Abstract
The hypothesis that single low dose exposures (0.025–0.5 Gy) to low LET radiation, given at either high (about 150 mGy/min) or low (1 mGy/min) dose rate, would promote aortic atherosclerosis was tested in female C57BL/6J mice genetically predisposed to this disease (ApoE−/− ). Mice were exposed either at early stage disease (2 months of age) and examined 3 or 6 months later, or at late stage disease (8 months of age) and examined 2 or 4 months later. Changes in aortic lesion frequency, size and severity, as well as total serum cholesterol levels and the uptake of lesion lipids by lesion associated macrophages were assessed. Statistically significant changes in each of these measures were observed, depending on dose, dose rate and disease stage. In all cases, the results were distinctly non-linear with dose, with maximum effects tending to occur at 25 or 50 mGy. In general, low doses given at low dose rate at either early or late stage disease were protective, slowing the progression of the disease by one or more of these measures. Most effects appeared and persisted for months after the single exposures, but some were ultimately transitory. In contrast to exposure at low dose rate, high dose rate exposure at early stage disease produced both protective and detrimental effects, suggesting that low doses may influence this disease by more than one mechanism, and dose rate is an important parameter. These results contrast with the known, generally detrimental effects of high doses on the progression of this disease in the same mice, and in humans, suggesting that a linear extrapolation of the known increased risk from high doses to low doses is not appropriate.
Key words for indexing: Radiation, low doses, heart disease, atherosclerosis, mice, apolipoprotein E
INTRODUCTION
Studies on non-cancer mortality in the Japanese atomic bomb survivors have shown a statistically significant increase in non-cancer disease death rates with radiation dose, with increasing trends observed for diseases of the circulatory, digestive and respiratory systems (1, 2). The data show statistically significant increases for heart disease, but no direct evidence of radiation effects for doses less than about 0.5 Sv (3, 4). Another study examined the risk of coronary heart disease following radiotherapy for peptic ulcer (5). Part of the heart was in the irradiated tissue volume and received very high doses of 7.6–18.4 Gy, corresponding to volume-weighted cardiac doses ranging from 1.6 to 3.9 Gy. The authors concluded that disease risk increased with dose. Similarly, childhood exposure of the scalp for Tinea Capitis has been shown to significantly elevate risk for adult carotid atherosclerosis disease (6). The mean dose to the heart was not described but could be either large, since one report of this exposed population estimated a mean dose to the brain of 1.5 Gy (7) or relatively small, since another report estimated a dose of 9 cGy to the thyroid (8).
The position regarding occupational exposure is less clear. In one report, a strong positive and statistically significant association between radiation dose (mean dose ~30 mSv) and deaths from atherosclerotic heart disease, including coronary heart disease was observed in a cohort of occupationally exposed workers (9). Assuming that a positive correlation of excess risk of cardiovascular disease at low radiation doses existed, Little et al. (10) produced a mechanistic model where multiple small radiation doses cause mean chemo-attractant (MCP-1) concentration to increase linearly with cumulative dose, consistent with a linear relationship between risk and dose at low doses. However, a review of the epidemiological literature, other than the literature on atomic bomb survivors, did not provide clear evidence of an increased risk of circulatory diseases at doses of ionizing radiation in the range 0–4 Sv (11). In contrast, other epidemiological evidence indicates that exposures to low LET radiation (60Co-γ) at very low dose (median dose, 35.8 mGy) and dose rate (median dose rate, 2.80 mGy/y) protected workers (p<0.05) generally from all diseases of the circulatory system (Standard Mortality Ratio (SMR) 0.73–0.76) and specifically from arteriosclerotic heart disease (SMR 0.74–0.79) (12). Similarly, the IARC 15-nation study showed an approximately 35% deficit in cardiovascular disease at low occupational doses (20 mSv), but a 10% increase at higher doses (200 mSv) indicating a highly non-linear dose response (13).
From the human data it is therefore unclear whether an association between heart disease and radiation exposure at low dose levels exists. If such an association is real, it is also unclear whether there is a general radiation-induced change in risk for the whole population, or whether any change is associated with particular dietary circumstances, radiation quality or sensitive subgroups. For example, it is possible that the increased risk from radiation exposure occurs only in persons genetically predisposed to heart disease, or is related to the mixed LET nature of their exposure.
One approach to testing these possibilities is the use of animal models, and the effect of radiation exposure plus dietary circumstance has been tested by Tribble et al. (14). They showed that high doses (2–8 Gy) of low LET ionizing radiation, given at high dose rate accelerated aortic lesion formation in normal C57BL/6 mice, but only if they were fed a high fat diet within 1–2 weeks after exposure. The increase in mean lesion area was a function of dose, but this effect was suppressed in mice overexpressing CuZn-SOD suggesting a link to oxidative stress. Pakala et al. (15) found a similar increase in lesion size in hypercholesterolemic rabbits that had their iliac arteries exposed, but the doses used were very high (15 Gy). A similar experiment by Cottin et al. (16) also using a local 15 Gy aortic dose in hypercholesterolemic rabbits reported increased plaque burden and more advanced plaque types.
Animals can also be used to model genetic susceptibility to heart disease. Apolipoprotein E acts as the main ligand mediating removal of cholesterol enriched chylomicron- and VLDL -remnants. Mice that are deficient in the production of apoE are susceptible to atherosclerosis when fed a normal low fat diet, and the progression of the disease is further exacerbated when such mice are fed a diet enriched in fat and cholesterol. This sensitivity to atherosclerosis in the ApoE−/− mice is due directly to the apoE deficiency, since apoE (derived from macrophages) reduced hypercholesterolemia in the apoE-deficient mice, leading to a decreased susceptibility to atherosclerosis (17).
Stewart et al. (18) also conducted a high dose study of radiation-induced atherosclerosis, and examined the carotid arteries (14 Gy neck exposure) of atherosclerosis-prone ApoE−/− mice fed a normal fat diet. They showed that cholesterol levels in irradiated mice were not significantly different from age-matched controls and markers of systemic inflammation were not elevated. However, compared to age-related atherosclerotic lesions, irradiation accelerated the development of macrophage-rich, inflammatory atherosclerotic lesions prone to intraplaque hemorrhage. Hoving et al. (19) showed similar results in ApoE−/− mice exposed to high dose fractionated (20 X 2 Gy) radiation. Schiller et al. (20) also conducted a high dose study and compared aortic lesion formation in lethally irradiated (10 Gy) low density lipoprotein receptor knockout mice (Ldlr−/− ). The mice were rescued by reconstitution with bone marrow from syngeneic Ldlr−/− mice and fed a high fat diet. Compared to non-reconstituted and non-irradiated mice, thoracic aorta lesion areas were smaller but aortic root lesion areas were greater in the transplanted mice than in non-transplanted mice.
