MAPK and Cdk1 play compensatory roles in suppressing APC/C activity early in prometaphase, thereby allowing accumulation of APC/C substrates essential for meiosis I.
Abstract
Female meiosis is driven by the activities of two major kinases, cyclin-dependent kinase 1 (Cdk1) and mitogen-activated protein kinase (MAPK). To date, the role of MAPK in control of meiosis is thought to be restricted to maintaining metaphase II arrest through stabilizing Cdk1 activity. In this paper, we find that MAPK and Cdk1 play compensatory roles to suppress the anaphase-promoting complex/cyclosome (APC/C) activity early in prometaphase, thereby allowing accumulation of APC/C substrates essential for meiosis I. Furthermore, inhibition of MAPK around the onset of APC/C activity at the transition from meiosis I to meiosis II led to accelerated completion of meiosis I and an increase in aneuploidy at metaphase II. These effects appear to be mediated via a Cdk1/MAPK-dependent stabilization of the spindle assembly checkpoint, which when inhibited leads to increased APC/C activity. These findings demonstrate new roles for MAPK in the regulation of meiosis in mammalian oocytes.
Introduction
Meiosis in mammalian oocytes is controlled by changes in the activity of Cdk1–cyclin B and MAPK. Oocytes remain arrested in a gap phase 2 (G2)/prophase–like state for much of their existence and are only stimulated to progress into metaphase of meiosis I (metaphase I) by an increase in Cdk1–cyclin B activity (Hashimoto and Kishimoto, 1988; Conti et al., 2012). Continued progression through meiosis I is driven by a slow rise in Cdk1–cyclin B activity that results in a protracted prometaphase I: 6–7 h compared with tens of minutes in somatic cells (Polanski et al., 1998; Ledan et al., 2001; Jones, 2008; Davydenko et al., 2013). On reaching metaphase I, a transient decrease in Cdk1–cyclin B activity leads to exit from metaphase I and extrusion of the first polar body (PB1). Oocytes then proceed directly to metaphase of the second meiotic division (metaphase II) at which MAPK activity and Emi2 (early mitotic inhibitor 2) cooperate to inhibit the anaphase-promoting complex/cyclosome (APC/C), thereby stabilizing Cdk1 activity (Tung et al., 2005; Shoji et al., 2006; Inoue et al., 2007; Nishiyama et al., 2007; Wu et al., 2007).
The APC/C is an E3 ubiquitin ligase that targets key regulatory proteins, such as securin and cyclin B, for degradation by the 26S proteasome via polyubiquitinating them (Peters, 2006; Pines, 2011; Homer, 2013). The APC/C-mediated destruction of securin and cyclin B activates separase and inhibits Cdk1–cyclin B, respectively, thereby providing a mechanism of coordinating chromosome segregation and cell cycle exit (Clute and Pines, 1999; Zur and Brandeis, 2001; Peters, 2002; Herbert et al., 2003; Terret et al., 2003; Madgwick et al., 2004). The timely activation of the APC/C is controlled via direct binding of two coactivators, Cdc20 (cell division cycle 20) and Cdh1 (Cdc20 homologue 1; Visintin et al., 1997). The APC/C is also subject to other regulatory mechanisms, such as binding to the components of spindle assembly checkpoint (SAC), which inhibits the APC/C until the establishment of stable bipolar microtubule attachments (Homer et al., 2005a; Musacchio and Salmon, 2007; McGuinness et al., 2009; Hoffmann et al., 2011).
In mitosis, phosphorylation of key elements, such as the APC/C subunits, its coactivators, and the SAC proteins, plays key roles in regulation of the APC/C (Hershko et al., 1994; Kotani et al., 1998; Zachariae et al., 1998; Jaspersen et al., 1999; Chung and Chen, 2003; D’Angiolella et al., 2003; Kraft et al., 2003; Wassmann et al., 2003a; Zhao and Chen, 2006; Kim et al., 2010). In mammalian meiosis, however, this remains largely unexplored. Here, we investigate the role of Cdk1 and MAPK on APC/C activity and uncover new roles for Cdk1/MAPK in regulation of meiotic progression.
