Abstract
Purpose
Obesity in adolescence increases the risk for early adult cardiovascular disease. We recently showed that 6 months of diet, exercise, and metformin resulted in reductions in adiposity and that diet/exercise alone reduced proinflammatory factors and intrahepatic fat in pubertal children with uncomplicated obesity. The purpose of the present study was to determine whether changes in cardiorespiratory fitness (CRF) after 6 months of structured diet and exercise (DE) or DE plus metformin are related to the previously observed changes in adiposity, markers of inflammation, and intrahepatic fat.
Methods
Sixteen obese pubertal adolescents between the ages of 10 and 17 were randomized into a structured lifestyle program consisting of DE or DE plus metformin. Subjects performed aerobic and resistance exercise 3 d·wk−1, 30 min per session. Cycle ergometer maximal oxygen consumption (V̇O2max), body composition, blood markers (glucose, insulin, homeostatic model assessment–insulin resistance, interleukin-6, hsCRP), and intrahepatic fat were measured at baseline and 6 months.
Results
In the cohort, as whole-body weight decreased by 4.0% (P = 0.009), body mass index decreased by 4.9% (P = 0.003), percent body fat decreased by 8.8% (P < 0.001), and V̇O2max improved in 10 of 16 subjects. The addition of metformin provided no further effect on body composition, CRF, or inflammatory factors. More favorable changes in adiposity, adiponectin, and a trend toward blood glucose and interleukin-6 concentrations (P = 0.07) were observed in subjects who increased V̇O2max at 6 months (n = 10) compared with no change in these variables in those who did not improve V̇O2max.
Conclusions
Metformin did not provide benefits above lifestyle modification for improving CRF in obese adolescents. Improvements in V̇O2max seem to be associated with more favorable metabolic outcomes.
Keywords: PUBERTY, V̇O2MAX, BODY COMPOSITION, METABOLIC SYNDROME
The prevalence of obesity among adolescents has increased threefold during the past 30 yr (30). Alarmingly, this trend has led to an increase in cardiovascular comorbidities not commonly observed until adulthood (i.e., hypertension, hyperlipidemia, type 2 diabetes, and fatty liver disease). We recently reported that obesity in childhood is associated with a proinflammatory and prothrombotic state even before the onset of puberty (27). Although the causes are not fully understood, physical inactivity and poor dietary behavior are likely key contributing factors (4,39). Data suggest that adolescents spend considerable time viewing television (40), are exposed to many energy-dense foods low in nutritional value (4), and overwhelmingly fall short of recommended physical activity levels, with only 8% of older children (12–15 yr) and adolescents (16–19 yr) accumulating 60 min of at least moderate-intensity physical activity per day (39). Moreover, objectively measured physical activity data from Troiano et al. (39) suggest that the time spent in structured physical activity of moderate or greater intensity (e.g., 8- to 10-min bouts) is less than 20 min·d−1 for older male children and adolescents and is less than 10 min·d−1 for females, with both genders falling short of the 30 min·d−1 recommended for health promotion in adults. Although a specific “dose–response” pattern between adiposity and physical activity has yet to be defined, data in the literature support the notion that higher levels of habitual physical activity are moderately protective against obesity in children and adolescents (20).
There are emerging data to suggest that cardiorespiratory fitness (CRF) attenuates some of the factors contributing to metabolic syndrome in adolescence, oftentimes independent of adiposity (12–14). Maximal oxygen consumption (V̇O2max), an objective measure of CRF, has been shown to be a strong predictor of cardiovascular disease (CVD) risk in both adults and children (7,15). Moreover, evidence from adult populations shows that higher levels of fitness afford greater protection against early morbidity and mortality attributable to CVD (23). For these reasons, lifestyle interventions aimed at increasing physical activity levels are the cornerstone treatment of childhood obesity. However, few intervention studies have examined the effect of V̇O2max on clinical outcomes in adolescence.
Recently, the use of the insulin-sensitizing drug metformin in obese children and adolescents has become more widespread in clinical practice. Although not currently approved for weight loss, studies suggest metformin has a favorable effect on body composition (9,24,33,38). However, it should be realized that metformin has several potential adverse effects, including hypoglycemia, nausea, vomiting, and abdominal pain. Furthermore, whether metformin also affords protection against systemic inflammation is unclear (9,24,33,38). We recently found that the addition of metformin administration to lifestyle intervention, in obese children with normal glucose tolerance, did not result in additional improvement in adiposity, adiponectin, homeostatic model assessment–insulin resistance (HOMA–IR), highly sensitive C-reactive protein (hsCRP), interleukin-6 (IL-6), or intrahepatic fat (IHF) compared with lifestyle alone (10). However, when data from all subjects were grouped, we observed favorable changes in markers related to inflammation and thrombosis and IHF contents with lifestyle intervention. Whether the improvements in metabolic risk were related in part to the improvement in V̇O2max and associated favorable changes in CRF has not been previously studied.