While the high dose data in animal models seems generally consistent with at least some of the high dose human data, there are no animal data examining the effects of low doses. Given the uncertainty regarding the ability of lower doses of low LET radiation exposure to promote atherosclerosis, we report here the results of an in vivo mouse study to test the hypothesis that low doses of ionizing radiation, in the range of 0.025–0.5 Gy, would promote the progression of atherosclerotic lesion formation in mice that are genetically predisposed to this disease (ApoE−/−). Because of the close link between this disease and circulating lipoprotein levels in this mouse model, we further tested the hypothesis that any radiation induced change in the progression of atherosclerotic lesions was linked to associated changes in serum lipoprotein levels. We report measures of high and low dose rate radiation effects on changes in lesion frequency, size and severity, along with general measures of total serum cholesterol levels as well as specific measures of the fraction of lesion lipid within associated macrophages.
METHODS
Mice
The ApoE−/− (B6.129P2-Apoe tm1lUnc/J) female mice were obtained at 4–6 weeks of age from Jackson Laboratories, USA. These mice are homozygous null for a functional ApoE gene (in a C57BL/6J background). They display marked atherosclerosis when fed a normal diet that is low in fat and these lesions can be accelerated in both size and complexity if the mice are fed a diet enriched fat. The morphological features of early stage lesions in ApoE−/− (21–23) mice are very similar to those found in humans (24–26). Mice of the same genetic background, but wildtype for ApoE (ApoE+/+) were obtained from the same supplier.
The mice were barrier-raised at Jackson Laboratories and then maintained as specific pathogen free mice at the Chalk River Laboratories. Health monitoring during and at the end of the study indicated no change in specific pathogen free status. Mice were group-housed, 6 per standard shoebox cage, in ventilated micro-isolators. They were provided with Bed-O-Cob bedding (Frisco Enterprises, Boulton, Ontario) and ad-lib reverse osmosis, UV treated water and Charles River Rodent Chow #5075 (Charles River Canada, St. Constant, Quebec), which is a normal, low fat (4.5%) diet. The mice were divided into groups and ear punched to identify them by group and as individuals within a cage. Mice were monitored daily for general health.
All housing, handling and experimental procedures were conducted in accordance with the guidelines of the Canadian Council on Animal Care and with the pre-approval of the local Animal Care Committee.
Irradiation
The unrestrained mice in their plastic cages were exposed, beginning at either 7–8 weeks of age or 8 months of age to 60Co-γ-irradiation at low dose rate from an open beam source (GammaBeam 150, Atomic Energy of Canada Limited) or exposed singly in a plastic container to 60Co-γ-irradiation at high dose rate in an enclosed irradiator (GammaCell 200, Atomic Energy of Canada Limited). The groups were exposed to either 0.025, 0.05. 0.10 or 0.50 Gy, either at low dose rate (1.0 mGy/min, GammaBeam 150, Atomic Energy of Canada Limited) or at high dose rate (about 0.15 Gy/min, GammaCell 200, Atomic Energy of Canada Limited). Animals received their designated dose as a single exposure, except those receiving 0.5 Gy at low dose rate which received 100 mGy per day at 1 mGy/min, for 5 consecutive days. Control (0 Gy) mice were handled and transported in the same manner as exposed mice and were placed in a shielded space beside the irradiation source (and sharing the same atmosphere) during the exposure of the test group. Following the treatments, the mice were monitored for general health once daily. Typically, samples from 8 animals were analyzed per dose point, at each dose rate and time of examination, except typically 6 animals were analyzed for the percentage of lipid within lesion macrophages, or as otherwise noted.
Study termination/timeline
Mice were exposed either at early stage disease (2 months of age) or at late stage disease (8 months of age). Mice exposed at early stage disease were euthanized and tissues collected either 3 or 6 months after exposure (at 5 or 8 months of age). Mice exposed at late stage disease (8 months of age) were euthanized and tissues collected 2 or 4 months after exposure (at 10 or 12 months of age).
Tissue/Blood Collection
Just prior to sampling of blood and tissue, the mice were euthanized with an intraperitoneal injection of sodium pentobarbital. Terminal blood samples were collected by puncture of the right ventricle. Blood was allowed to clot at room temperature for 30 minutes, and then centrifuged at 1000 x g for 25 min at 4 °C. Serum samples were immediately tested for total cholesterol.
Serum Cholesterol
Individual serum total cholesterol concentrations were determined using a commercially available colorimetric assay (Wako Bioproducts, Richmond, VA), following a slight modification of the manufacturer’s instructions to allow for quantification using a 96 well microtiter plate format. For this analysis, serum from ApoE+/+ mice as well as ApoE−/− was collected and measured.
Heart Tissue Collection
Mice were perfused with PBS via a cannula placed in the left ventricle, with perfusate drained from a severed right atrium. Hearts were separated from the aorta at the base, embedded in optimum cutting temperature (OCT) medium, and snap-frozen on a metal plate that was cooled with liquid nitrogen.
Quantification of Atherosclerotic Lesions in Tissue Sections
The size of atherosclerotic lesions in the ascending aorta was determined from five Sudan IV stained serial sections, countered stained with Weigart’s haematoxylin, from each mouse, cut 10 μm thick and separated by 100 μm. Lesion analysis began with the first section of tissue that contained the ostia for the coronary arteries; a region defining the boundary between the aortic sinus and ascending aorta. Using the staining as a guide, lesion area defined as intimal tissue within the internal elastic lamina was determined using Image-Pro Plus software (V6.2, Media Cybernetics, Silver Springs, MD) on images that were created using a digital CoolSNAP cf camera (Roper Scientific Inc., Duluth, GA). The mean lesion cross-sectional area derived from the five serial sections was taken as the average lesion size for each animal as described previously (27–31). We calculated the average number of lesions that could be counted within the five histological sections used per mouse to determine the mean lesion cross sectional area in the control (0 Gy) and experimental (0.025–0.5 Gy) treated mice.
Based on the staining patterns of lesions for neutral-lipid (Sudan IV), each lesion identified in each of the five serial sections was classified as being at a stage of severity in the range 1–5, This process uses the human lesion classification format, (32, 33) as being either in the early stages of severity (stages 1 to 3), consisting mainly of macrophage derived foam cells, or advanced stages of severity (stages 4 and 5) which are characterized by a well-defined necrotic core either with or without a fibrous cap.
