Abstract
Background
The Dog erythrocyte antigen (DEA) 1 blood group system was thought to contain types DEA 1.1 and 1.2 (and possibly 1.3 [A3]). However, DEA 1.2+ dogs are very rare and newer typing methods reveal varying degrees of DEA 1 positivity.
Objectives
To assess if variation in DEA 1 positivity is because of quantitative differences in surface antigen expression. To determine expression patterns in dogs over time and effects of blood storage (4°C). To evaluate DEA 1.2+ samples by DEA 1 typing methods.
Animals
Anticoagulated blood samples from 66 dogs in a research colony and from a hospital, and 9 previously typed DEA 1.2+ dogs from an animal blood bank.
Methods
Research study: Samples were analyzed by flow cytometry and immunochromatographic strip using a monoclonal anti‐DEA 1 antibody.
Results
Twenty dogs were DEA 1−, whereas 46 dogs were weakly to strongly DEA 1+. Antigen quantification revealed excellent correlation between strip and flow cytometry (r = 0.929). Both methods reclassified DEA 1.2+ samples as weakly to moderately DEA 1+, but they were not retyped with the polyclonal anti‐DEA 1.1/1.X antibodies. Dogs and blood samples retained their relative DEA 1 antigen densities over time.
Conclusions and Clinical Importance
The blood group system DEA 1 is a continuum from negative to strongly positive antigen expression. Previously typed DEA 1.2+ appears to be DEA 1+. These findings further the understanding of the DEA 1 system and suggest that all alleles within the DEA 1 system have a similarly based epitope recognized by the monoclonal antibody.
Keywords: Blood groups, Blood typing, Crossmatching, Hemolytic reaction
Abbreviations
- ABRI
Animal Blood Resources International
- DEA
dog erythrocyte antigen
- EDTA
ethylenediaminetetraacetic acid
- FACS
fluorescence‐activated cell sorter
- FITC
fluorescein isothiocyanate
- FSC
forward scatter
- MFI
mean fluorescence intensity
- PBS
phosphate buffered saline
- RBC
red blood cell
- SSC
side scatter
Immunohematologic studies over half a century suggest the existence of at least a dozen blood group systems in dogs. Experimental transfusions and alloantibody studies led to the initial international recognition of 7 blood groups, termed dog erythrocyte antigens (DEA).1, 2, 3 Acute hemolytic transfusion reactions against DEA 1.1 and 4, Dal, and other red, cell antigens are observed in dogs previously sensitized by transfusion.4, 5, 6, 7, 8, 9 Currently, polyclonal typing reagents are only available on a limited basis for DEA 1.1, 3, 4, and 5, and Dal, and there are only a couple of monoclonal DEA 1.1 antibodies used in blood typing kits.2, 4, 5, 10, 11 Because of the relative paucity of typing reagents, little is known about the biochemistry and molecular genetics of blood group systems in dogs.5, 12
While most blood group systems in dogs are thought to be simple 2 allele systems with a positive and negative blood type, the DEA 1 blood group system differs. Based upon 2 polyclonal typing reagents (anti‐DEA 1.1 and 1.X) raised in dogs, the DEA 1 system includes at least 2 types, DEA 1.1 and DEA 1.2. The DEA 1.1 antigen appears to be dominant to DEA 1.2, such that only a dog that is DEA 1.1− can be DEA 1.2+.4, 10 In addition, a DEA 1.3(A3) antigen has been proposed in 1 study, but reagents are not available for further comparison.13 The prevalence of DEA 1.1+ dogs varies both geographically and among breeds from 100% to <10% DEA 1.1+ dogs, but has been estimated at ~50% overall internationally.2
The proportion of DEA 1.2+ dogs was originally described at ~20% and then 7% in the United States, but currently DEA 1.2+ dogs are very rarely found.1 , 5, 14, 15, 16 Recently, flow cytometry with the anti‐DEA 1.X polyclonal antibody was used experimentally to type erythrocytes for DEA 1.1, but DEA 1.1 and 1.2 expression levels or their variation among dogs were not examined.17 Originally, DEA 1.1 typing was done with a polyclonal DEA 1.1 antiserum derived by alloimmunizing dogs with different blood types; this reagent was a weak agglutinin and required canine antiglobulin (Coombs') reagent to better visualize the agglutination reaction in the tube or microtiter assay.5, 6, 10, 13 Two murine monoclonal anti‐DEA 1.1 antibodies, introduced in the 1990s, are used in typing cards,2 gel columns,3 and immunochromatographic strips.4 , 13, 17, 18, 19, 20, 21 It has been suspected that DEA 1.2+ blood gives a weakly positive DEA 1.1 result.13 Because these monoclonal antibodies were never properly evaluated against dogs which tested DEA 1.1+ or DEA 1.2+, we will refer to them as anti‐DEA 1 rather than anti‐DEA 1.1 antibodies. Furthermore, while these kits provide reliable DEA 1 typing results, the observed agglutination or binding (chromatographic) reactions vary from strongly to weakly positive to negative.