In the present study, we determined 1) whether the addition of metformin to lifestyle modification leads to greater improvement in aerobic fitness in obese adolescents than lifestyle intervention alone and 2) whether changes in fitness are related to changes in adiposity, adiponectin, CHO metabolism (insulin, glucose, HOMA-IR), inflammatory factors (hsCRP, IL-6), and IHF content.
METHODS
Subjects
Thirty-seven adolescents in late puberty (Tanner stages IV and V, age = 14.3 ± 2.4 yr) with uncomplicated obesity were recruited for the present study. Subjects were participants in a larger longitudinal study that examined whether lifestyle modification with structured diet and exercise, with or without metformin, improves proinflammatory and prothrombotic-related markers in children with uncomplicated obesity before the establishment of comorbidities of the metabolic syndrome.
Recruitment and screening
Subjects in the present study as well as the larger study were recruited from extensive regional institutional review committee–approved advertising and from the pediatric endocrinology clinics (10). Obesity without metabolic syndrome comorbidities was defined as a body mass index (BMI) greater than the 95th percentile for North American standards, based on year 2000 data, but without elevation of the blood pressure for age (using National Institutes of Health published percentiles (http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.pdf)), with normal fasting glucose (<100 mg·dL−1), with no increase in cholesterol (<200 mg·dL−1), and with normal triglycerides (<150 mg·dL−1). All other causes of endocrine or genetic obesity were excluded, and obese subjects had to have been overweight for less than 5 yr. Subjects could not have any history of chronic illness, chronic medications, or smoking. To avoid illness-related elevations of the markers of interest, subjects were studied only if they had no history of recent illness, bone fracture, or viral syndrome within 2 wk of blood draws. They were instructed not to consume any medications, including vitamins, herbal medications, or anti-inflammatory drugs within 10 d of anticipated blood draws. Menstruating girls must have completed their period at least 2 wk previously and could not be pregnant. Studies were approved by the Wolfson Children’s Hospital institutional review board. Informed written consent of the parent/guardian and the child’s assent were obtained.
Study design
Eligible subjects were given a 6-month program of structured lifestyle modification consisting of either diet and exercise alone (DE) or diet and exercise plus metformin (DEM). Group assignment to DE or DEM was random. For each group, body composition, aerobic fitness, and physical activity level were assessed at baseline and after the 6-month intervention. Resting energy expenditure (REE) was obtained at baseline. Before testing, subjects were familiarized with all laboratory procedures. Body composition and REE measurements were performed by skilled health care professionals. When feasible, pre- and postintervention measurements were performed by the same person.
Body composition
A dual-energy x-ray absorptiometry (DEXA) scan was performed to measure fat mass (FM), lean mass, and percent body fat (%BF) (model S/N 45903, Hologic Discovery A; Hologic, Inc., Bedford, MA).
Resting Energy Expenditure (REE)
REE was determined by indirect calorimetry (MedGraphics BreezeSuite Ultima CPX). The average of three calorimetry tests, each 10 min in duration, was obtained after an overnight fast.
Aerobic fitness assessment
Subjects completed a continuous incremental V̇O2max protocol on a bicycle ergometer (MedGraphics BreezeSuite Ultima CPX, St. Paul, MN; and Lode BV Corival Recumbent V2, Groningen, The Netherlands). Bicycle ergometer testing was selected because of the ease of administration, although maximal treadmill exercise is well tolerated in obese adolescents. Initial power output was 20 W, and power output was increased by 10 to 20 W every minute until volitional fatigue. Pedal rate was maintained between 60 and 100 rpm during the test. V̇O2max was chosen as the highest V̇O2 attained during the test and was considered valid when at least two of the following criteria were satisfied: 1) respiratory exchange RER > 1.00, 2) HR > 95% of age-predicted maximum (220 – age), or 3) a plateau of V̇O2max. An OMNI-RPE score was obtained (1–10, 10 being maximum exhaustion) at the end of each stage to determine the level of exhaustion (35). HR was monitored continuously by telemetry monitoring (Hewlett-Packard Model M1204A, Hewlett-Packard, Palo Alto, CA), and blood pressure was measured manually every 2 min during the test.
Lifestyle intervention
After baseline testing, all subjects began a structured 6-month lifestyle intervention consisting of dietary modification and exercise. Intensive dietary counseling was provided weekly for the first 4 wk of the intervention, monthly subsequently until 3 months, and then again at 6 months. A target caloric deficit of ~250–500 cal·d−1 (individually calculated on the basis of REE) was recommended throughout dietary counseling. Subjects were instructed to keep food records daily. Subjects and their families were given a free membership to a local Young Men’s Christian Association (YMCA). They were encouraged to use the YMCA facilities to exercise at least three times per week for 30 min per session. Sessions consisted of structured exercise including both aerobic and strength training. Exercise consisted of 5–10 min for warm-up and stretching, followed by 15–30 min of cardiovascular exercise (i.e., treadmill, bicycle ergometer, rower, NuStep (NuStep Inc., Ann Arbor, MI), etc.), 10–20 min of strength training (supervised using weight stack equipment), and 5–10 min of cooldown and stretching. Participants were started at 15 min of cardiovascular exercise and 10 min of strength training exercise and encouraged to progress by 2–3 min every week until 30 and 20 min, respectively, was achieved. All sessions were supervised by an American Council on Exercise–certified personal trainer, and subjects received instruction on the safe use of exercise equipment. Exercise logs were maintained and included average time per session and number of sessions performed per week.