Quantification of the Percent Lesion-Associated Lipid within Macrophages
Histological analysis was performed as described previously, (27, 31) using four sequential sections of the ascending aorta of each mouse to detect neutral lipid and macrophages. Sudan IV was used to stain for neutral lipid and immune-histochemistry was used to detect macrophages. For the latter, detection was achieved by first incubating tissue with rabbit antisera to mouse macrophages (1:5000 dilution, Accurate Chemical Co.) followed by a biotinylated secondary antibody against rabbit, and finally incubation with avidin-peroxidase (Vectastain Elite ABC kit, Vector Laboratories) for visualization of the positive staining. The lesion area staining positive for macrophages and for neutral lipid was quantified using ImagePro Plus. An imprint was made for each area and superimposed using Adobe Photoshop CS2 (Version 9.0.2). Both imprints were still distinguishable at this point, only the overlapping areas appeared darker. The superimposed image was then imported to ImagePro Plus to measure the amount of overlap and the percentage of lipid associated within macrophages calculated by dividing the overlapping area with the total area of lesion-associated lipid. It is important to note that each section used for the overlay of lesion-associated lipid and macrophage areas was only separated by 10 μm.
Statistics
Data for lesion severity (as measured by lesion stage) as well as for the mean cross-sectional areas (size) or numbers of lesions in the aortic roots, plus lipid in lesion macrophages of the exposed and unexposed groups of mice, and mean serum cholesterol values were tested for normal distribution and variance. When the data were normally distributed and had equal variance, comparisons of unexposed control animals with mice exposed to individual doses were made using a student’s t-test. Data not normally distributed or with unequal variances were compared using the Mann-Whitney Rank Sum Test. T-tests and Mann-Whitney Rank Sum Tests were done with the statistical program SigmaStat 3.10 (Systat Software Inc., Point Richmond CA). Two-tailed p-values of ≤ 0.05 were considered significant.
In addition to the univariate tests of significance above, global tests of significance of the modifying effects of dose rate and time were performed via regression modelling using the statistical program Stata (Release 10, 2007, StataCorp., College Station, TX). The only explanatory variable under consideration was dose, with modifying effects of dose rate and assay time after exposure. Simple linear regressions provided a poor fit due to substantial non-linearity (often more than linear-quadratic curvature) in data and so we considered spline models in which the relationship between an endpoint and dose is estimated as a piecewise linear function, with a variable number of knots where linear segments join.
Specifically, we assumed a model of the form Y=β0 +β1 D1 +β2 D2 + β3 D3 +β4 D4. The βi measure the slopes of dose response at the knot points ki and the created variables Di are given by D1=min{D,k1}, Di= max {min(D,ki), ki-1} - ki-1 (i=2,3,4). The model with the optimal combination of knots is selected that yields the smallest value of Akaike information criteria (AIC), defined as −2ln L + 2p, where L is the likelihood for an estimated model with p parameters. For each endpoint, we stratified the dataset by high or low dose rate and by 3 or 6 months post-irradiation for early disease stages and by 2 and 4 months for late disease stages. Formal statistical tests for heterogeneity between these groups were conducted.
RESULTS
1. Unexposed ApoE−/− mice
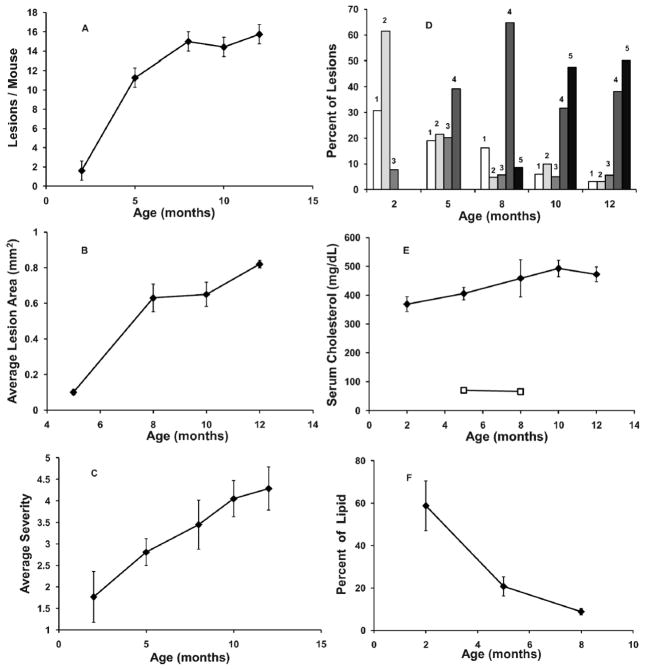
Aortic atherosclerotic lesion frequency was initially low in young ApoE−/− mice (2 months of age), but increased rapidly with time in the unexposed animals (Figure 1A), reaching a maximum at about 8 months of age. The average size of each lesion also increased with the age of the animals, (by about 0.003 mm2/day), but did not show any indication of reaching a maximum within 12 months (Fig. 1B). Similarly, average lesion severity continued to increase as the unexposed mice aged (Fig. 1C). Between 2 and 8 months of age, lesion severity increased by an average of 1.68 stage units, or about 0.0093 stage units/day. Figure 1D indicates the distribution of lesion stages of severity with increasing age. Most lesions were not severe (stage 1–2) in unexposed 2 month old mice, but progressed to more severe stages with time. At 8 months of age, most aortic lesions had reached stage 4, and by 10 months of age about half the lesions had reached the most severe level, stage 5. Animals wildtype for ApoE (ApoE+/+) were also examined for aortic lesions. None were observed.
FIG. 1.
Disease progression with increasing age in unexposed ApoE−/− mice. Panel A: Aortic root lesion frequency. Panel B: Average lesion cross-sectional area in the aortic root. Panel C: Average lesion severity stage. Panel D: Distribution of lesions by severity stage. The numbers on the histogram bars indicate the lesion severity stage (increasing severity 1–5) at the various mouse ages. Panel E: Total serum cholesterol. Closed diamonds, ApoE−/− mice; open squares, ApoE+/+ mice. n = 6–10. Panel F: The percentage of total lesion lipids contained within lesion-associated macrophages. Error bars indicate ± SE.
Total serum cholesterol increased slightly as the unexposed mice aged (Fig. 1E), and reached a maximum at about 10 months of age, significantly higher than at 2 months of age (p<0.01). Animals wildtype for ApoE (ApoE+/+, open squares, Fig. 1E) were also tested for total serum cholesterol at 5 and 8 months of age, and had levels 6–7 fold lower than the ApoE−/− mice of the same age.
Figure 1F shows, as a function of unexposed animal age, the percentage of total lesion lipid that was contained by macrophages that were within aortic atherosclerotic lesions. The figure shows that in young mice, at a time when atherosclerotic lesions are small and generally not severe (2 months of age), the majority of lesion lipid was contained within macrophages. This high percentage of lipid in lesion macrophages declined rapidly with time, and at 8 months of age, when most lesions (about 65%) were generally classified as severe (Stage 4, Fig. 1D), less than 10 percent of the total lesion lipid was contained within lesion macrophages.