Results and discussion
The roles of Cdk1 and MAPK in regulation of APC/C activity in prometaphase I were investigated by applying specific inhibitors during a 2-h window, 3–5 h after release from prophase I arrest. Inhibition of the kinases was performed using small molecule inhibitors, roscovitine and UO126, which have been used widely in many cell types, including oocytes (Meijer et al., 1997; Favata et al., 1998; Phillips et al., 2002; Gorr et al., 2006; Yu et al., 2007; Nabti et al., 2008). Western blots performed on these oocytes demonstrate that inhibition of Cdk1 or MAPK during this window did not impact on securin levels, suggesting that APC/C activity was not affected. In contrast, inhibition of Cdk1 and MAPK caused an ∼60% decrease in securin level (Fig. 1, A and B). To verify this role of Cdk1 and MAPK, we used alternative approaches to inhibit the kinases (Fig. S1). Cdk1 activity was inhibited with flavopiridol (Potapova et al., 2006; Holt et al., 2012) and MAPK by depletion of Mos, an oocyte-specific MAPK kinase kinase, through injection of a Mos morpholino (Coonrod et al., 2001). We confirmed in positive control experiments that flavopiridol inhibits germinal vesicle (GV) breakdown (GVBD), and Mos morpholino–treated oocytes progress through metaphase II (Fig. S1, A and B). Western analysis of securin levels in flavopiridol and Mos morpholino–treated oocytes revealed a similar decrease in protein levels as seen with roscovitine and UO126 (Fig. S1, C and D). Together, these findings provide strong evidence that Cdk1 or MAPK activity is essential for securin accumulation during prometaphase I.
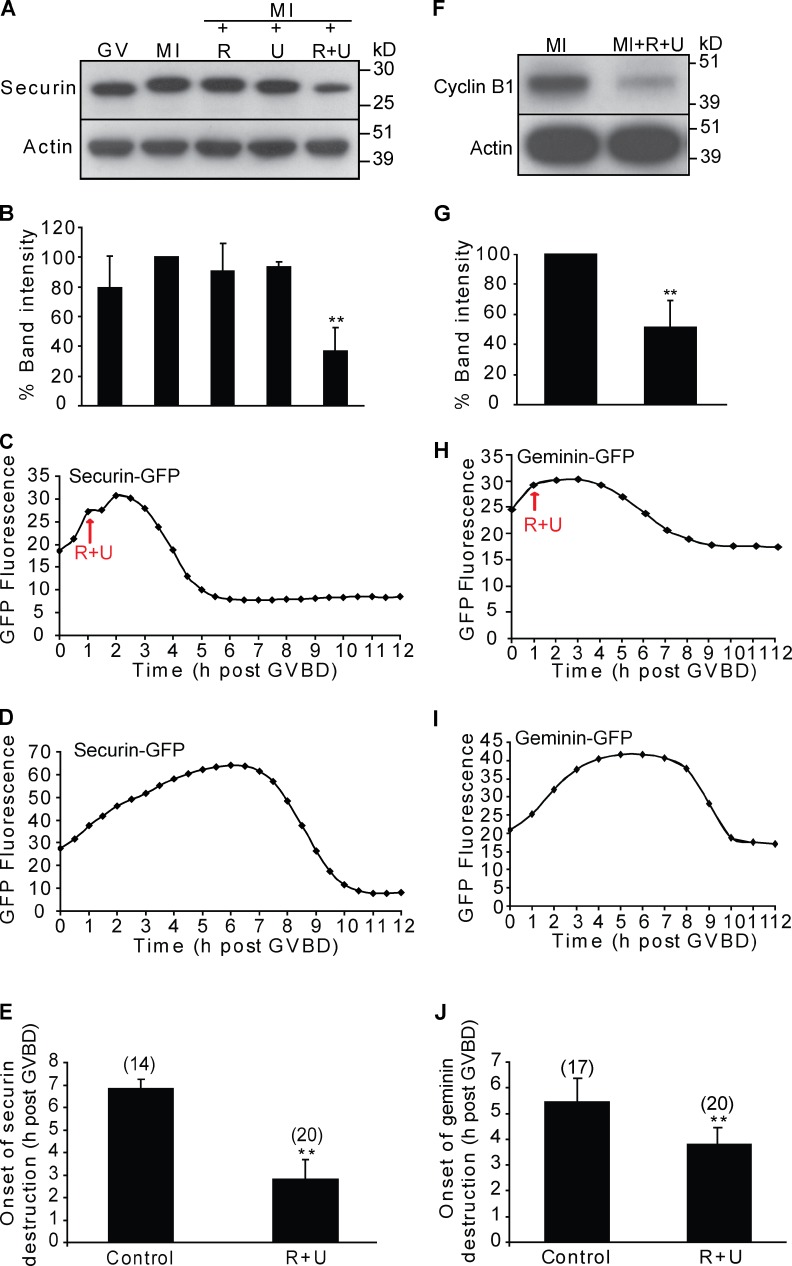
Figure 1.