Assessment of physical activity level
At baseline, subjects were issued an Accusplit Eagle 170s pedometer (Livermore, CA) and were instructed on proper use and to wear the pedometer at all applicable times except at night and during YMCA visits. Subjects were instructed to keep pedometer logs daily. Daily step count was calculated at 3 and 6 months. Although we do not have reliability data for the specific pedometer used in the present study, a recent review including 25 articles investigating the validity, reliability, and feasibility of pedometers for children concluded that pedometers generally have high intra- and interunit reliability (intra-unit range: intraclass correlation coefficient = 0.51–0.92, interunit range: intraclass correlation coefficient = 0.73–0.8) and correlate strongly with direct observation–based (range: r = 0.74–0.92) and accelerometer-based (range: r = 0.47–0.99) measurements of physical activity (29). As such, pedometer data in the present study are provided as an estimate of daily step rate.
Medication
Subjects in the DEM group initially received 250 mg of metformin orally twice a day, titrating to 500 mg twice a day in subjects <12 yr and 1000 mg twice a day as tolerated on the basis of adverse effects in the older subjects. Some subjects reported mild abdominal discomfort, decreased appetite, nausea, and loose stools when initiating metformin therapy; however, this was resolved by subjects titrating the dose slowly. The average dose of metformin was ~1000 mg·d−1 by 6 months.
Assays
At baseline and within 6 wk of screening, a 3-h oral glucose tolerance test was performed, and those with impaired glucose tolerance (IGT; 2-hour plasma glucose between 140 and 200 mg·dL−1) were excluded from further participation. Glucose was measured using a glucose oxidase method using a Beckman (Beckman Coulter, Inc., Indianapolis, IN) glucose analyzer. Subsequently, finger stick blood sugars were measured using a OneTouch finger stick glucometer (LifeScan, Inc., Milpitas, CA). Insulin was determined by radioimmunoassay (Diagnostic Systems Laboratories, Inc., Webster, TX). Oral glucose tolerance test insulin levels were determined by chemiluminescent detection. The HOMA for insulin resistance was calculated using the following formula: fasting insulin concentration (μIU·mL−1) × fasting glucose concentration (mg·dL−1) / 405. HOMA assumes that normal young subjects have an insulin resistance value of 1.0 (26). HsCRP concentrations were measured in our laboratory by immunonephelometry (Siemens Health-care Diagnostics, Deerfield, IL) with a lower sensitivity of 0.156 mg·L−1 as reported previously (3). IL-6 was measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN), and adiponectin was measured by radioimmunoassay.
Statistical analysis
Statistical analyses were conducted using SPSS v 17.0 (Chicago, IL). Data are presented as means ± SD. Only subjects completing both baseline and 6-month V̇O2max testing were included in the analysis. Mixed-model ANOVA with repeated measures over time was used to assess within- and between-group differences. Paired t-tests were used to analyze the effects of lifestyle intervention on all subjects. Significance was established a priori at P < 0.05.
RESULTS
Of the 37 subjects recruited for V̇O2max testing, 15 did not complete the study because of family and social issues, one subject was dropped from the study at baseline because of IGT, and five subjects did not complete a valid baseline V̇O2max test, leaving a total of nine subjects in the DE group (three males, six females; age = 13.4 ± 1.7 yr) and seven in the DEM group (four males, three females; age = 15.0 ± 2.0 yr) who completed the 6-month study. There was no significant difference in age between groups (P = 0.10).
Physical activity
Average pedometer step count (DE = 7935.8 ± 1736.3 steps, DEM = 7976.0 ± 2602 steps, P = 0.98), aerobic exercise time per YMCA session (DE = 31.7 ± 6.2 min, DEM = 31.7 ± 16.6 min, P = 0.99), average strength training time per session (DE = 23.0 ± 14.7 min, DEM = 27.7 ± 19.3 min, P = 0.72), and the number of YMCA sessions per week (DE = 0.9 ± 0.6 sessions, DEM = 1.0 ± 0.8 sessions, P = 0.71) were not significantly different between groups at 6 months (Table 1).
TABLE 1.