2. Exposed ApoE−/− mice
2.1. Exposure at Early Stage Disease
When atherosclerosis was at an early stage of development (at 2 months of age, Fig. 1) ApoE−/− mice were exposed to low doses of radiation delivered at either low or high dose rate, and then examined 3 or 6 months after exposure (at 5 and 8 months of age), time points at which the disease had significantly advanced in unexposed mice (Fig. 1).
a. Exposure at low dose rate
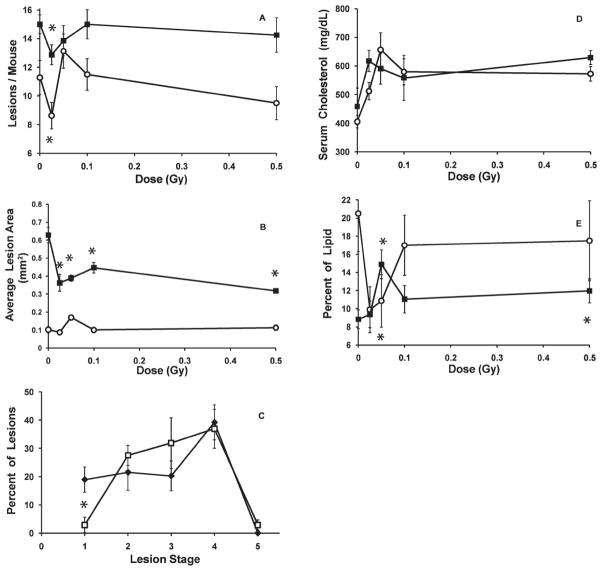
Compared to unexposed controls, (Fig. 1A) low dose exposure generally reduced lesion frequency (Fig. 2A), but the reduction was restricted to the lowest doses used. At one or other of the lowest doses examined (25 or 50 mGy), radiation exposure reduced lesion frequency in animals examined 3 months after exposure, and this effect persisted until 6 months after exposure. This protective effect tended to be reduced or disappear when the dose increased above these doses.
FIG. 2.
Influence of low-dose-rate exposure at an early stage of disease (2 months of age). Panel A: Lesion frequency.*P < 0.01 at 5 months of age and P < 0.04 at 8 months of age. Panel B: Average lesion cross-sectional area. *P < 0.001. Open circles, examined at 5 months of age; solid squares, examined at 8 months of age. Error bars indicate ± SE.
The effect of radiation exposure on the average cross sectional area of the lesions measured in the aortic root is shown in Fig. 2B. At 5 months of age, lesions were small in unexposed mice (Fig. 1B), and at that time no significant effect of radiation on lesion area was seen. However, by 8 months of age, lesion size in unexposed mice had increased considerably (Fig. 1B) and a significant radiation effect was then evident. Average lesion size for each group of animals receiving any dose between 0.025 to 0.5 Gy at low dose rate (Fig. 2B) had smaller lesions than the unexposed control mice. Again, 25 and 50 mGy were the most effective doses.
After the radiation exposures at 2 months of age, lesion severity was assessed as both dose dependent changes in average severity and as changes in lesion stage frequency. While the average lesion severity increased with mouse age between ages 5 months and 8 months in unexposed controls (Fig. 1C,D), average lesion severity showed no additional statistically significant change with increasing dose, given at low dose rate, when measured at either 3 or 6 months after exposure (at 5 or 8 months of age). A similar conclusion was reached when the analysis was based on the relative frequency of lesions at each stage (data not shown).
Serum cholesterol was high in the unexposed ApoE−/− mice at all ages (Fig. 1E). Exposure to any dose of radiation at low dose rate, up to 0.5 Gy, had no significant effect on that high level of total serum cholesterol, measured either 3 or 6 months after exposure at early stage disease, 2 months of age (data not shown).
Compared to unexposed control mice (Fig. 1F), there were no statistically significant changes in the percentage of the lesion lipids that were contained in macrophages within the aortic lesions of the exposed mice, when examined either 3 or 6 months after any dose at early stage disease (data not shown).
b. Exposure at high dose rate
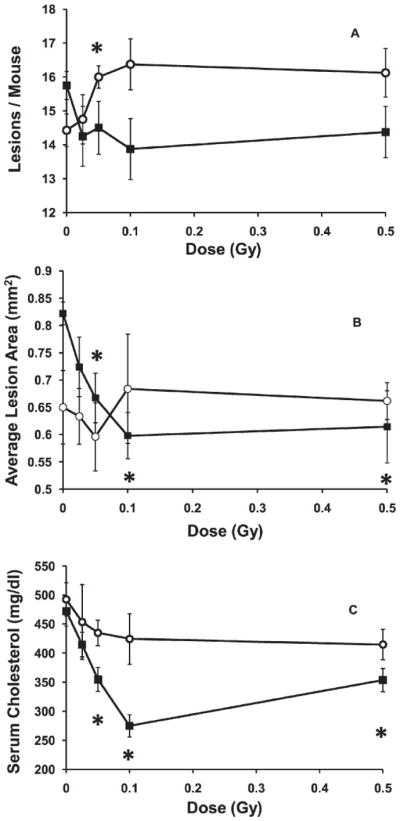
The effects on lesion frequency and size of low dose exposures given at high dose rate (Fig. 3A, B) were qualitatively similar to the inhibitory effects observed after the same doses given at low dose rate (Fig. 2A, B). The high dose rate effects were additionally similar to the low dose rate effects when compared at different times after exposure. As observed after the low dose rate exposure, the lowest doses used (25–50 mGy) tended to be the most effective at reducing both lesion frequency and size.
FIG. 3.
Influence of high-dose-rate exposure given at an early stage of disease (2 months of age). Panel A: Lesion frequency. Open circles. examined at 5 months of age; solid squares, examined at 8 months of age. *P < 0.05. Panel B: Average aortic root lesion cross-sectional area. Open circles, examined at 5 months of age; solid squares, examined at 8 months of age. *P ≤ 0.003. Panel C: Percentage of lesions at various stages of severity examined at 5 months of age. Solid diamonds, control, unexposed mice; open squares, mice that received 0.025 Gy. *P = 0.009. Panel D: Serum cholesterol. Open circles, examined at 5 months of age, P < 0.02 for all doses; solid squares, examined at 8 months of age, P < 0.05 for 0.025 and 0.5 Gy. Panel E: Percentage of lesion lipid within lesion associated macrophages. Open circles, examined at 5 months of age. *P < 0.03 at 0.050 Gy. Solid squares, examined at 8 months of age. *P = 0.005 at 0.05 Gy and P < 0.04 at 0.5 Gy. Error bars indicate ± SE.