Inhibition of CDK1 and MAPK in prometaphase I induces premature destruction of APC/C substrates. (A and B) Western blot (20 oocytes/lane; A) and analysis of securin (B) in oocytes during GV arrest, meiosis I (MI), and meiosis I after treatment with roscovitine (R), UO126 (U), or both roscovitine and UO126 (R + U). (C and D) Representative fluorescence traces of oocytes injected with securin-GFP in the presence (C) or absence (D) of roscovitine and UO126. In C, the inhibitors were added at 1 h after GVBD (red arrow). (E) Time of onset of securin-GFP destruction in the treated (roscovitine + UO126; n = 20) and nontreated oocytes (control; n = 14). The peak of the trace was taken as a marker for the start of destruction. (F and G) Western blot (30 oocytes/lane; F) and analysis of cyclin B (G) in oocytes at meiosis I in the presence or absence of roscovitine and UO126. (H and I) Representative fluorescence traces of oocytes injected with geminin-GFP in the presence (H) or absence (I) of roscovitine and UO126, as in C and D. (J) Time of onset of geminin-GFP destruction in the inhibitor-treated (roscovitine + UO126; n = 20) and nontreated oocytes (control; n = 17 from two experiments); data were analyzed as in E. In B and G, results are compared with the control meiosis I lane, which is set at 100%. Error bars show SDs. **, P < 0.01.
Next, we used time-lapse microscopy of oocytes injected with securin-GFP cRNA to examine whether the loss of securin was caused by premature destruction. In oocytes treated with roscovitine and UO126, securin-GFP destruction is initiated ∼3 h after GVBD compared with 7 h in untreated control oocytes (Fig. 1, C–E). We then examined whether two other APC/C substrates, cyclin B1 and geminin (McGarry and Kirschner, 1998; Herbert et al., 2003), also undergo premature destruction on Cdk1 and MAPK inhibition. Western analysis of cyclin B1 in roscovitine- and UO126-treated oocytes showed a similar premature decrease in protein levels as seen with securin (Fig. 1, F and G). Similarly, time-lapse imaging of inhibitor-treated oocytes expressing geminin-GFP showed destruction starting ∼3 h after GVBD compared with 8 h in control oocytes, a pattern very similar to that seen with securin (Fig. 1, H–J). These data demonstrate that Cdk1/MAPK activity is essential for suppression of APC/C activity early in prometaphase I, which is essential to allow the accumulation of cyclin B1 to levels that are sufficient to achieve a metaphase state (Polanski et al., 1998; Gavet and Pines, 2010).
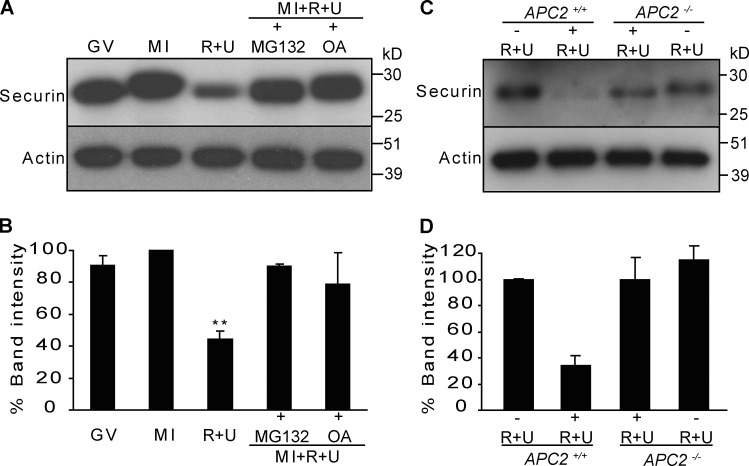
To prove that the APC/C is the site of Cdk1/MAPK action, we set out to test whether phosphorylation was the mode of regulation and whether the 26S proteasome was necessary for increased destruction. Western analysis of roscovitine- and UO126-treated oocytes that were co-incubated with the phosphatase inhibitor okadaic acid or the proteasome inhibitor MG132 shows a complete reversal of the premature securin destruction (Fig. 2, A and B). To provide a genetic evidence that securin destruction was mediated by the APC/C, we examined securin levels in roscovitine- and UO126-treated oocytes lacking APC/C activity as a result of conditional deletion of the Apc2 gene (McGuinness et al., 2009). The experiments revealed that inhibition of MAPK and Cdk1 in Apc2-deleted (Apc2−/−) oocytes was without effect on securin stability during prometaphase I (Fig. 2, C and D). This confirms that the APC/C is responsible for the observed decrease in securin when M-phase kinases are inhibited.
