tris(8-carboxy-2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiol-4-yl)methanol (6)
Compound 6 was prepared by analogy to the literature protocol.[12] To a stirred suspension of 3 (6.265 g, 7.10 mmol) in n-hexane (7 mL) and freshly distilled anhydrous TMEDA (25 mL) cooled to −78 °C under argon was added by syringe n-butyllithium (2.5 M in hexane, 28.4 mL, 0.071 mol, 10 equiv.) slowly over 30 min. After stirring for 20 min, the cooling bath was removed, and the reaction was stirred at room temp. for 2 h 30 min. The mixture gradually changed to a dark brown solution and then a thick pale brown slurry. To lessen the viscosity, anhydrous toluene (24 mL) was added by syringe. The resulting fluent gel was stirred for 30 min and then transferred to solid carbon dioxide (350–500 g) that was soaked in anhydrous toluene (100 mL). The mixture was left to attain ambient temperature. The solvents and TMEDA were evaporated in vacuo. The resulting solid cake was triturated with toluene (20 mL), which then was evaporated again to remove the residual TMEDA. Methyl tert-butyl ether (50 mL), water (150 mL), and NaOH (2 m solution, 20 mL) were added to the residue. The biphasic mixture was vigorously shaken and transferred to a separatory funnel, to which brine (60 mL) was added. The water phase was separated, and the organic phase was extracted in the order of water (1 × 30 mL), NaOH (2 m solution, 1 × 2 mL), and brine (1 × 10 mL). The combined water extracts were left at room temp. until there was complete precipitation of the solid impurities (typically overnight). The mixture was then filtered through slow-filtering paper (e.g., Whatman Grade 50) and acidified to pH = 2 by the slow addition of hydrochloric acid (4 m aqueous solution) to give a lemon yellow slurry. After 5 h, the solid was collected on a filter, washed with HCl (0.1 m aqueous solution, 5 × 20 mL) and water (50 mL), and dried in vacuo. The dry residue was heated at reflux in acetonitrile (25 mL) for 20 min. After standing at 5 °C for 2 h, the solid was collected on a filter, washed with cold acetonitrile (5 × 3 mL), and dried in vacuo to give 6 (4.479 g, 62%) as a lemon yellow solid [product may have contained residual acetonitrile (30–40 mol-%, 1.5–2 wt.-%)]; m.p. >280 °C (gradually turned black, decomposition). HPLC purity >95%. C40H40O7S12 (1017.47): calcd. C 47.21, H 3.96, S 37.82; found C 46.91, H 4.12, S 37.36. MS (ESI): calcd. for C40H39O7S12 [M – H]− 1014.935; found 1014.946. IR (KBr): ṽ = 2963 (m), 2918 (m), 2860 (m), 1688 (s), 1508 (m), 1453 (m), 1431 (m), 1367 (m), 1317 (m), 1261 (m), 1223 (s), 1167 (m), 727 (w) cm−1. 1H NMR (400 MHz, [D6]DMSO): δ = 1.59 (s, 9 H, CH3), 1.62 (s, 9 H, CH3), 1.69 (s, 9 H, CH3), 1.72 (s, 9 H, CH3), 2.07 (s, 0.8–1.2 H, acetonitrile), 6.79 (s, 1 H, OH) ppm. 13C NMR (100 MHz, [D6]-DMSO): δ = 27.52 (CH3), 28.56 (CH3), 31.35 (CH3), 33.92 (CH3), 60.72 (SCS), 60.80 (SCS), 83.59 (COH), 122.11 (C), 133.40 (C), 138.33 (C), 139.87 (C), 140.60 (C), 140.94 (C), 167.06 (CO2H) ppm.