While exposure at low dose rate had no significant effect on lesion severity, exposure at high dose rate, produced a different result. Figure 3C shows the distribution of lesions within the five stages of severity, and compares unexposed animals to animals that had received 0.025 Gy at high dose rate, when examined 3 months after exposure, at 5 months of age. The exposure resulted in a significant decrease in lesions at the least severe stage 1, with a commensurate increase in lesions at stages 2 and 3. Interestingly, while about 40% of the lesions were at the more severe stage 4, the exposure had no effect on the severity of those lesions. This acceleration of early stage lesion severity by the 0.025 Gy dose lost statistical significance as the disease progressed with time, when the animals were examined 6 months after exposure, at 8 months of age (data not shown). Exposure to higher doses at high dose rate had no significant effect (data not shown).
In contrast to the lack of effect of radiation exposure given at low dose rate, every dose between 0.025 and 0.5 Gy, given at high dose rate (Fig. 3D) significantly elevated the total serum cholesterol when the mice were examined 3 months after the exposure When examined 6 months after the high dose rate exposures, a similar trend of increased serum cholesterol was observed, but the increases were statistically significant only for doses 0.025 and 0.5 Gy.
Also in contrast to the lack of effect seen after a low dose rate exposure, when mice were exposed at high dose rate and examined 3 months later an exposure of 0.025 Gy produced a marginally non-significant decrease in the percentage of the lesion lipid contained within macrophages (p=0.065), but which became a significant decrease at 0.050 Gy (Fig. 3E). As the dose increased further, that result became not significantly different from controls. However, measurements taken 6 months after the high dose rate exposures suggested an opposite trend, with the mice exposed to 0.05 Gy or 0.5 Gy showing a significantly elevated percentage of lesion lipids contained within macrophages.
2.2 Exposure at Late Stage Disease
Mice were also exposed to doses between 0.025 and 0.5 Gy at high or low dose rate, when the mice were 8 months of age. At that age the mice were at late stage disease and the lesions in the aortic root were near maximum number (Fig. 1A) and maximum size (Fig. 1B), and most lesions had progressed to stage 4, considered severe (Fig. 1D). After radiation exposure, the mice were examined 2 or 4 months later, when they were either 10 or 12 months of age, time points at which further changes in the lesions of the unexposed mice were relatively smaller.
a. Exposure at low dose rate
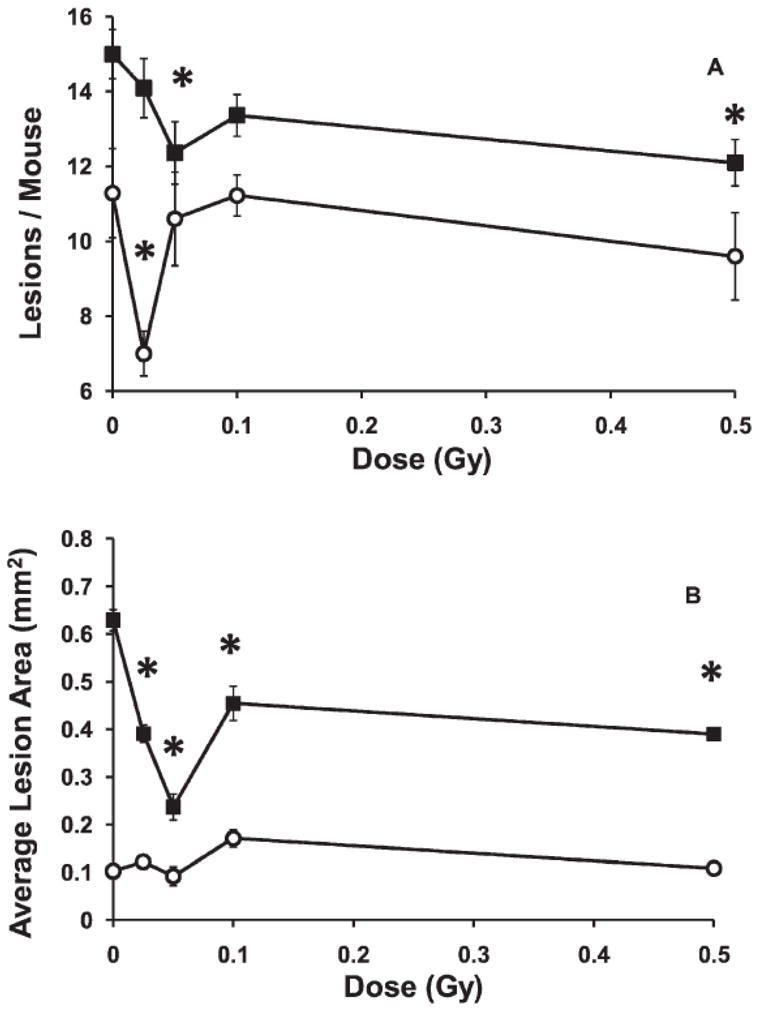
Exposure to 0.05 Gy at low dose rate had a small (10–14%) but statistically significant effect (Fig. 4A) of increasing the lesion frequency when the mice were examined 2 months after exposure, at 10 months of age (when compared to unexposed controls). Increasing the dose to 0.10 or 0.50 Gy resulted in marginally non-significant increases (p=0.056 and 0.072 respectively). When subsequently examined at 12 months of age, there was a trend to lower frequencies when compared to the unexposed animals, but this was not statistically significant. This result suggested that some low doses given at low dose rate in late stage disease could have a small, transient influence on accelerating the appearance of aortic lesions.
FIG. 4.
Influence of low-dose-rate exposure given at a late stage of disease (8 months of age). Panel A: Lesion frequency. Open circles, examined at 10 months of age, solid squares, examined at 12 months of age.*P ≤ 0.02. Panel B: Average lesion cross-sectional area. Open circles, examined at 10 months of age, solid squares, examined at 12 months of age; *P ≤ 0.007. Panel C: Total serum cholesterol. Examined at 10 months of age (open circles, p>0.05 for all doses) or at 12 months of age (solid squares, P ≤ 0.003 for doses 0.05–0.5 Gy). n = 11–17. Error bars indicate ± SE.
Exposure to low doses at low dose rate significantly reduced average lesion size, by about 30%, for doses from 0.05 to 0.5 Gy, when the mice were examined at 12 months of age, i.e. 4 months after exposure, (Fig. 4B). No significant effect was evident if the mice were examined earlier, at 10 months of age.