Exercise test parameters.
| DE (n = 9)
|
DEM (n = 7)
|
|||||
|---|---|---|---|---|---|---|
| 0 Months | 6 Months | P | 0 Months | 6 Months | P | |
| Max power (W) | 91.0 ± 48.0 | 107.0 ± 26.0 | 0.81 | 105.0 ± 31.0 | 116.0 ± 26.0 | 0.51 |
| HRmax (beats·min −1) | 156.0 ± 25.0 | 150.0 ± 20.0 | 0.59 | 159.0 ± 22.0 | 158.0 ± 16.0 | 0.93 |
| RER | 1.06 ± 0.17 | 1.03 ± 0.06 | 0.78 | 1.14 ± 0.17 | 1.04 ± 0.06 | 0.21 |
| OMNI-RPE rating | 8.0 ± 3.0 | 10 ± 1.0 | 0.17 | 10.0 ± 1.0 | 10.0 ± 1.0 | 0.99 |
| V̇O2max (mL·kg; −1·min−1) | 16.0 ± 4.3 | 17.5 ± 3.6 | 0.32 | 19.3 ± 5.6 | 20.9 ± 6.5 | 0.38 |
Data are shown as mean ± SD. P value < 0.05 is significant.
CRF
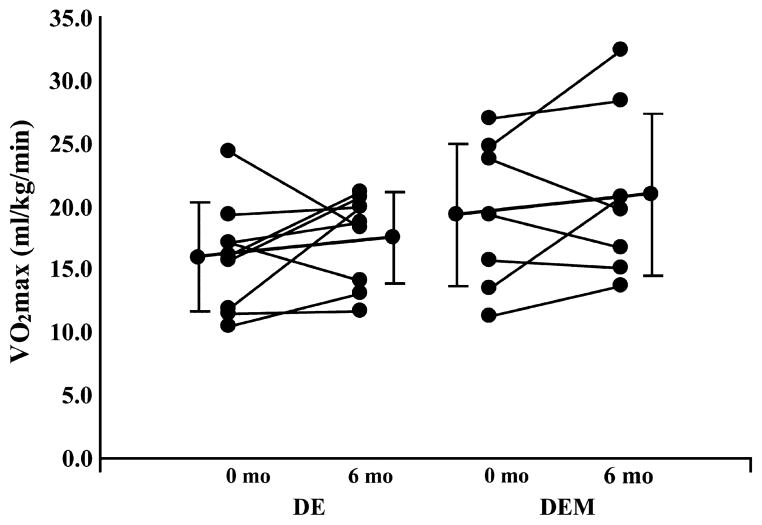
Mean and individual subject changes in V̇O2max are shown in Table 1 and Figure 1, respectively. V̇O2max relative to body mass was not different between groups at baseline or 6 months (baseline, P = 0.22; 6 months, P = 0.22; Table 1). Within-group change in V̇O2max from baseline was not statistically significant (DE, P = 0.32; DEM, P = 0.38; Fig. 1).
FIGURE 1.
Effects of 6 months of DE or DEM on CRF; individual subject change in V̇O2max (solid lines) and mean change (closed circles) ± SD are shown.
Because the addition of metformin did not affect CRF, we analyzed the effects of lifestyle intervention on all subjects combined (n = 16, paired t-test). In this subanalysis, V̇O2max tended to be, on average, 12.6% higher after the intervention (baseline = 17.4 ± 5.3 mL·kg−1·min−1, 6 months = 19.0 ± 5.4 mL·kg−1·min−1, P = 0.16), with 10 of 16 subjects improving fitness from baseline (5 males/5 females; Fig. 1).
Body composition
Weight decreased by 5.4% ± 4.8% in the DEM group (baseline = 90.2 ± 21.8 kg, 6 months = 85.1 ± 20.1 kg, P = 0.04) versus 3.0% ± 5.6% in the DE group (baseline = 93.9 ± 13.8 kg, 6 months = 90.9 ± 12.9 kg, P = 0.14, P = 0.47 between groups). A significant decrease in BMI was observed in the DEM group (baseline = 33.6 ± 7.2 kg·m−2, 6 months = 30.9 ± 6.5 kg·m−2, P = 0.02) but not in the DE group (baseline = 33.6 ± 3.4 kg·m−2, 6 months = 32.6 ± 2.6 kg·m−2, P > 0.05). %BF as measured by DEXA was significantly lower at 6 months in both groups (DE: baseline = 39.5% ± 4.1%, 6 months = 37.0% ± 3.2%, P = 0.01; DEM: baseline = 37.7% ± 7.4%, 6 months = 33.3% ± 8.7%, P = 0.04).
For the groups combined (n = 16), weight decreased by 4.0% (baseline = 92.3 ± 18.1 kg, 6 months = 88.3 ± 17.0 kg, P = 0.009), %BF decreased by 8.8% (baseline = 38.7% ± 6.0%, 6 months = 35.3% ± 6.7%, P = 0.002), and BMI decreased by 4.9% (baseline = 33.6 ± 5.5 kg·m−2, 6 months = 31.8 ± 4.9 kg·m−2, P = 0.003).