Tris(8-carboxy-2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiol-4-yl)methyl (5) and Quinone Methide 7
A suspension of 6 (0.375 g, 0.37 mmol) in freshly distilled TFA (4 mL) was stirred for 16 h at room temp. under argon.[24] The deep colored greenish-brown solution was concentrated in vacuo to give a black cake. The cake was dissolved in NaOH (2 m solution, 5 mL, 10 mmol), and the resulting solution was diluted with water (10 mL) to afford a reddish-brown solution.[25] The addition of brine (10 mL) resulted in the formation of an abundant amount of a fine precipitate. The mixture was left under argon for 4 h and then filtered through slow-filtering paper. The deep green clear filtrate was acidified to pH = 3 by the addition of HCl (2 m solution) to give 5 (0.206 g, 56%), which was isolated as reported above. The solid material collected on the filter and was washed with water/brine (1:1 v/v, 3 × 5 mL). The solid was then dissolved in acidified methanol [concentrated HCl (25 µL) in methanol (50 mL)]. The resulting deep purple solution was concentrated in vacuo, and the crude product was purified by column chromatography on silica gel (dichloromethane/methanol, from 20:1 to 3:1 v/v) to afford quinoide 7 (0.078 g, 22%) as a reddish-black powder; m.p. >280 °C (decomposition). Data for 7: MS (ESI): calcd. for C39H37O5S12 [M – H+]− 968.929; found 968.935. IR (KBr): ṽ = 2957 (m), 2920 (s), 2851 (m), 1686 (m), 1659 (m), 1603 (s), 1585 (s), 1495 (m), 1452 (s), 1385 (s), 1366 (s), 1231 (s), 1150 (s), 1105 (m), 733 (m) cm−1. UV/Vis (methanol): λmax (ε, Lmol−1cm−1) = 276 (38100), 369 (11700), 477 (9600), 529 (10800) nm. 1H NMR (400 MHz, [D6]DMSO): δ = 1.57 (s, 6 H, CH3), 1.63 (s, 6 H, CH3), 1.69 (s, 6 H, CH3), 1.71 (s, 6 H, CH3), 1.72 (s, 6 H, CH3), 1.76 (s, 6 H, CH3) ppm. 1H NMR (600 MHz, CD3OD): δ = 1.64 (s, 6 H, CH3), 1.72 (s, 6 H, CH3), 1.74 (s, 6 H, CH3), 1.76 (s, 6 H, CH3), 1.82 (s, 6 H, CH3), 1.85 (s, 6 H, CH3) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 29.95 (CH3), 28.27 (CH3), 30.45 (CH3), 31.18 (CH3), 33.35 (CH3), 34.52 (CH3), 60.97 (SCS), 61.67 (SCS), 62.63 (SCS), 127.30 (C), 128.49 (C), 131.29 (C), 137.08 (C), 138.94 (C), 140.44 (C), 141.79 (C), 142.89 (C), 157.40 (C), 169.07 (CO), 171.55 (CO) ppm. 13C NMR (150 MHz, CD3OD): δ = 29.50 (CH3), 31.08 (CH3), 32.24 (CH3), 33.25 (CH3), 34.66 (CH3), 62.86 (SCS), 63.77 (SCS), 64.00 (SCS), 129.30 (C), 130.44 (C), 131.01 (C), 139.22 (C), 140.36 (C), 140.99 (C), 141.59 (C), 143.82 (C), 145.54 (C), 157.39 (C), 173.96 (CO), 174.63 (CO) ppm. Spectra of quinoide 7 are given in Supporting Information.
Tris(2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiol-4-yl)methyl (11) and Quinone Methide 12
A solution of 3 (0.200 g, 0.226 mmol) in anhydrous dichloromethane (2 mL) and freshly distilled TFA (2 mL)[26] was stirred at room temp. overnight under argon. The deep green solution was concentrated in vacuo to give a black cake. The cake was dissolved in dichloromethane (4 mL), and then water (2 mL) was added. The mixture was stirred for 7 h under argon, and the green organic solution slowly changed to a deep brown color. The organic phase was separated, and water phase was extracted with DCM (3 × 2 mL). The combined organic extracts were filtered through a short cotton plug and concentrated in vacuo. Column chromatography on silica gel (dichloromethane/hexane, from 1:1 to 5:1) afforded trityl 11 (0.115 g, 58.6%) as a greenish-black powder (green in DCM solution) and quinoide 12 (0.054 g, 27.0%) as a reddish-black powder. Data for 11: MS (ESI): calcd. for C37H39S12 [M]+ 866.970; found 866.964. MALDI-TOF: m/z = 867.04. IR (KBr): ṽ = 2974 (m), 2954 (m), 2920 (m), 2910 (m), 1452 (m), 1363 (s), 1342 (m), 1248 (s), 1169 (m), 1148 (s), 1103 (m), 849 (m), 644 (m)cm−1. UV/Vis (CH2Cl2): λmax (ε, Lmol−1cm−1) = 273 (55300), 325 (22300), 443 (21500) nm. ESR: 1:3:3:1 quartet αH = 2.27 G; linewidth, 258 mG for 1 mm solution in DCM; g = 2.0055. Spectra of trityl 11 are given in the Supporting Information. Data for 12: m.p. >260 °C (decomposition). MS (ESI): calcd. for C37H39OS12 [M + H]+ 882.965; found 882.964. IR (KBr): ṽ = 2957 (m), 2920 (s), 2912 (m), 1605 (s), 1468 (s), 1452 (s), 1364 (s), 1252 (m), 1150 (s), 1090 (m), 1030 (m), 733 (m) cm−1. UV/Vis (CH2Cl2): λmax (ε, Lmol−1cm−1) = 273 (47600), 517 (18000) nm. 1H NMR (600 MHz, CDCl3): δ = 1.70 (s, 6 H, CH3), 1.74 (s, 6 H, CH3), 1.77 (s, 6 H, CH3), 1.79 (s, 6 H, CH3), 1.87 (s, 6 H, CH3), 1.89 (s, 6 H, CH3), 7.07 (s, 2 H, CH) ppm. 13C NMR (150 MHz, CDCl3): δ = 29.66 (CH3), 30.07 (CH3), 31.42 (CH3), 31.66 (CH3), 31.88 (CH3), 33.63 (CH3), 62.81 (SCS), 64.72 (SCS), 65.86 (SCS), 119.08 (CH), 127.74 (C), 129.45 (C), 136.50 (C), 137.66 (C), 138.85 (C), 139.04 (C), 141.22 (C), 144.05 (C), 153.20 (C), 172.85 (CO) ppm. Spectra of quinoide 12 are presented in the Supporting Information.