Low dose rate exposure of mice with late stage disease to doses between 0.025 and 0.5 Gy resulted in a generally dose dependent decrease in total serum cholesterol, that was a non-statistically significant trend 2 months after exposure, but became statistically significant when measured at 4 months after exposure. The decreases at each dose became larger with increased time after exposure (Fig. 4C). The largest decrease was observed 4 months after exposure to 0.1 Gy, when the serum cholesterol levels were about 40% below those of the unexposed animals (Fig. 1E, p<10−5). The levels in these 0.1 Gy exposed animals were actually about 25% lower than the values observed in even the very young (2 month old) unexposed mice (Fig. 1E).
At 10 months of age, most lesions were at either stage 4 or stage 5 in unexposed mice (Fig. 1D). A small inhibitory effect on the progression of lesion severity was seen when mice were exposed at low dose rate, but the slowing was only significant at 0.025 Gy (Fig. 5A). This slowing of progression of stage 4 lesions to stage 5 lesions was no longer significant when the mice reached 12 months of age (data not shown).
FIG. 5.
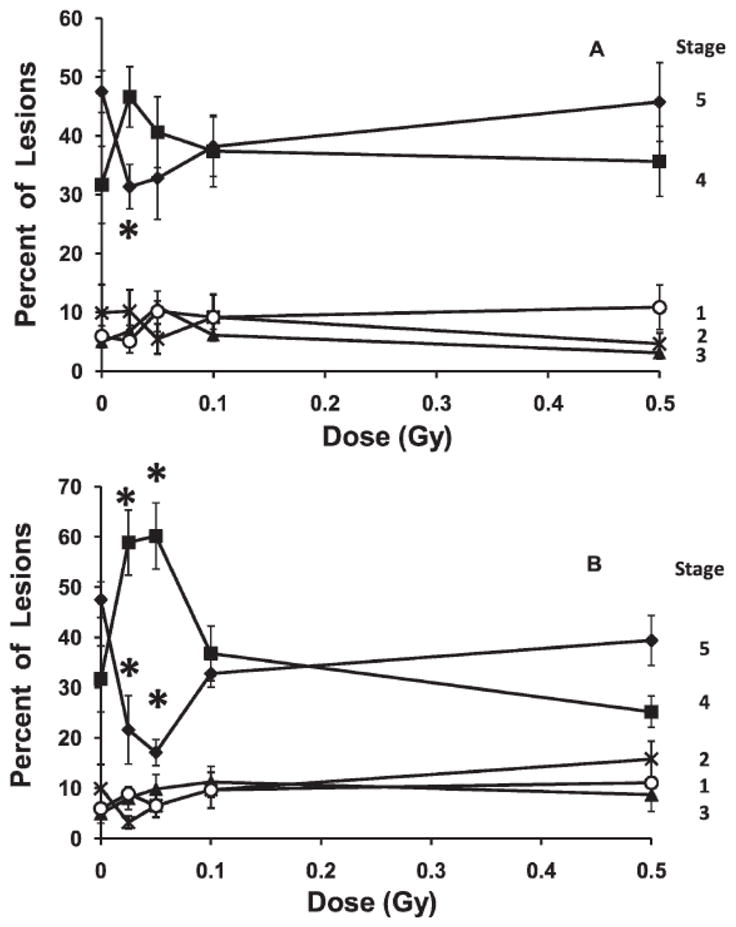
Changes in lesion severity with increasing high- or low-dose-rate exposure given to mice at a late stage of disease (8 months of age) and examined at 10 months of age. The figure shows the change in the percentage of aortic lesions at each severity stage of the disease. Panel A: Low-dose-rate exposure *P ≤ 0.02. Panel B: High-dose-rate exposure; *P ≤ 0.01. Error bars indicate ± SE.
b. Exposure at high dose rate
Exposure at late stage disease, to any dose at high dose rate, had no significant effect on lesion frequency or on average lesion size, regardless of the dose or time of examination (data not shown). Exposure to the same doses and dose rate likewise produced no significant changes in total serum cholesterol, whether measured 2 or 4 months after exposure at 8 months of age.
A high dose rate exposure of either 0.025 or 0.05 Gy given at late stage disease (at 8 months of age) significantly slowed, by about half, the fraction of lesions that had progressed in severity from stage 4 to stage 5 disease, when examined at 10 months of age (Fig. 5B). This slowing of the progression of the disease was temporary, since when the mice reached 12 months of age it was no longer significant (data not shown).
Groups of animals that were wildtype for ApoE (ApoE+/+) were also exposed at 8 months of age to doses from 0.025 Gy to 0.5 Gy, at low and high dose rates. The aortas were examined for lesions and total serum cholesterol measured 3 and 6 months later. No lesions were observed and no significant changes from the cholesterol values measured in unexposed mice (Fig. 1E) were seen (data not shown).
2.3. Analysis of Heterogeneity
We analyzed the data for heterogeneity. Analysis of aortic root data among mice aged 2 months at irradiation (early stage disease) demonstrated statistically significant differences (p<0.01) by dose rate overall and within the data taken 6 month after exposure. Likewise, for the mice exposed at 8 months of age (late stage disease), there were statistically significant differences (p<0.01) by dose rate overall (combining mice examined at 10 months and at 12 months) and by dose rate (p<0.05) within the mice examined at 12 months, but not within the mice examined at 10 months. Analysis of cholesterol data among mice aged 2 months at irradiation demonstrated statistically significant (p<0.01) differences by dose rate overall and within the data taken 3 months after exposure. Analysis of lipid in macrophages among mice aged 2 months at irradiation indicated that there are statistically significant (p<0.05) differences overall by dose rate.
DISCUSSION
In the experiments presented here, ApoE−/− mice were used to test the influence of a low LET radiation exposure on atherosclerotic disease progression in a genetically predisposed group. In the absence of radiation exposure, the disease progressed rapidly, with lesion number and size reaching near maximum values by the time the mice were 8 months of age (Fig. 1A,B). Similarly, by that age, most lesions had progressed to a stage 4 level of severity (Fig. 1C,D). Because of their genetic deficiency, serum cholesterol was already high in young animals and also increased to a maximum between 8 and 10 months of age (Fig. 1E). Conversely, the percentage of lesion lipid contained in lesion associated macrophages dropped with age, and reached a low level when the mice were 8 months old (Fig. 1F).
Exposure of the ApoE−/− mice to doses between 0.025 and 0.5 Gy influenced many of the above noted parameters of atherosclerosis, but the effects of the exposures varied with the dose, dose rate, time of measurement and the stage of the disease when the mice received the radiation exposure. A summary of the effects on the measured endpoints (as compared to unexposed controls), grouped by dose rate and disease state at exposure is given in Table 1.
TABLE 1.