In this study, we used flow cytometry and immunochromatographic strip (with densitometry) techniques to further assess the DEA 1 expression among dogs. On the basis of the use of a single anti‐DEA 1 antibody, we hypothesized that the variation in the DEA 1 system is quantitative rather than qualitative, involving the same DEA 1 epitope with different surface expression. To test this hypothesis, we determined (1) if DEA 1 expression varied among dogs, (2) if DEA 1.2+ blood typed as DEA 1+, and (3) if DEA 1 is stably expressed in each animal and (4) not affected by red blood cell (RBC) storage in ethylenediaminetetraacetic acid (EDTA).22
Materials and Methods
Animals and Samples
Sixty‐six dogs were studied with samples collected from a research dog colony at the School of Veterinary Medicine and obtained as leftover samples from dogs at the Clinical Pathology Laboratory at the Veterinary Hospital of the University of Pennsylvania. Samples from DEA 1.2+ dogs were sent for typing from Animal Blood Resources International (ABRI).1 Blood (1–10 mL) was collected from the blood donor and research colony dogs in EDTA‐anticoagulated tubes and stored at 4°C before initial analysis the same day or as specified on later dates at the PennGen Laboratories of the University of Pennsylvania, Philadelphia, PA. In the case of leftover samples from clinical cases, 1–3 mL EDTA‐anticoagulated blood was stored for ≤3 days at 4°C before analysis and those from ABRI were tested within <8 days. All samples were kept stored at 4°C in their original EDTA collection tubes. The studies were approved by the institutional animal care and use committee.
DEA 1 Blood Typing by Immunochromatographic Strip and Flow Cytometry
After the preparation of 20% RBC suspensions from each blood sample, blood typing was performed by the immunochromatographic strip technique, which only differs from the previously described cartridge method in terms of packaging (strip alone versus strip in a cartridge).19 The band strength was read on a scale from 0 (no band) to 4+ (as strong as control band) by one author (MA) before densitometric analysis of the strip (Fig 1). The subjective scale was based on previous typing modalities (tube and cartridge methods) used routinely in the laboratory. For flow cytometry, diluted monoclonal murine anti‐DEA 1 antibody (identical to the antibody impregnated on the immunochromatographic strip) was incubated with a 10% RBC suspension washed with phosphate buffered saline (PBS) followed by labeling with fluorescein isothiocyanate (FITC) conjugated polyclonal goat antimouse antibody.5 Flow analysis was performed on a FACSCalibur and the data were analyzed with CellQuest Pro software6 , 7 (Fig 2). Detailed protocol information can be found in Data S1.
Figure 1.
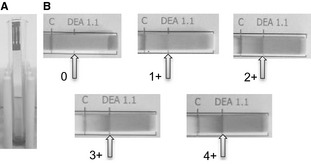
Immunochromatographic typing strip. (A) Example of a test in progress as the erythrocytes are diffusing up the strip to bind to the control and DEA 1 binding sites. (B) Blood samples, corrected to a PCV of 20%, were assigned to a subjective category based on band strength, ranging from 0 (negative) to 4+ (strongly positive).