Figure 2.
Proteasome, phosphatase inhibitor, and APC/C deletions restore securin levels. (A and B) Western blot (20 oocytes/lane; A) and analysis of securin (B) in oocytes during GV arrest, meiosis I (MI), and meiosis I after incubation with roscovitine (R), UO126 (U), MG132, or okadaic acid (OA). Results are compared with the meiosis I lane, which is set at 100%. (C and D) Western blot (20 oocytes/lane, n = 2; C) and analysis of securin (D) in Apc2-deleted (Apc2−/−) and control (Apc2+/+) oocytes at meiosis I in the presence or absence of roscovitine and UO126. Error bars show SDs. **, P < 0.01.
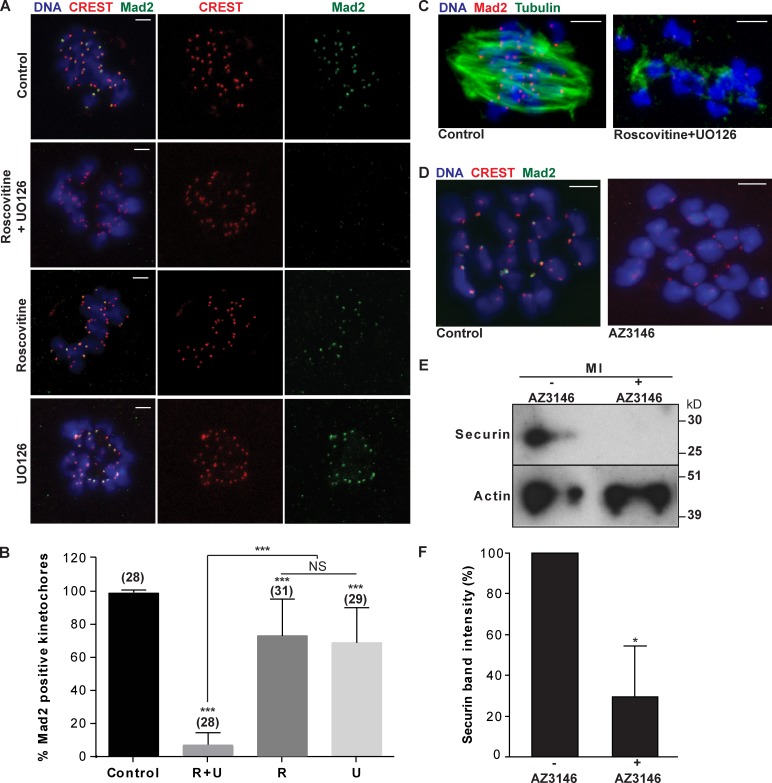
Next, we set out to determine how phosphorylation by Cdk1/MAPK suppresses APC/C activity during prometaphase I. Given the major role of the SAC in suppressing APC/C activity, we focused on a role for phosphorylation in suppressing APC/C via spindle checkpoint regulation (Schwab et al., 2001; Wassmann et al., 2003a; Zhao and Chen, 2006; Kim et al., 2010; Morin et al., 2012). As a readout of the SAC status, we examined the localization of the checkpoint protein Mad2, which binds to unattached kinetochores and is released upon stable microtubule–kinetochore attachments (Waters et al., 1998; Wassmann et al., 2003b; Homer et al., 2009). The effect of inhibition of Cdk1 and MAPK on Mad2 localization was examined using the same treatment regimen that destabilizes APC/C substrates. At 5 h after release, bipolar spindles are forming, and as previously reported (Homer et al., 2009), strong Mad2 staining is seen at virtually every kinetochore (99%; Fig. 3, A–C). After inhibition of Cdk1 or MAPK, Mad2 was present on 70% of kinetochores (Fig. 3, A and B), whereas inhibition of both Cdk1 and MAPK resulted in Mad2 localization on only 5% of kinetochores, including those that lacked microtubules attachments (Fig. 3, A–C). This strongly links Cdk1/MAPK to SAC function in this prometaphase period.