Fasting glucose, insulin, HOMA-IR, and adiponectin
At baseline, subjects in both groups had normal glucose tolerance (2-h glucose during OGTT: DE = 102 ± 24.0 mg·dL−1, DEM = 119 ± 28 mg·dL−1) and normal fasting glucose (DE = 88 ± 7 mg·dL−1, DEM = 92 ± 7 mg·dL−1) and, as expected, were hyperinsulinemic (DE = 22 ± 14.3 μIU·mL−1, DEM = 24.7 ± 12.0 μIU·mL−1) and moderately insulin resistant as measured by HOMA-IR (DE = 4.9 ± 3.2, DEM = 5.3 ± 1.6). After the intervention, there were no changes in fasting glucose within or between groups. Fasting insulin levels were significantly higher at 6 months in the DE group compared with baseline (DE = 38.6 ± 10.7 μIU·mL−1, P = 0.049). HOMA-IR tended to be higher at 6 months in the DE group (P = 0.07). Adiponectin concentrations, a measure of insulin sensitivity, were significantly higher (i.e., improved insulin sensitivity) at 6 months in the DEM group (P = 0.02), although not in the DE group (P = 0.12).
IL-6, hsCRP, and IHF
Inflammation-related markers hsCRP and IL-6 at 6 months were not significantly different from baseline values in either group (P values > 0.1). However, as a whole (n = 16), hsCRP concentrations were significantly lower after the intervention (percent decrease = 35.4%, P = 0.046). IL-6 concentrations were not different from baseline in the total sample (n = 16, P = 0.38). IHF contents after 6 months were not different from baseline in either group (DE, P = 0.16; DEM, P = 0.36) or when the groups were pooled (n = 16, P = 0.25). The results for fasting glucose, insulin, HOMA-IR, adiponectin, IL-6, hsCRP, and IHF, in the present subset, are similar to what we reported in the larger sample (28).
Effect of CRF on outcomes
The effect of CRF on the metabolic risk factors was examined by comparing subjects who increased V̇O2max after 6 months (n = 10, six subjects from DE (three males/three females) and four subjects from DEM (three males/one female)) with those subjects whose measured V̇O2max values either decreased or did not change from baseline (n = 6, three subjects from DE (one male/two females) and three subjects from DEM (one male/two females)). DE and DEM groups were pooled for this analysis because metformin did not affect 6-month outcome measures. Subjects who increased V̇O2max (4.1 ± 2.7 mL·kg−1·min−1) had more favorable changes in weight (P = 0.02), BMI (P = 0.01), %BF (P = 0.01), and adiponectin (P = 0.02) and tended to have lower blood glucose (P = 0.07) and lower IL-6 concentrations at 6 months (P = 0.07, Table 2). Changes in these outcomes during 6 months were not significantly different from baseline in subjects who did not increase V̇O2max (−2.2 ± 2.2 mL·kg−1·min−1) (Table 2).
TABLE 2.
Change in body composition, CHO metabolism, and blood markers related to inflammation in obese children at 6 months.
| ↑ V̇O2max at 6 Months
|
No Change or ↓ V̇O2max at 6 Months
|
|||
|---|---|---|---|---|
| 6 Months – Baseline | P | 6 Months – Baseline | P | |
| n | 10 | 6 | ||
| V̇O2max (mL·kg−1·min−1)* | 4.1 ± 2.7 | 0.001 | −2.7 ± 2.2 | 0.03 |
| Weight (kg) | −4.3 ± 4.8 | 0.02 | −3.2 ± 6.4 | 0.27 |
| BMI (kg·m−2) | −2.0 ± 2.0 | 0.01 | −1.4 ± 2.1 | 0.17 |
| %FM (DEXA) | −4.2 ± 3.9 | 0.01 | −1.9 ± 2.1 | 0.08 |
| Glucose (mg·dL−1) | −3.7 ± 5.7 | 0.07 | 0.0 ± 10.5 | 1.00 |
| Insulin (μIU·mL−1) | 5.7 ± 17.6 | 0.33 | 9.7 ± 16.1 | 0.20 |
| HOMA-IR | 0.9 ± 4.0 | 0.49 | 2.1 ± 3.3 | 0.18 |
| hsCRP (mg·dL −1) | −0.7 ± 3.8 | 0.59 | −1.4 ± 3.4 | 0.35 |
| IL-6 (pg·mL−1) | −1.1 ± 1.7 | 0.07 | −0.6 ± 1.9 | 0.44 |
| Adiponectin (μg·mL−1) | 3.7 ± 4.1 | 0.02 | 2.3 ± 4.2 | 0.24 |
| %IHF | −0.7 ± 3.9 | 0.59 | −1.5 ± 2.5 | 0.19 |
Data grouped by change in V̇O2max (mL·kg−1 ·min−1) at 6 months.
P < 0.001, between groups.
DISCUSSION
Obesity has emerged as a significant pediatric disorder in the United States (2,30). The targeted approaches for improving these trends among children and adolescents have been lifestyle modification (diet, exercise, and behavior) and, more recently, pharmacotherapy, including the insulin-sensitizing drug metformin (22,28,36). We recently reported that 6 months of intensive diet and exercise with metformin resulted in reduced adiposity, and those on diet and exercise alone demonstrated improvements in markers related to inflammation (IL-6, hsCRP), thrombosis (fibrinogen), and IHF contents in obese children with normal glucose tolerance (10,28). In these studies, the addition of metformin did not show added benefit compared with lifestyle intervention alone. These improvements were, however, modest, and subjects did not normalize these outcomes as compared with lean age-matched controls.