Figure 2.
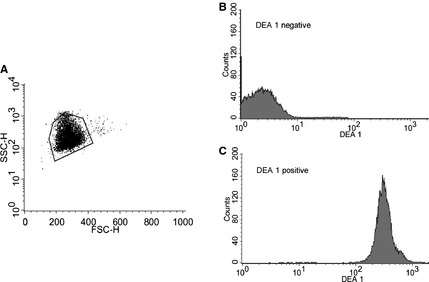
DEA 1 blood typing by flow cytometry. (A) Electronic settings were initially adjusted to give a reproducible erythrocyte population cluster. Then samples were gated using the same DEA 1 negative sample during each run. (B) A typical tracing obtained from a DEA 1 negative and (C) strongly DEA 1 positive sample.
DEA 1 Expression Analysis
Samples from 6 dogs with DEA 1 band strength ranging from 0 to 4+ were collected in EDTA tubes 6 times over 42 days. Blood was analyzed by the above protocols the day of collection. Data were compared over time to assess expression patterns of DEA 1 density on erythrocytes in dogs.
Analysis of the Effect of Storage on DEA 1 Expression
Samples from 6 dogs with DEA 1 band strength ranging from 0 to 4+ (same dogs as expression analysis above) were collected in 10 mL EDTA tubes and analyzed immediately by the above protocol. After initial analysis by immunochromatographic strip, densitometry, and flow cytometry, the remaining blood was stored at 4°C in the original collection tubes. Subsequent analyses were performed 4 more times over a 41 day period as above. Data were compared over time to assess any effects of storage on DEA 1 density of the samples over the 41 days.
Statistical Analysis
Data from the immunochromatographic strip (subjective and densitometric) and flow cytometry were statistically compared with Pearson product moment correlation. Probability values P < .01 were considered statistically significant.
Results
DEA 1 Blood Typing by Immunochromatographic Strip and Densitometry
Of the PCV‐adjusted blood samples from 66 purebred and mixed‐breed dogs prospectively tested at PennGen by the novel immunochromatographic strip technique, 20 were typed as DEA 1− and 46 were typed as DEA 1+ by visual examination. Instructions for the strip typing method were simple, the assay was easy to perform, and took <10 minutes per sample, and the control band was uniformly strong. The band strength of 46 DEA 1+ samples varied in the binding intensity despite adjustments of the PCV, whereas the DEA 1− were completely negative (Fig 1). The correlation between band strength, as determined by densitometry, and the subjective visual categorization of 0 to 4+ was statistically significant (P < .01). The overall intensity of the test bands ranged from 0 to 116% of the strip's control band, as quantified by densitometry (Table 1).
Table 1.
Comparison of flow cytometry and densitometry results
| Immunochromatographic Strip‐Subjective Category | Number of Dogs/Samples | Densitometry (units) | Flow Cytometry | |||
|---|---|---|---|---|---|---|
| FACSCalibur I | FACSCalibur II | |||||
| n | MFI | n | MFI | |||
| 0 | 20/28 | 0.0 (0.0–0.3) | 22 | 2.6 (2.18–3.77) | 6 | 3.3 (3.15–3.5) |
| 1+ | 10/18 | 26.7 (10.8–50) | 8 | 25.0 (20.7–35.46) | 10 | 42.5 (33.4–55.05) |
| 2+ | 7/15 | 49.2 (26.4–67) | 4 | 77.2 (58.4–109.0) | 11 | 107.1 (36.8–125.8) |
| 3+ | 20/30 | 72.2 (42.4–89.8) | 17 | 161.9 (115.7–210.8) | 13 | 347.8 (107.4–663.6) |
| 4+ | 9/27 | 89.9 (72.2–116) | 12 | 280.3 (225.3–331.1) | 15 | 504.0 (256.4–683.6) |
Flow Cytometry Typing for DEA 1
Consistent with the DEA 1 blood typing results obtained by the strip method, flow cytometry results of the RBCs from 66 dogs typed showed wide variation in mean fluorescence intensity (MFI) (Fig 3) that correlated significantly with the chromatographic strip results (Fig 4). The histograms show narrow peaks indicating uniform populations of labeled cells. Those that were DEA 1− fell between 1 and 10, the range of unlabeled RBCs. All samples that were typed as DEA 1+ by the strip had MFIs that were well above 10. It appeared that there was a gap in MFI between the negative and weakly positive samples, with none of the 1+ samples falling between an MFI of 4 and 20. Among DEA 1+ samples, there was great variation between and within the subjective categories preassigned by the strip method and the maximal MFI was nearly 700. The chromatographic and flow typing results of a representative sample from each category are plotted in Figure 3 for easy comparison.