Summary of Measured Responses to Low-Dose Exposure
| LDR Early stage | HDR Early stage | LDR Late stage | HDR Late stage | |
|---|---|---|---|---|
| Lesion frequency | ↓ | ↓ | ↑ | — |
| Lesion size | ↓ | ↓ | ↓ | — |
| Lesion severity | — | ↑ | ↓ | ↓ |
| Serum cholesterol | — | ↑ | ↓ | — |
| % Lesion lipid in macrophages | — | ↑ ↓ | N.D. | N.D. |
Notes. LDR, low-dose-rate exposure. HDR, high-dose-rate exposure. Downward arrows indicate a decrease and upward arrows an increase in the measured parameter. A horizontal line indicates no change, and N.D. indicates that the end point was not measured. The arrows indicate that the response was observed at some dose and time after exposure but do not indicate that there may have also been a lack of response at some other dose or time.
Exposure at Early Stage Disease
In some experiments the ApoE−/− mice were exposed to a single low dose at 2 months of age, when the disease was at an early stage of development. At that age, existing lesions are few (Fig. 1A) and small (Fig. 1B), and most had not yet progressed beyond a relatively mild stage 2 level of severity (Fig. 1D).
Exposure at either low or high dose rate induced a protective effect that slowed both the formation of new lesions (Figs. 2A, 3A) and the increase in size of existing lesions (Figs. 2B, 3B). At both dose rates, maximum inhibition of lesion growth was produced by a 25 or 50 mGy dose. However, while the low dose rate exposures had no influence on lesion severity, high dose rate exposure to 25 mGy significantly accelerated progression of the least severe stage 1 lesions to more severe stage 2 and 3 lesions, when measured 3 months after the exposure, at 5 months of age (Fig. 3C). The observed increase in lesion severity appeared to be linked to an influence of the high dose rate exposure on lesion lipid metabolism and lipid uptake by macrophages, since, also at 3 months after exposure less lesion lipid appeared in lesion associated macrophages (Fig. 3E) and more in the lesion itself. This impairment of lesion lipid uptake by macrophages was accompanied by a persistent increase in total serum cholesterol (Fig. 3D) to levels higher than those ever seen in the unexposed mice (Fig. 1E). That result suggested that low doses, when given at high dose rate at early stage disease resulted in an even further impairment in the ability of these ApoE−/− mice to metabolize and clear serum lipids. While the impairment of lesion lipid uptake by macrophages appeared to dissipate with time (Fig. 3E), the impairment in the ability to clear serum lipids persisted for up to 6 months after exposure (Fig. 3D).
These observations indicate that while low doses given at either high or low dose rate at early stage disease can induce effects that protect against lesion formation and growth, there is a distinct dose rate dependent difference in the ability of low doses to influence and modulate lipid metabolism and affect lesion severity. Consequently, while low doses given at low dose rate induce only protective effects, the same doses given at high dose rate appear to induce both protective and detrimental effects (Table 1). The results therefore suggest that the influence of low doses on lesion formation and size may proceed by a mechanism that is independent of the effect of low doses on lipid metabolism and lesion severity. The results also indicate a potential long term detrimental effect of high dose rate exposure at early stage disease, even when the doses are as low as 25 mGy.
Exposure at Late Stage Disease
In some experiments, the ApoE−/− mice were exposed at 8 months of age when the disease was in its later stages. At that age, lesion frequency and size were near maximum, and most lesions had reached a stage 4 level of severity (Fig. 1). Again, exposure to low doses of radiation was at either high or low dose rate. The mice were examined 2 or 4 months later, at 10 or 12 months of age.
The effect of exposure of mice with late stage disease to low doses at low dose rate generally produced protective effects, as was observed for low dose rate exposure at early stage disease (Table 1). However, while lesion growth (Fig. 4B) was retarded as in early stage disease, the protective effects at late stage disease also manifested as a reduction in serum cholesterol (Fig. 4C) and as a slowing in the progression of lesion severity (Fig. 5A), neither of which were seen when the mice were exposed at early stage disease. If, as suggested by the results of exposures at early stage disease, modulation of lipid metabolism and macrophage uptake of lipids represents one mechanism by which low doses influence atherosclerosis, then the reduction in serum cholesterol by low doses given at late stage disease would seem consistent with the observed parallel slowing in the progression of lesion severity.
Again as suggested by the observations from exposures at early stage disease, there is a second mechanism by which low doses affect atherosclerosis, and that mechanism influences both new lesion formation and the growth of those lesions. While low dose, low dose rate exposures given at early stage disease slowed advancement of both those endpoints, the same doses given at late stage disease also slowed the growth of lesions, but appeared to temporarily accelerate the formation of new lesions (Fig. 4A). That temporary increase in lesion frequency appears inconsistent with the generally protective effects seen, and this observation could suggest that the influence of low doses on these two endpoints proceeds by different mechanisms. However, we hypothesize that this observation may be an analysis artefact resulting from the other observed effects. Since the exposures slowed both the growth and the progression of the lesions to more severe disease, the lesions may have been relatively smaller, potentially making it easier to distinguish separate lesions at this late stage of lesion development, and consequently resulting in an apparent increase in frequency.
The effects on changes in lesion severity of low doses given at high dose rate when the mice had late stage disease were strongly protective against increases in lesion severity (Fig. 5B) and opposite those seen on lesion severity at early stage disease (Fig. 3C). This result suggests that some of the effects of low dose radiation on atherosclerosis depend on the state of progression on the disease, and exposures at early or late stage should be considered separately. They also suggest that low dose exposures may influence this disease by a third mechanism, which is protective and specific for late stage disease.
CONCLUSIONS
To date, the effects of low doses on atherosclerosis have not been studied in animal models. The data presented here clearly show that single doses of 25–500 mGy, given at either early or late stage disease, can have a significant impact on the development of atherosclerosis in mice fed a normal diet low in fat, but genetically predisposed to the disease (ApoE−/−). The effects of the single doses often persisted for months, but some were ultimately transient in nature. All effects were distinctly nonlinear with dose, and were generally protective for low dose rate exposures given at either early or late stage disease. However, high dose rate exposures given at an early stage of disease development showed both protective and detrimental effects, indicating multiple mechanisms may influence the outcome. Most effects occurred below about 100 mGy, and many of the endpoints measured showed maximum effects at 25–50 mGy, the lowest doses used here, indicating that even lower doses need to be examined.