Tris(2,2,6,6-tetramethylbenzo[1,2-d;4,5-d′]bis[1,3]dithiol-4-yl)methyl (11) and Trityl 15
A solution of 3 (0.134 g, 0.150 mmol) in anhydrous dichloromethane (2 mL) and freshly distilled TFA (2 mL) was stirred at room temp. overnight under argon. The deep green solution was concentrated in vacuo to give a black cake. The cake was dissolved in dichloromethane (4 mL), and the flask was flushed with argon. A solution of diethylamine (0.055 g, 0.750 mmol) in anhydrous DCM (2 mL) was added by a syringe. The resulting green solution was stirred overnight and then concentrated in vacuo. Trityls 11 and 15 were isolated by column chromatography on silica gel (TFA in DCM, 1:1000 v/v and then DCM saturated with aqueous ammonia) to give pure 11 (0.062 g, 47%) and 15 (0.057, 42%) as a black powder (bluish-green in DCM solution). Data for 15: MS (ESI): calcd. for C41H49NS12 [M + H]+ 939.051; found 939.040. MALDI-TOF: calcd. for C41H48NS12 [M]+ 938.043; found 938.00. IR (KBr): ṽ = 2959 (s), 2922 (s), 2912 (s), 1450 (s), 1381 (s), 1363 (s), 1251 (s), 1167 (s), 1148 (s), 853 (m), 704 (m) cm−1. UV/Vis (CH2Cl2): λmax (ε, L mol−1 cm−1) = 270 (61100), 322 (16200), 445 (9120) nm. ESR: broad 1:2:1 triplet αH = 2.29 G; linewidth, 609 mG for 1 mm solution in DCM; g = 2.0055. Spectra of trityl 15 are presented in the Supporting Information.
Alternative Preparation for Trityl 15
A solution of 3 (0.132 g, 0.146 mmol) in anhydrous dichloromethane (3 mL) and CF3SO3H (0.044 g, 0.293 mmol) was stirred at room temp. for 2 h under argon. The resulting deep green solution was added by syringe slowly over 30 min to a stirred solution of diethylamine (0.320 g, 4.38 mmol) in DCM (1 mL). The homogeneous solution was stirred overnight at room temp., and then water (6 mL) was added. The mixture was stirred and left in the air for 30 min. The organic phase was separated, and the water phase was extracted with CH2Cl2 (3 × 3 mL). The combined organic extracts were filtered through a short cotton plug and concentrated in vacuo. Column chromatography on silica gel (DCM/hexane, 1:1 v/v and then DCM) afforded trityl 15 (0.111 g, 82%) as the only product.
Supplementary Material
Acknowledgments
The authors thank Drs. Leonid A. Shundrin and Denis A. Komarov for recording the ESR spectra and Dr. V. V. Koval for the registration of the MALDI-TOF spectra. The authors wish to thank Professor Michael K. Bowman (University of Alabama, USA), Dr. Alexander M. Genaev and G E. Sal’nikov for the helpful discussion and suggestions. This study was supported by The Russian Foundation for Basic Research (project 13-04-00680A), The Ministry of Education and Science of the Russian Federation (project 8466) and the National Institute of Biomedical Imaging and Bioengineering, National Institute of Health (NIH), grant number 5P41EB002034. NMR, IR, high resolution ESI-MS, and ESR experiments were carried out in the Chemical Service Center of the Siberian Branch of the Russian Academy of Sciences (RAS).