Figure 3.
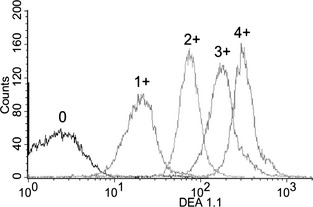
DEA 1 expression among different dogs by flow cytometry. DEA 1− and DEA 1+ dogs were easily distinguished. Among DEA 1+ dogs, there was a large variation along a continuum for antigen level.
Figure 4.
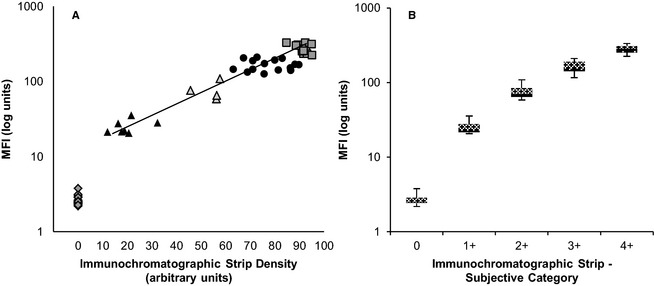
Comparison between subjective and densitometric DEA 1 blood typing and flow cytometric techniques. (A) Correlation between densitometry of the immunochromatographic strip and flow cytometry was excellent (r = 0.929). diamond = 0, filled triangle = 1 +, empty triangle = 2+, filled circle = 3+, empty square = 4+. (B) Correlation between subjective (0–4+) and densitometric immunochromatographic strip results showed a strong clustering of samples.
DEA 1 Expression over Time in Dogs
The degree of DEA 1 expression on erythrocytes from 6 dogs tested 6 times remained practically unchanged over the course of 42 days, as analyzed by flow cytometry (Fig 5A).
Figure 5.
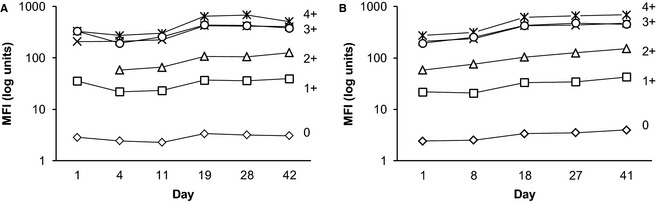
Longitudinal studies of DEA 1 expression as analyzed by immunochromatographic strip and flow cytometry. (A) Over a period of 42 days, 6 separate samples collected from 6 dogs gave a constant level of antigen expression. (B) Samples from the same 6 dogs when stored at 4°C for 41 days maintained DEA 1 expression. For both studies, 2 different FACSCaliburs had to be used, starting on day 19 for (A) and day 18 for (B) which accounts for the small artificial increase in DEA 1 expression across all samples.
Effects of Storage on DEA 1 Expression
Blood samples stored at 4°C retained their relative antigen density over the course of 41 days, as analyzed by flow cytometry on a weekly basis (Fig 5B).