The results of these animal studies have implications for human health. Many human epidemiological studies have shown the detrimental effects of high dose exposure for a variety of cardiovascular or circulatory diseases. However, most such studies have either failed to show statistically significant increased risk at doses below about 0.5 Gy (3, 4, 11, 34), or have shown protective effects at doses similar to those used in this mouse study (12, 13). Interestingly, the highly non-linear dose response evident in the disease progression data shown here, very much resembles the nonlinear (protective then detrimental) cardio-vascular mortality seen with similar increasing low doses in one human study (13), suggesting that the animal responses are paralleling the human response. This parallel exists in spite of the fact that these mouse studies measured markers of disease progression, rather than the risk of lethality, as usually reported in the human studies. It needs to be recognized, however, that this study deals with single exposures. The effects of multiple or chronic low dose exposures, such as modelled by Little et al. (10) remain to be determined.
Acknowledgments
This work was performed as part of the European Commission FP6 NOTE project “Non-targeted effects of ionising radiation” (FP6-036465) and was funded by Atomic Energy of Canada Limited, the European Commission and Health Canada. This manuscript is dedicated to the memory of Dr. Stewart Whitman.
References
- 1.Shimizu Y, Pierce DA, Preston DL, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, part II. Noncancer mortality: 1950–1990. Radiat Res. 1999;152:374–389. [PubMed] [Google Scholar]
- 2.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–632. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 3.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 4.Yamada M, Naito K, Kasagi F, Masunari N, Suzuki G. Prevalence of atherosclerosis in relation to atomic bomb radiation exposure: an RERF Adult Health Study. Int J Radiat Biol. 2005;81:821–826. doi: 10.1080/09553000600555504. [DOI] [PubMed] [Google Scholar]
- 5.Carr ZA, Land CE, Kleinerman RA, Weinstock RW, Stovall M, Griem ML, Mabuchi K. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 6.Shai E, Siegals S, Michael Z, Itzhak K, Ronen R, Dror M, Sylvia B, Adina BH, Ramzia AH, Marina F, Angela C, Dov Y, Joshua W, Arie B. Carotid atherosclerotic disease following childhood scalp irradiation. Atherosclerosis. 2009;204:556–560. doi: 10.1016/j.atherosclerosis.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163:424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 8.Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice JD., Jr Thyroid neoplasia following low-dose radiation in childhood. Radiat Res. 1989;120:516–531. [PubMed] [Google Scholar]
- 9.Howe GR, Zablotska LB, Fix JJ, Egel J, Buchanan J. Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res. 2004;162:517–526. doi: 10.1667/rr3258. [DOI] [PubMed] [Google Scholar]
- 10.Little MP, Gola A, Tzoulaki I. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure. PLoS Comput Biol. 2009 Oct;5(10):e1000539. doi: 10.1371/journal.pcbi.1000539. Epub 2009 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGale P, Darby SC. Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res. 2005;163:247–257. doi: 10.1667/rr3314. [DOI] [PubMed] [Google Scholar]
- 12.Sponsler R, Cameron JR. Nuclear shipyard worker study (1980 1988): a large cohort exposed to low-dose-rate gamma radiation. Int J Low Radiation. 2005;1:463–478. [Google Scholar]
- 13.Vrijheid M, Cardis E, Auvinen P, Bae AJM, Engels H, Gilbert E, Gulis G, Habib R, Howe G, Kurtinaitis J, Malker H, Muirhead C, Richardson D, Rodriguez-Artalejo F, Rogel A, Schubauer-Berigan M, Tardy H, Telle-Lamberton M, Usel M, Veress K. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol. 2007;36:1126–1135. doi: 10.1093/ije/dym138. [DOI] [PubMed] [Google Scholar]
- 14.Tribble DL, Barcellos-Hoff MH, Chu BM, Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol. 1999;19:1387–1392. doi: 10.1161/01.atv.19.6.1387. [DOI] [PubMed] [Google Scholar]
- 15.Pakala R, Leborgne L, Cheneau E, Chan RC, Yazdi H, Fournadjiev J, Weber D, Hellinga D, Kolodgie F, Virmani R, Waksman R. Radiation-induced atherosclerotic plaque progression in a hypercholesterolemic rabbit: a prospective vulnerable plaque model? Cardiovasc Radiat Med. 2003;4:146–151. doi: 10.1016/S1522-1865(03)00182-3. [DOI] [PubMed] [Google Scholar]
- 16.Cottin Y, Kollum M, Kolodgie FD, Chan RC, Kim HS, Vodovotz Y, Virmani R, Waksman R, Yazdi H. Intravascular radiation accelerates atherosclerotic lesion formation of hypercholesteremic rabbits. Cardiovasc Radiat Med. 2001;2:231–240. doi: 10.1016/s1522-1865(02)00129-4. [DOI] [PubMed] [Google Scholar]
- 17.Van Eck M, Herijgers N, Yates J, Pearce NJ, Hoogerbrugge PM, Groot PH, Van Berkel TJ. Bone marrow transplantation in apolipoprotein E-deficient mice. Effect of ApoE gene dosage on serum lipid concentrations, (beta)VLDL catabolism, and atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:3117–3126. doi: 10.1161/01.atv.17.11.3117. [DOI] [PubMed] [Google Scholar]
- 18.Stewart FA, Heeneman S, Te Poele J, Kruse J, Russell NS, Gijbels M, Daemen M. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649–658. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoving S, Heeneman S, Gijbels MJ, te Poele JA, Russell NS, Daemen MJ, Stewart FA. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(−/−) mice. Int J Radiat Oncol Biol Phys. 2008;71:848–857. doi: 10.1016/j.ijrobp.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Schiller NK, Kubo N, Boisvert WA, Curtiss LK. Effect of gamma-irradiation and bone marrow transplantation on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;10:1674–1680. doi: 10.1161/hq1001.096724. [DOI] [PubMed] [Google Scholar]
- 21.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 24.Stary HC. Macrophages, macrophage foam cells, and eccentric intimal thickening in the coronary arteries of young children. Atherosclerosis. 1987;64:91–108. doi: 10.1016/0021-9150(87)90234-6. [DOI] [PubMed] [Google Scholar]
- 25.Stary HC. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990;11(Suppl E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- 26.Stary HC. Composition and classification of human atherosclerotic lesions. Virchows. Arch A Pathol Anat Histopathol. 1992;421:277–290. doi: 10.1007/BF01660974. [DOI] [PubMed] [Google Scholar]
- 27.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 28.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 29.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 31.Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A. Depletion of Natural Killer Cell Function Decreases Atherosclerosis in Low-Density Lipoprotein Receptor Null Mice. Arterioscler Thromb Vasc Biol. 2004;6:1049–1054. doi: 10.1161/01.ATV.0000124923.95545.2c. [DOI] [PubMed] [Google Scholar]
- 32.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 33.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, Grant EJ, Sugiyama H, Sakata R, Moriwaki H, Hayashi M, Konda M, Shore RE. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]