In the present study, we observed a modest increase in CRF as measured by V̇O2max in the majority of children (n = 10 of 16) participating in 6 months of lifestyle modification with dietary counseling and structured exercise. In agreement with our previous findings, the addition of metformin did not have additional effects above lifestyle modification alone on CRF. This suggests that in adolescents with simple obesity (obesity without the presence of IGT, impaired fasting glucose, or type 2 diabetes where the use of metformin is clinically indicated), lifestyle intervention alone could limit the need for metformin, which should have clinical implications because the effect of long-term use of pharmacotherapy at such an early age is unknown. Subjects who increased V̇O2max during the 6-month intervention had significant reductions in weight, %BF, and BMI, as well as more favorable changes in adiponectin, IL-6, and blood glucose (Table 2). It is interesting to note that these favorable changes occurred with an average of only one supervised exercise setting per week.
Exercise training is vital to the prevention of CVD. In adolescence, fitness has been shown to independently predict body fatness (16) as well as physical activity behavior in adulthood (25). Moreover, high levels of fitness carried into adulthood are strongly associated with decreased morbidity and mortality attributable to CVD, independent of body fatness (6,31). According to National Heath and Nutrition Examination Survey data, V̇O2max is lower in overweight–obese adolescents age 12–19 yr old compared with their lean counterparts (32). In agreement with National Heath and Nutrition Examination Survey data, the objectively measured values for V̇O2max obtained by subjects in the present study are considerably lower than values reported in comparable samples of lean pubertal adolescents (34,37). Although we observed an encouraging 12.5% mean increase in V̇O2max after 6 months of an intensive exercise program, the fitness of the subjects remained relatively low (Fig. 1).
The finding that metformin provided no additional benefits to improving aerobic fitness above and beyond diet and structured exercise alone is not surprising. However, metformin has been shown to have an inhibitory effect on complex I of the electron transport chain (5), suggesting that maximal exercise capacity may be lower when the drug is taken concomitantly with exercise training. Studies in adults suggest either no effect (21) or a minor negative effect of metformin on exercise capacity (~3% decrease in V̇O2max) (8).
The observed changes in V̇O2max during the intervention were not likely affected by changes in chronological age. Cross-sectional and longitudinal data from Armstrong and Welsman (1) suggest that V̇O2max increases linearly with age in both boys and girls beginning around the age of 10. However, when expressed relative to body mass, V̇O2max remains fairly constant across age in boys but tends to decrease by ~4.0–5.0 mL·kg−1·min−1 in girls during adolescence due in part to an increase in inert fat tissue. These factors probably did not affect our findings, given that the majority of subjects (n = 10), including pubertal females, increased V̇O2max by ~4.0 mL·kg−1·min−1 during the study.
Improvements in fitness in the present study were associated with decreased adiposity (weight, BMI, and %FM; Table 2), whereas no improvement in V̇O2max was associated with weight maintenance. The role of physical activity in weight loss is well documented in adults (11), particularly because aerobic exercise is effective means for inducing a caloric deficit and stimulating fat oxidation both during and after exercise. Although we were not able to quantify exercise intensity, it is plausible that subjects who increased V̇O2max also performed higher intensity exercise sessions. Compared with low- to moderate-intensity exercise, interventions that incorporate higher intensity exercise bouts seem to lead to greater fat loss, even when sessions are calorically equated (18).
Evidence from adult studies suggests a role of physical activity in lowering blood markers related to inflammation. The present study examined the effects of improving fitness on IL-6 and hsCRP and observed a trend for lower concentrations of the proinflammatory cytokine IL-6 but not hsCRP. Few studies have examined the effects of exercise on IL-6 in youth. Cross-sectional data suggest that IL-6 concentrations are higher in sedentary compared with active adolescent girls (19). Balagopal et al. (3) reported a significant decrease in IL-6 after a 3-month lifestyle intervention in obese adolescents aged 14–18. Other studies have reported no change in IL-6 after exercise intervention. The interaction between physical activity, IL-6, and adiposity is complex and requires further investigation.
Improved fitness was also associated with a significant increase in adiponectin concentrations as well as a trend for decreased fasting glucose levels. Both outcomes indicate improved insulin sensitivity. It is well known that exercise training results in improved insulin sensitivity but seems to have a transient effect that lasts between 24 and 48 h after the last exercise bout (17). Adiponectin levels are associated with improved insulin sensitivity in adults, but the role of exercise in modulating circulating adiponectin levels is not clear in children or adolescents. Again, further study will be necessary to elucidate these mechanisms, specifically the manner in which exercise, weight loss, and nontraditional markers of insulin sensitivity (i.e., adiponectin) interact.
The nonsignificant change we observed in IHF during 6 months of DE or DEM may have been a statistical power issue due to such a small sample of adolescents. In our recent randomized controlled trial (10), which includes a much larger cohort of children (including subjects in the present study), we observed a trend (P = 0.09) for decreased IHF in the pubertal DE group but not the DEM group.