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/ejoc.201300176.
Supporting Information: TLC analyses of crude products obtained from triarylmethanols 6 and 3. MS (ESI), 1H and 13C NMR, and UV/Vis spectroscopic data for quinoides 7 and 12. MS (ESI), MALDI-TOF, ESR, and UV/Vis spectroscopic data for trityls 11 and 15. MS (ESI), ESR, and UV/Vis spectroscopic data for trityl 5 (Method D).
References
- 1.a) Andersson S, Radner F, Rydbeck A, Servin R, Wistrand L-G. 5530140. U S. Patent. 1996; b) Ardenkjaer-Larsen JH, Leunbach I. wo/9709633. PCT Int. Patent Appl. 1997; c) Andersson S, Radner F, Rydbeck A, Servin R, Wistrand L-G. 5728370. U S. Patent. 1998; d) Thaning M. wo/9839277. PCT Int. Patent Appl. 1998
- 2.a) Khan N, Swartz H. Mol. Cell. Biochem. 2002;234:341–357. [PubMed] [Google Scholar]; b) Kuppusamy P, Wang P, Chzhan M, Zweier JL. Magn. Reson. Med. 1997;37:479–483. doi: 10.1002/mrm.1910370402. [DOI] [PubMed] [Google Scholar]
- 3.a) Halevy R, Tormyshev V, Blank A. Biophys. J. 2010;99:971–978. doi: 10.1016/j.bpj.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Elas M, Ahn K-H, Parasca A, Barth ED, Lee D, Haney C, Halpern HJ. Clin. Cancer Res. 2006;12:4209–4217. doi: 10.1158/1078-0432.CCR-05-0446. [DOI] [PubMed] [Google Scholar]; c) Elas M, Williams DD, Parasca A, Mailer C, Pelizzari CA, Lewis MA, River JN, Karczmar GS, Barth ED, Halpern HJ. Magn. Reson. Med. 2002;49:682–691. doi: 10.1002/mrm.10408. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Villamena FA, Song Y, Sun J, Rockenbauer A, Zweier JL. J. Org. Chem. 2010;75:7796–7802. doi: 10.1021/jo1016844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Liu Y, Villamena FA, Sun J, Wang TY, Zweier JL. Free Radical Biol. Med. 2009;46:876–883. doi: 10.1016/j.freeradbiomed.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu Y, Villamena FA, Sun J, Xu Y, Dhimitruka I, Zweier JL. J. Org. Chem. 2008;73:4190–4197. doi: 10.1021/jo7022747. [DOI] [PubMed] [Google Scholar]
- 6.Rizzi C, Samouilov A, Kutala VK, Parinandi NL, Zweier JL, Kuppusamy P. Free Radical Biol. Med. 2003;35:1608–1618. doi: 10.1016/j.freeradbiomed.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Dhimitruka I, Bobko AA, Hadad CM, Zweier JL, Khramtsov VV. J. Am. Chem. Soc. 2008;130:10780–10787. doi: 10.1021/ja803083z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talmon T, Shtirberg L, Harneit W, Rogozhnikova OYu, Tormyshev V, Blank A. Phys. Chem. Chem. Phys. 2010;12:5998–6007. doi: 10.1039/b922060g. [DOI] [PubMed] [Google Scholar]
- 9.a) Leggett J, Hunter R, Granwehr J, Panek R, Perez-Linde AJ, Horsewill AJ, McMaster J, Smith G, Köcken-berger W. Phys. Chem. Chem. Phys. 2010;12:5883–5892. doi: 10.1039/c002566f. [DOI] [PubMed] [Google Scholar]; b) Lerche MH, Meier S, Jensen PR, Baumann H, Petersen BO, Karlsson M, Duus JØ, Ardenkjær-Larsen JH. J. Magn. Reson. 2010;203:52–56. doi: 10.1016/j.jmr.2009.11.020. [DOI] [PubMed] [Google Scholar]; c) Ardenkjær-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc. Natl. Acad. Sci. USA. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy TJ, Iwama T, Halpern HJ, Rawal VH. J. Org. Chem. 2002;67:4635–4639. doi: 10.1021/jo011068f. [DOI] [PubMed] [Google Scholar]
- 11.Dhimitruka I, Velayutham M, Bobko AA, Khramtsov VV, Villamena FA, Hadadd CM, Zweier JL. Bioorg. Med. Chem. Lett. 2007;17:6801–6805. doi: 10.1016/j.bmcl.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tormyshev VM, Genaev AM, Sal’nikov GE, Rogozhnikova OYu, Troitskaya TI, Trukhin DV, Mamatyuk VI, Fadeev DS, Halpern HJ. Eur. J. Org. Chem. 2012:623–629. doi: 10.1002/ejoc.201101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Chadwick ST, Rennels RA, Rutherford JL, Collum DB. J. Am. Chem. Soc. 2000;122:8640–8647. [Google Scholar]; b) Chalk AJ, Hoogeboom TJ. J. Organomet. Chem. 1968;11:615–618. [Google Scholar]
- 14.In principle, this could be accomplished by following the earlier method of a one-electron reduction of the carbocation that was generated from 6 (for example, see Scheme 1, step d) by treating it with a strong protic or Lewis acid. Unfortunately, the insolubility of the substrate in nonpolar solvents (e.g., chloroform or dichloromethane) obstructed the application of this attractive approach.