Blood Typing of DEA 1.2+ Dogs
Of the 9 samples that were previously typed by ABRI as DEA 1.2+, all were classified as DEA 1+ with the immunochromatographic strip method and by flow cytometry. Interestingly, all samples were weakly to moderately DEA 1+ by both typing methods, with 4 samples being 2+ (moderately) and 5 samples only 1+ (weakly positive) for DEA 1. The monoclonal flow cytometry results were once again in good agreement, with the MFI ranging from 39 to 109 of an overall observed range from 1 to 691.
Discussion
In the past the DEA 1 blood group system was identified and assessed by polyclonal alloantibodies and was thought to be composed of 2–3 types known as DEA 1.1, 1.2, and possibly 1.3 (A3), with decreasing antigen strengths.2, 8, 9, 13 Utilizing quantitative flow cytometry and an immunochromatographic technique with one monoclonal anti‐DEA 1 alloantibody, we found greatly varied, but stable quantitative DEA 1 expression on erythrocytes in 66 dogs. This continuum of DEA 1− to weakly to strongly DEA 1+ is in sharp contrast to the originally described DEA 1 system. This broad range of DEA 1 expression appears similar to erythrocytic antigen expression of the human Rh system, for instance, and will likely change blood typing practices and incompatibility assessments when transfusing dogs.23Although varied agglutination and band strength reactions were previously noted after performance of DEA 1.1 blood typing with tube, card, strip, or column methods using polyclonal or monoclonal anti‐DEA 1.1 antibodies, they were mostly ascribed to differences in sample PCVs and variation in test performance.19, 24 By quantitatively assessing antigen binding of erythrocytes at a fixed PCV of 20% and antigen expression of single RBCs by flow cytometry, we were able to exclude differences in RBC concentration as the cause for varied DEA 1 reaction strengths. In addition, DEA 1 typing results remained unaffected by blood storage in EDTA at 4°C or repeated collection over a 42 day period from healthy dogs. Thus, the observed variation in DEA 1 expression is stable in individual dogs over time and is not altered by prolonged blood storage. While the samples were stored in EDTA, it is likely that the same constant DEA 1 expression is observed in citrated and preservative solutions used in blood banking.
While the number of samples tested in this study was limited, samples covered a wide spectrum from DEA 1− to weakly to strongly DEA 1+. And although there was a clear distinction between DEA 1− and weakly DEA 1+ samples, we observed a continuum from samples that reacted weakly (1+) to strongly (4+) positive by chromatography (subjective and densitometric) and flow cytometry. Using the same monoclonal anti‐DEA 1 antibody, there was a significant correlation between test results from both methods, with a complete agreement between strip and flow methods categorizing dogs as DEA 1+ and DEA 1−. We did not assess the monoclonal anti‐DEA 1 antibodies from Kansas State University that are used in the DEA 1.1 typing cards.2 However, weak and strong typing reactions have also been observed with that typing kit.13, 19
Although we did not directly identify any DEA 1.2+ dogs, the 9 dogs which were previously typed as DEA 1.2+ (and thus DEA 1.1−) by the reference laboratory1 for extended blood typing in dogs, typed as weakly to moderately DEA 1+ by both methods with the monoclonal antibody used in this study. Searches for additional DEA 1.2+ dogs at our laboratory and the reference laboratory1 by the tube method were also unsuccessful. The tube or microtiter typing method used to differentiate DEA 1.1+ from DEA 1.2+ is not robust, frequently gives weak agglutination reactions that can readily break up, and requires Coombs' reagent to potentially better identify the agglutination reaction, leaving some doubts when reading the results. Because polyclonal antibodies are inherently variable, the agglutination reaction also seems different depending on the batch of antisera used. Moreover, the DEA 1.3 (A3) type was only described in 1 study and anti‐DEA 1.3 (A3) antibodies are not available.13 Hence, we conclude that a more appropriate typing scheme for the DEA 1 system would be simply DEA 1− and DEA 1+ with weak to strong antigen expression (if a standardized PCV of 20% is used), thus eliminating the poorly defined DEA 1.2 and 1.3 (A3) types.