Figure 3.
Inhibition of CDK1 and MAPK induces Mad2 dissociation from kinetochores. (A) Representative images of immunostaining for DNA, CREST, and Mad2 in nontreated control oocytes and oocytes treated with roscovitine, UO126, or both roscovitine and UO126. (B) Proportion of Mad2-positive kinetochores in control (n = 28), both roscovitine- and UO126 (R + U; n = 28)-, roscovitine (R; n = 31)-, and UO126 (U; n = 29)-treated oocytes. (C) Representative images of meiotic spindles from control nontreated meiosis I oocytes (n = 10) and oocytes treated with roscovitine and UO126 (n = 20). (D) Representative images of control nontreated meiosis I oocytes (n = 10) and oocytes treated with the Mps1 inhibitor AZ3146 (n = 10) immunostained for DNA, CREST, and Mad2. (E and F) Western blot (20 oocytes/lane; E) and analysis of securin (F) in oocytes at meiosis I in the presence or absence of AZ3146. In B and F, results were compared with the nontreated control or to each other where indicated and expressed as SEMs. NS, P > 0.05; *, P < 0.05; ***, P < 0.001. Bars, 5 µm.
A strong candidate of the SAC that may be a substrate of Cdk1/MAPK activity is Mps1 (monopolar spindle 1). Recent work in Xenopus laevis egg extracts has shown phosphorylation by Cdk1 or MAPK is important for kinetochore localization of the SAC proteins, including Mad2 (Zhao and Chen, 2006; Morin et al., 2012). Also, mouse oocytes depleted of Mps1 show premature activation of APC/C (Hached et al., 2011). Here, we have asked whether inhibition of Mps1 using the well-characterized inhibitor AZ3146 (Hewitt et al., 2010), during the same prometaphase window, phenocopies the effects of inhibition of Cdk1 and MAPK. The data clearly show that inhibiting Mps1 causes loss of kinetochore-localized Mad2 (Fig. 3 D) and also phenocopies the effect of inhibiting Cdk1 and MAPK in causing a similar premature destruction of securin (Fig. 3, E and F). This study strongly implicates Mps1 as the downstream target for Cdk1/MAPK-mediated suppression of the APC/C.
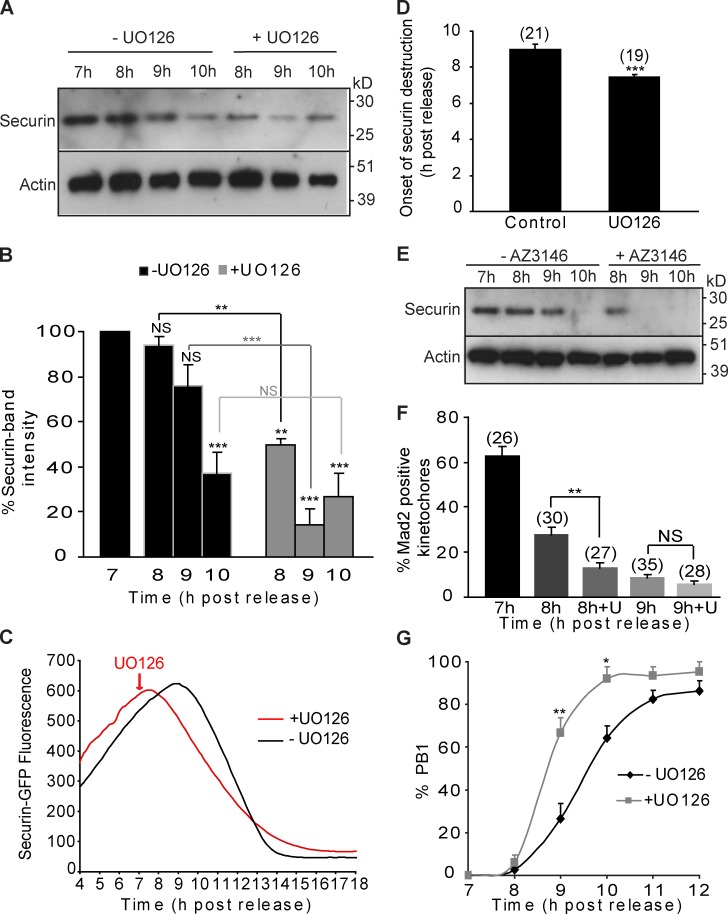
The redundancy of Cdk1 and MAPK in regulating APC/C activity early in prometaphase I raises the question as to whether MAPK plays a role in restraining APC/C activity during the meiosis I to meiosis II (MI–MII) transition when Cdk1 activity is declining. We therefore designed experiments to target this window of APC/C activity during meiosis I. To this end, oocytes were treated with UO126 at 7 h after release, close to the time APC/C activity increases, and the MI–MII transition is initiated in the majority of oocytes (Fig. 1 D). Securin levels were analyzed over the next 3 h to examine whether MAPK inhibition modulates APC/C activity. Western blots show that in control oocytes only 10% of endogenous securin is degraded at 8 h compared with nearly 50% in UO126-treated oocytes (Fig. 4, A and B). This difference is maintained at 9 h when ∼20% and just over 80% of securin is