- 15.Driesschaert D, Robiette R, Lucaccioni F, Gallez B, Marchand-Brynaert J. Chem. Commun. 2011;47:4793–4795. doi: 10.1039/c1cc10988j. [DOI] [PubMed] [Google Scholar]
- 16.a) Decroos C, Li Y, Bertho G, Frapart Y, Mansuy D, Boucher JL. Chem. Res. Toxicol. 2009;22:1342–1350. doi: 10.1021/tx9001379. [DOI] [PubMed] [Google Scholar]; b) Decroos C, Li Y, Bertho G, Frapart Y, Mansuy D, Boucher JL. Chem. Commun. 2009:1416–1418. doi: 10.1039/b819259f. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Song Y, Pascali FDe, Liu X, Villamena FA, Zweier JL. Free Radical Biol. Med. 2012;53:2081–2091. doi: 10.1016/j.freeradbiomed.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Zhang Y, Gustafson LO, Chen EY-X. J. Am. Chem. Soc. 2011;133:13674–13684. doi: 10.1021/ja2053573. [DOI] [PubMed] [Google Scholar]; b) Dyker G, Hagel M, Muth O, Schirrmacher C. Eur. J. Org. Chem. 2006:2134–2144. [Google Scholar]; c) Nishimae Y, Kurata H, Oda M. Angew. Chem. 2004;116:5055. doi: 10.1002/anie.200460480. Angew. Chem. Int. Ed. 2004, 43, 4947–4950. [DOI] [PubMed] [Google Scholar]
- 19.Decroos C, Prange T, Mansuy D, Boucher JL, Li Y. Chem. Commun. 2011;47:4805–4807. doi: 10.1039/c1cc10426h. [DOI] [PubMed] [Google Scholar]
- 20.Decroos C, Li Y, Soltani A, Frapart Y, Mansuy D, Boucher JL. Arch. Biochem. Biophys. 2010;502:74–80. doi: 10.1016/j.abb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 21.During chromatography, trityl 11 and quinoide 12 separated into compact sharp-edged bands. In addition, these bands were deeply colored (e.g., see Supporting Information), and, therefore, it was easy to control the course of the chromatographic isolation/purification.
- 22.Irradiation of the triplet at δ = 1.43 ppm simplified the ABX3 multiplet at δ = 4.41 ppm to give the AB system, which resulted from the coupling of the geminal methylenic protons of the ethoxy group at 4.39 (J = 10.8 Hz, 1 H, OCHaHb), 4.42 (J = 10.8 Hz, 1 H, OCHaHb) ppm.
- 23.We found that larger amounts of SnCl2 gave a notable yield of the triarylmethane as the product of a formal two-electron reduction. The presence of the triarylmethane is detected either by MS (ESI) or the appearance of a characteristic series of three singlet signals (1.6–1.75 ppm) in the 1H NMR spectrum or four singlets (25–36 ppm) in the 13C NMR spectrum. Therefore, it is strongly recommended to limit the amount of reductant to 0.5 equiv.
- 24.Product distributions and their yields were shown to be independent of the presence of atmospheric oxygen and the variation of reaction times over a range of 6–36 h.
- 25.A drop of this solution was acidified to pH = 3. TLC analysis of the sample obtained after removal of water clearly indicated the two-component nature of the crude product (see Supporting Information).
- 26.Also following the above protocol, trityl 11 and quinone methide 12 were obtained by using other strong acids such as a 1:1 ethereal solution of HBF4 [3 equiv. in DCM (4 mL)] and CF3SO3H [3 equiv. in DCM (4 mL)]. The yields and product distribution were similar as those for the reaction with TFA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.