Little is known about the biochemical and molecular basis of the DEA 1 blood group system. Few studies have examined membrane proteins from DEA 1+ and DEA 1− erythrocytes and different apparent molecular weights for DEA 1 have been obtained, depending on the antibody and electrophoretic methods used.9, 12, 13 By immunoblotting with a monoclonal antibody from Kansas State University, DEA 1.1 appeared to run as a 50 and 200 kDa band,12 whereas studies using less specific antisera identified a protein band at 85 kD in a DEA 1.2+ dog.13 Those studies were not extended any further and clearly, additional work is required to define the biochemical and molecular genetic characteristics of this erythrocytic antigen.
The DEA 1 blood group in dogs and the Rh blood group system in humans bear some similarities, including the presence of weakly to strongly Rh+ individuals because of a quantitative polymorphism that dictates the amount of Rh D antigen on the erythrocyte membrane.1 Rh30‐like polypeptides, which are Rh‐related integral membrane proteins with molecular mass of 33 kDa, have been detected in the erythrocyte membranes of both dogs and humans.25 Interestingly, a monoclonal antibody specific for an epitope on the human erythrocyte Rh D surface antigen immunoprecipitated a protein from canine erythrocyte membranes with the molecular weight of 33 kDa.26 The amount of Rh D antigen, encoded by the RHD and RhCE genes, dictates the Rh phenotype (weak to strong) observed in humans.27 The Rh system has only recently been defined at the molecular level to involve 2 genes with multiple alleles, and varied expression and antigenicity have been found.23 There are also other blood group systems with varied degree of antigen expression in humans, such as the ABO system.23 Studies with the monoclonal anti‐DEA 1 antibody used here are needed to further define the DEA 1 antigen(s).
Finally, little is known about the inheritance of the DEA 1 blood group system: DEA 1.1+ is considered dominant over DEA 1.2+. While in certain breeds DEA 1.1+ is predominant, in other breeds different proportions of DEA 1.1+ and DEA 1.1− dogs are observed.8 However, these surveys were done with the polyclonal and not monoclonal antibodies and thus do not provide information on the degree of DEA 1 expression. Based on the varied DEA 1+ expression, families with weakly to strongly DEA 1+ and DEA 1− dogs need to be investigated. Ultimately, molecular characterization of these molecules is required to completely understand the genetics of the DEA 1 system and show similarities to any human blood group system.
The discoveries in the study presented here have several important and immediate clinical implications. Because of the close correlation between strip and flow data, we recommend that typing results be recorded not only as DEA 1+ or DEA 1− as currently outlined by the manufacturer's guidelines, but include the degree of DEA 1+ (weak to strong). This grading will likely require standardizing the amount of erythrocytes used in an assay, ie, set the PCV to 20% for comparison (washing of RBCs is not necessary for in‐clinic typing); and there is no need to type for DEA 1.2+ dogs, but one has to be diligent to detect the weak DEA 1+ reactions by the chromatographic strip technique. The commercial reference laboratory in the United States1 for extended typing no longer offers routine DEA 1.2 typing as of 2012, based upon them not identifying any DEA 1.2+ dogs over the past years and our study results that retyped their DEA 1.2+ dogs as DEA 1+.
There is experimental and clinical evidence in the literature that strong DEA 1+ erythrocytes (from dogs currently typed as DEA 1.1+) will trigger an immune response in DEA 1− dogs.5 Interestingly, there are no clinical reports of any hemolytic transfusion reactions because of DEA 1.2 incompatibility, but in early experimental studies DEA 1.2+ blood given to DEA 1.2− dogs apparently elicited an incompatibility reaction.16 Evaluation of the immune responses to mismatched transfusions based upon varied DEA 1 expression is needed to see if there are differences between weakly to strongly positive dogs.
The DEA 1 expression remains constant in healthy dogs, and thus a single typing should definitively determine the dog's blood type. However, because of typing and clerical errors, it might still be advisable to repeat typing at each transfusion event (as in humans), and crossmatching on subsequent transfusions >4 days from the first transfusion to assure blood compatibility related to other blood groups.