This study has some limitations: daily physical activity as measured by pedometer step count was not obtained at baseline; the sample size in each group was small, limiting our availability to analyze gender and pubertal age differences; the study was controlled but did not include placebo; and the intervention was of a relatively short duration (6 months). However, it also has important strengths, including the use of a community YMCA exercise program, frequent dietary counseling, and the measurement of body fatness, V̇O2max, inflammatory factors, and CHO metabolism before and after the intervention.
CONCLUSIONS
In summary, we report that a 6-month lifestyle intervention program results in favorable changes in body composition and aerobic fitness in adolescents with uncomplicated obesity and that the addition of metformin to a combined exercise and diet intervention provides no additional benefits above that of exercise and diet alone. We further report that the favorable changes associated with lifestyle intervention are more pronounced in obese adolescents who increase V̇O2max with training. We conclude that treatment of uncomplicated obesity (i.e., free of comorbidities associated with the metabolic syndrome) should continue to focus on lifestyle modification as the primary intervention and that more aggressive exercise training programs should be designed to increase V̇O2max.
Acknowledgments
This project was supported in part by the Thrasher Research Fund, YMCA, Nemours Research Programs, and a development research donation from W. J. Wadsworth to Nelly Mauras, principal investigator. Corey Rynders, Arthur Weltman, Charles DelGiorno, Prabhakaran Balagopal, Ligeia Damaso, and Kelleigh Killen have no disclosures.
The authors thank Shiela Smith and the expert nursing staff of the Clinical Research Center at the Wolfson Children’s Hospital for the expert care of our patients, Katie Black for research assistance, Mr. Ray Purvis at the YMCA for membership support for our subjects, and all the families and children who participated in these studies.
Footnotes
The study’s clinical trial registration number is NCT00139477.
The authors declare no conflicts of interest.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Armstrong N, Welsman JR. Peak oxygen uptake in relation to growth and maturation in 11- to 17-year-old humans. Eur J Appl Physiol. 2001;85(6):546–51. doi: 10.1007/s004210100485. [DOI] [PubMed] [Google Scholar]
- 2.August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93(12):4576–99. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balagopal P, George D, Patton N, et al. Lifestyle-only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. J Pediatr. 2005;146(3):342–8. doi: 10.1016/j.jpeds.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Barnard ND. Trends in food availability, 1909–2007. Am J Clin Nutr. 2010;91(5):1530–6S. doi: 10.3945/ajcn.2010.28701G. [DOI] [PubMed] [Google Scholar]
- 5.Batandier C, Guigas B, Detaille D, et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38(1):33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 6.Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262(17):2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 7.Boreham C, Twisk J, Murray L, Savage M, Strain JJ, Cran G. Fitness, fatness, and coronary heart disease risk in adolescents: the Northern Ireland Young Hearts Project. Med Sci Sports Exerc. 2001;33(2):270–4. doi: 10.1097/00005768-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Braun B, Eze P, Stephens BR, et al. Impact of metformin on peak aerobic capacity. Appl Physiol Nutr Metab. 2008;33(1):61–7. doi: 10.1139/H07-144. [DOI] [PubMed] [Google Scholar]
- 9.De Jager J, Kooy A, Lehert P, et al. Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2005;257(1):100–9. doi: 10.1111/j.1365-2796.2004.01420.x. [DOI] [PubMed] [Google Scholar]
- 10.DelGiorno C, Weltman A, Damaso L, Killen K, Mauras NM. Aerobic fitness in adolescents with simple obesity: effects of lifestyle modification with and without metformin. Horm Res. 2009;72(Suppl 3):293. [Google Scholar]
- 11.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand: appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 12.DuBose KD, Eisenmann JC, Donnelly JE. Aerobic fitness attenuates the metabolic syndrome score in normal-weight, at-risk-for-overweight, and overweight children. Pediatrics. 2007;120(5):e1262–8. doi: 10.1542/peds.2007-0443. [DOI] [PubMed] [Google Scholar]
- 13.Eisenmann JC. Aerobic fitness, fatness and the metabolic syndrome in children and adolescents. Acta Paediatr. 2007;96(12):1723–9. doi: 10.1111/j.1651-2227.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 14.Eisenmann JC, Katzmarzyk PT, Perusse L, Tremblay A, Despres JP, Bouchard C. Aerobic fitness, body mass index, and CVD risk factors among adolescents: the Quebec family study. Int J Obes (Lond) 2005;29(9):1077–83. doi: 10.1038/sj.ijo.0802995. [DOI] [PubMed] [Google Scholar]
- 15.Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc. 2007;39(8):1251–6. doi: 10.1249/MSS.0b013e318064c8b0. [DOI] [PubMed] [Google Scholar]