degraded in control and UO126-treated oocytes, respectively. By 10 h, securin destruction has reached a maximum and is similar in both groups (Fig. 4, A and B). Time-lapse imaging of securin-GFP in oocytes treated at 7 h with UO126 show that the faster destruction observed in Western blots appears to be caused by an earlier onset of securin destruction rather than an increased rate of destruction (Fig. 4, C and D). This early onset of APC/C activity is consistent with inhibition of MAPK causing early satisfaction of the SAC. Subsequent to our findings in early prometaphase, we have inhibited Mps1 over the 7–10-h time window of the MI–MII transition and found that it causes premature securin destruction, similar to the effects of inhibiting MAPK (Fig. 4 E).
Figure 4.
Inhibition of MAPK in late prometaphase I induces early destruction of securin, faster Mad2 dissociation, and accelerated PB1 extrusion. (A and B) Western blot (20 oocytes/lane; A) and analysis of securin (B) in oocytes at 7, 8, 9 and 10 h after release from IBMX, in the presence or absence of UO126. (C) Representative fluorescence traces of oocytes injected with securin-GFP in the presence or absence of UO126. (D) Time of onset of securin-GFP destruction in the UO126-treated oocytes (n = 19 from two experiments) and nontreated control oocytes (n = 21 from two experiments). The peak of the graph was taken as a marker for the start of destruction. (E) Western blot (20 oocytes/lane, n = 2) of securin in oocytes at 7, 8, 9, and 10 h after release from IBMX, in the presence or absence of AZ3146. (F) The proportion of Mad2-positive kinetochores at 7, 8, and 9 h after release from IBMX, in the presence or absence of UO126 (U). (G) Timing of PB1 extrusion in control (n = 116) and UO126-treated oocytes (n = 112). In B, D, F, and G, results were compared with the nontreated controls or to each other where indicated and expressed as SEMs. UO126 and AZ3146 were added to the media at 7 h after release from IBMX. NS, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further implicate MAPK-dependent regulation of the SAC in the timing of meiosis, we tested whether inhibition of MAPK from 7 h leads to premature loss of Mad2 from kinetochores and acceleration of polar body extrusion. In control oocytes, Mad2 is lost from kinetochores over the 7–9-h treatment window, whereas in UO126-treated oocytes, Mad2 loss from kinetochores is significantly accelerated by 8 h (Fig. 4 F). Consistent with this premature satisfaction of the SAC, analysis of the timing of PB1 extrusion reveals that PB1 extrusion was consistently faster in UO126-treated oocytes (Fig. 4 G). Thus, inhibition of MAPK from 7 h after release causes premature securin destruction, accelerated loss of kinetochore-associated Mad2, and faster progression through meiosis I. These results suggest that MAPK, acting via the SAC, restrains APC/C activity and therefore refines the timing of meiosis I.