Future studies will need to answer the clinically important question: Do weakly to strongly DEA 1+ erythrocytes elicit a similarly severe transfusion reaction in DEA 1− dogs or not? Clearly DEA 1− dogs should only receive DEA 1− blood and for now any donor of any degree of DEA 1 positivity should be considered DEA 1+. However, it is likely that some of the weakly DEA 1+ (including DEA 1.2+) dogs were typed as DEA 1− in the past which could have affected blood compatibility. Because it has been suggested that DEA 1− dogs will mount immune responses against weakly DEA 1+ erythrocytes, we recommend classifying any weakly to moderately DEA 1+ donor dog as DEA 1+.16 In addition, it is advisable to transfuse weakly DEA 1+ dogs with DEA 1− blood, as it is yet unclear if weakly DEA 1+ dogs could mount an alloantibody response when given strongly DEA 1+ erythrocytes. Labeling weakly DEA 1+ dogs as DEA 1+ will undoubtedly reduce the DEA 1− donor pool, making the use of both DEA 1− and DEA 1+ blood donors critical. Additional studies are clearly warranted to define the inheritance mode and biochemical and molecular basis of the DEA 1 system.
Supporting information
Data S1.
Acknowledgments
The monoclonal DEA 1 antibody and immunochromatographic typing kits were kindly provided by Alvedia, Lyon, France. The assistance with blood samples by ABRI, Dixon, CA, and the staff in the clinical laboratory and research colony at the University of Pennsylvania are also thanked.
Conflict of Interest Declaration: Urs Giger has been a scientific advisor to Alvedia and reagents were received for these studies. However, the design and execution of the study and writing the manuscript has been done entirely independently.
This study was supported in part by NIH OD 010939 and the veterinary scholars program from NIH 2T35 OD 010919 and from Merial
Footnotes
Animal Blood Resources International, Dixon, CA
RapidVet‐H from DMS Laboratories, Inc, Flemington, NJ
DiaMed, Cressier, Switzerland
Alvedia, Lyon, France
Dakocytomation, Glostrup, Denmark
Becton Dickinson & Co, Franklin Lakes, NJ
Syngene USA, Frederick, MD
References
- 1. Vriesendorp HM, Albert ED, Templeton JW, et al. Joint report of the Second International Workshop on Canine Immunogenetics. Transplant Proc 1976;8:289–314. [PubMed] [Google Scholar]
- 2. Bell K. Blood groups of domestic animals In: Agar N, Board D, eds. Red Blood Cells of Domestic Mammals. Amsterdam: Elsevier Press; 1983:137–164. [Google Scholar]
- 3. Smith JE. Erythrocytes. Adv Vet Sci Comp Med 1991;36:9–55. [DOI] [PubMed] [Google Scholar]
- 4. Giger U. Blood typing and crossmatching to ensure compatible transfusions In: Bouagusa JD, Twedt D, eds. Kirk's Current Veterinary Therapy, 15th ed St. Louis, MO: :Elsevier Saunders; 2014:e143. [Google Scholar]
- 5. Giger U, Gelens CJ, Callan MB, et al. An acute hemolytic transfusion reaction caused by dog erythrocyte antigen 1.1 incompatibility in a previously sensitized dog. J Am Vet Med Assoc 1995;206:1358–1362. [PubMed] [Google Scholar]
- 6. Callan MB, Jones LT, Giger U. Hemolytic transfusion reactions in a dog with an alloantibody to a common antigen. J Vet Intern Med 1995;9:277–279. [DOI] [PubMed] [Google Scholar]
- 7. Melzer KJ, Wardrop KJ, Hale AS, et al. A hemolytic transfusion reaction due to DEA 4 alloantibodies in a dog. J Vet Intern Med 2003;17:931–933. [PubMed] [Google Scholar]
- 8. Hale AS. Canine blood groups and their importance in veterinary transfusion medicine. Vet Clin North Am Small Anim Pract 1995;25:1323–1332. [DOI] [PubMed] [Google Scholar]