- 16.Eisenmann JC, Wickel EE, Welk GJ, Blair SN. Relationship between adolescent fitness and fatness and cardiovascular disease risk factors in adulthood: the Aerobics Center Longitudinal Study (ACLS) Am Heart J. 2005;149(1):46–53. doi: 10.1016/j.ahj.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Frosig C, Richter EA. Improved insulin sensitivity after exercise: focus on insulin signaling. Obesity (Silver Spring) 2009;17(3 suppl):S15–20. doi: 10.1038/oby.2009.383. [DOI] [PubMed] [Google Scholar]
- 18.Irving BA, Davis CK, Brock DW, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40(11):1863–72. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ischander M, Zaldivar F, Jr, Eliakim A, et al. Physical activity, growth, and inflammatory mediators in BMI-matched female adolescents. Med Sci Sports Exerc. 2007;39(7):1131–8. doi: 10.1249/mss.0b013e318053e7a2. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Pavon D, Kelly J, Reilly JJ. Associations between objectively measured habitual physical activity and adiposity in children and adolescents: systematic review. Int J Pediatr Obes. 2010;5(1):3–18. doi: 10.3109/17477160903067601. [DOI] [PubMed] [Google Scholar]
- 21.Johnson ST, Robert C, Bell GJ, Bell RC, Lewanczuk RZ, Boule NG. Acute effect of metformin on exercise capacity in active males. Diabetes Obes Metab. 2008;10(9):747–54. doi: 10.1111/j.1463-1326.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamath CC, Vickers KS, Ehrlich A, et al. Clinical review: behavioral interventions to prevent childhood obesity: a systematic review and metaanalyses of randomized trials. J Clin Endocrinol Metab. 2008;93(12):4606–15. doi: 10.1210/jc.2006-2411. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Kuk JL, Katzmarzyk PT, Blair SN, Church TS, Ross R. Cardiorespiratory fitness attenuates metabolic risk independent of abdominal subcutaneous and visceral fat in men. Diabetes Care. 2005;28(4):895–901. doi: 10.2337/diacare.28.4.895. [DOI] [PubMed] [Google Scholar]
- 24.Lima LM, Wiernsperger N, Kraemer-Aguiar LG, Bouskela E. Short-term treatment with metformin improves the cardiovascular risk profile in first-degree relatives of subjects with type 2 diabetes mellitus who have a metabolic syndrome and normal glucose tolerance without changes in C-reactive protein or fibrinogen. Clinics (Sao Paulo) 2009;64(5):415–20. doi: 10.1590/S1807-59322009000500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malina RM. Tracking of physical activity and physical fitness across the lifespan. Res Q Exerc Sport. 1996;67(3 suppl):S48–57. doi: 10.1080/02701367.1996.10608853. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Mauras N, Delgiorno C, Kollman C, et al. Obesity without established comorbidities of the metabolic syndrome is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. 2010;95(3):1060–8. doi: 10.1210/jc.2009-1887. [DOI] [PubMed] [Google Scholar]
- 28.McGovern L, Johnson JN, Paulo R, et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93(12):4600–5. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 29.McNamara E, Hudson Z, Taylor SJ. Measuring activity levels of young people: the validity of pedometers. Br Med Bull. 2010;95:121–37. doi: 10.1093/bmb/ldq016. [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 31.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 32.Pate RR, Wang CY, Dowda M, Farrell SW, O’Neill JR. Cardio-respiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999–2002 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2006;160(10):1005–12. doi: 10.1001/archpedi.160.10.1005. [DOI] [PubMed] [Google Scholar]
- 33.Pradhan AD, Everett BM, Cook NR, Rifai N, Ridker PM. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302(11):1186–94. doi: 10.1001/jama.2009.1347. [DOI] [PubMed] [Google Scholar]
- 34.Rizzo NS, Ruiz JR, Hurtig-Wennlof A, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: the European youth heart study. J Pediatr. 2007;150(4):388–94. doi: 10.1016/j.jpeds.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Robertson RJ, Goss FL, Boer NF, et al. Children’s OMNI scale of perceived exertion: mixed gender and race validation. Med Sci Sports Exerc. 2000;32(2):452–8. doi: 10.1097/00005768-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 36.Rogovik AL, Chanoine JP, Goldman RD. Pharmacotherapy and weight-loss supplements for treatment of paediatric obesity. Drugs. 2010;70(3):335–46. doi: 10.2165/11319210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz JR, Ortega FB, Rizzo NS, et al. High cardiovascular fitness is associated with low metabolic risk score in children: the European Youth Heart Study. Pediatr Res. 2007;61(3):350–5. doi: 10.1203/pdr.0b013e318030d1bd. [DOI] [PubMed] [Google Scholar]
- 38.Stocker DJ, Taylor AJ, Langley RW, Jezior MR, Vigersky RA. A randomized trial of the effects of rosiglitazone and metformin on inflammation and subclinical atherosclerosis in patients with type 2 diabetes. Am Heart J. 2007;153(3):445, e1–6. doi: 10.1016/j.ahj.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman FJ, Bell JF. Associations of television content type and obesity in children. Am J Public Health. 2009;100(2):334–40. doi: 10.2105/AJPH.2008.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]