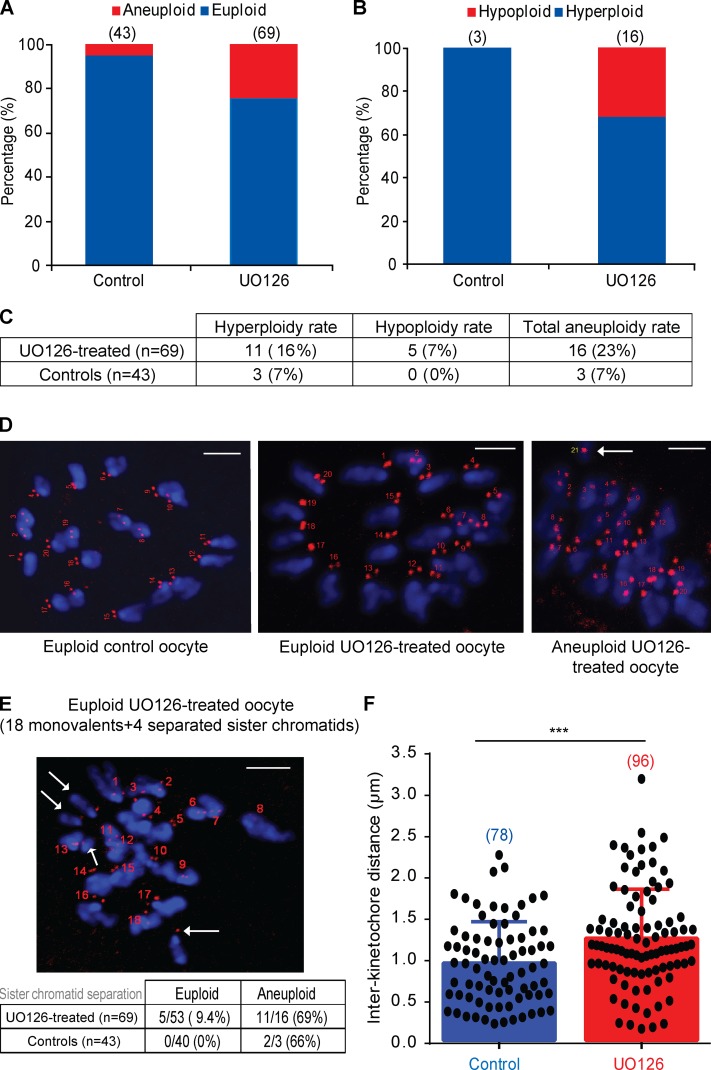
Accelerated progression through meiosis I in mouse oocytes has been associated with chromosome segregation errors during anaphase I, leading to aneuploid metaphase II–arrested eggs (Homer et al., 2005b; Reis et al., 2007). Therefore, we next examined whether inhibition of MAPK at 7 h leads to an increase in aneuploidy in oocytes after completion of meiotic maturation. The majority of controls had a normal chromosome count of 20 monovalents, with only 7% being hyperploid. In contrast, in the UO126-treated oocytes, hyperploidy was observed in 16% of the oocytes, whereas 7% were hypoploid, leading to a total of 23% being aneuploid. Thus, chromosome missegregation had occurred in nearly a quarter of the UO126-treated oocytes, a three- to fourfold increase over controls (Fig. 5, A–D). Furthermore, we observed that in 69% of the UO126-treated aneuploidy oocytes, there were single chromatids, a sign of premature sister separation. This was also observed in 9.4% of the UO126-treated euploid oocytes (Fig. 5 E), suggesting that inhibition of MAPK resulted in a reduced cohesion level. Subsequent measurement of the interkinetochore distance revealed a significantly increased separation of kinetochores in UO126-treated oocytes (mean distance of 1.23 µm) compared with nontreated controls (mean distance of 0.93 µm; Fig. 5 F). On the basis of these findings, we propose that MAPK activity during the MI–MII transition is required for normal segregation and can regulate chromosome cohesion. The increased rate of aneuploidy is probably caused by overriding the SAC, leading to a more rapid activation of the APC/C as well as a loss in SAC-mediated control over the fidelity of the first meiotic division. The increased occurrence of sister separation and increased interkinetochore distance may also be a result of premature degradation of securin, leading to aberrant separase activity (Yamamoto et al., 1996; Ciosk et al., 1998; Nabti et al., 2008). Alternatively, an effect of MAPK on the localization of I2PP2A, an endogenous inhibitor of PP2A (protein phosphatase 2A), cannot be discounted. I2PP2A was shown to colocalize with PP2A at metaphase II, during which it counteracts PP2A-mediated protection of centromeric cohesion (Chambon et al., 2013). Analysis of the mouse I2PP2A sequence has identified a consensus motif (serine 30) for MAPK phosphorylation, thereby providing a possible mechanism linking MAPK to cohesion.