- 9. Hohenhaus AE. Importance of blood groups and blood group antibodies in companion animals. Transfus Med Rev 2004;18:117–126. [DOI] [PubMed] [Google Scholar]
- 10. Blais MC, Berman L, Oakley DA, et al. Canine Dal blood type: A red cell antigen lacking in some Dalmatians. J Vet Intern Med 2007;21:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Symons M, Bell K. Expansion of the canine A blood group system. Anim Genet 1991;22:227–235. [DOI] [PubMed] [Google Scholar]
- 12. Corato A, Mazza G, Hale AS, et al. Biochemical characterization of canine blood group antigens: Immunoprecipitation of DEA 1.2, 4 and 7 and identification of a dog erythrocyte membrane antigen homologous to human Rhesus. Vet Immunol Immunopathol 1997;59:213–223. [DOI] [PubMed] [Google Scholar]
- 13. Andrews GA, Chavey PS, Smith JE. Production, characterization, and applications of a murine monoclonal antibody to dog erythrocyte antigen 1.1. J Am Vet Med Assoc 1992;201:1549–1552. [PubMed] [Google Scholar]
- 14. Swisher S, Bull R, Bowdler J. Canine erythrocyte antigens. Tissue Antigens 1973;3:164–165. [Google Scholar]
- 15. Suzuki Y, Stormont C, Morris BG, et al. New antibodies in dog blood groups. Transplant Proc 1975;7:365–367. [PubMed] [Google Scholar]
- 16. Swisher SN, Young LE. The blood grouping systems of dogs. Physiol Rev 1961;41:495–520. [DOI] [PubMed] [Google Scholar]
- 17. de A Lucidi C, Takahira RK, Gerlach JA, et al. Flow cytometric assessment of canine erythrocytes and platelets for dog erythrocyte antigen 1.1. Vet Clin Pathol 2011;40:435–443. [DOI] [PubMed] [Google Scholar]
- 18. Kessler RJ, Reese J, Chang D, et al. Dog erythrocyte antigens 1.1, 1.2, 3, 4, 7, and Dal blood typing and cross‐matching by gel column technique. Vet Clin Pathol 2010;39:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seth M, Jackson KV, Winzelberg S, et al. Comparison of gel column, card, and cartridge techniques for dog erythrocyte antigen 1.1 blood typing. Am J Vet Res 2012;73:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohn B, Classe G, Weingart C. Clinical evaluation of the QuickVet/RapidVet canine dog erythrocyte antigen 1.1 blood‐typing test. J Vet Diagn Invest 2012;24:539–545. [DOI] [PubMed] [Google Scholar]
- 21. Blois SL, Richardson DM, Abrams‐Ogg AC. Comparison of a gel column blood typing method and a point‐of‐care cartridge for Dog Erythrocyte Antigen 1.1. J Vet Emerg Crit Care (San Antonio) 2013;23:340–343. [DOI] [PubMed] [Google Scholar]
- 22. Patterson J, Rousseau A, Kessler RJ, et al. In vitro lysis and acute transfusion reactions with hemolysis caused by inappropriate storage of canine red blood cell products. J Vet Intern Med 2011;25:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reid M, Lomas‐Francis C, Olsson M. The Blood Group Antigen Facts Book, 3rd ed New York: Elsevier; 2012. [Google Scholar]
- 24. Giger U, Stieger K, Palos H. Comparison of various canine blood‐typing methods. Am J Vet Res 2005;66:1386–1392. [DOI] [PubMed] [Google Scholar]
- 25. Apoil P, Blancher A. Sequences and evolution of mammalian RH gene transcripts and proteins. Immunogenetics 1999;49:15–25. [DOI] [PubMed] [Google Scholar]
- 26. Paire J, Monestier M, Rigal D, et al. Establishment of human cell lines producing anti‐D monoclonal antibodies: Identification of rhesus D antigen. Immunol Lett 1986;13:137–141. [DOI] [PubMed] [Google Scholar]
- 27. Wagner FF, Gassner C, Muller TH, et al. Molecular basis of weak D phenotypes. Blood 1999;93:385–393. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.