Figure 5.
Inhibition of MAPK in late prometaphase I causes higher rates of aneuploidy and premature sister chromatid separation in metaphase II–arrested eggs. (A) Chromosome count in metaphase II–arrested eggs, at 14 h after release from IBMX, in the presence (UO126, n = 69) or absence (control, n = 43) of UO126. UO126 was added to the media at 7 h after release. (B) Rate of hypoploidy and hyperploidy within the aneuploid UO126-treated eggs (n = 16) and the aneuploid nontreated controls (n = 3). (C) Table summarizing the rate of hyperploidy, hypoploidy, and total aneuploidy in UO126-treated (n = 69) and control (n = 43) eggs. (D) Representative examples of the chromosome counting assay demonstrating a euploid control egg (20 monovalents, numbered in red), a euploid UO126-treated egg (20 monovalents), and an aneuploid UO126-treated egg (20 monovalents + one single chromatid pointed out with an arrow). DNA is shown in blue, and CREST-labeled kinetochores are shown in red. (E) Representative example of a euploid UO126-treated egg featuring premature sister chromatid separation (18 monovalents + four single chromatids pointed out with arrows) and a table summarizing the rates of premature sister separation within UO126-treated (n = 69) and control eggs (n = 43). (F) The mean distance between sister kinetochores in UO126-treated eggs (n = 96 pairs of kinetochores from 17 eggs) and controls (n = 78 pairs of kinetochores from 15 eggs). The results are mean + SEMs. ***, P < 0.001. Bars, 5 µm.
Conclusions
Cdk1 activity drives oocytes through meiosis I, whereas MAPK is best known for promoting arrest at metaphase II. Here, we show that Cdk1 and MAPK act in a complementary manner during prometaphase I to restrain APC/C activity and thereby drive the accumulation of APC/C substrates, including cyclin B1, which is required for completion of meiosis I (Polanski et al., 1998; Ledan et al., 2001; Davydenko et al., 2013). The mechanism, as revealed by a loss of kinetochore-associated Mad2 and a phenocopy of the effects by inhibition of Mps1, appears to be mediated via an effect on the ability to establish or stabilize the SAC. Therefore, our data provide a new feedback loop whereby Cdk1/MAPK activity promotes stabilization of cyclin B1, which is then available to further drive an increase in Cdk1 activity.
A second role uncovered for MAPK is during the MI–MII transition when persistent MAPK activity maintains the SAC as shown by its ability to modulate the timing of securin destruction, the localization of Mad2 at kinetochores, and the timing of progression through the MI–MII transition. Modulation of the timing of meiosis I by this MAPK-mediated maintenance of the SAC ensures a faithful segregation of chromosomes at meiosis I. These observations, together with the finding that Mps1 inhibition phenocopies premature APC/C activation, are consistent with a model in which MAPK-dependent phosphorylation of Mps1 maintains the SAC and controls the timing and fidelity of meiosis I. Finally, our data suggest an additional role for MAPK in the protection of centromeric cohesion, thereby preventing premature sister separation. This study may lead to new insights into the mechanisms of aneuploidy in human oocytes in which an increase in premature sister separation is a common feature (Angell, 1991, 1997; Nagaoka et al., 2012).
Materials and methods
Oocytes collection and culture
GV oocytes were collected from 21–24-d-old MF1 mice (Harlan). The females were superovulated by intraperitoneal injection of 7.5 IU pregnant mare’s serum gonadotropin (Intervet). Mice were killed by cervical dislocation at 46–48 h after pregnant mare’s serum gonadotropin injection. The ovaries were removed and immediately transferred to dissection medium, consisting of M2 medium supplemented with 200 µM 3-isobutyl-1-methylxanthine (IBMX), to keep the oocytes arrested at the GV stage. The cumulus-enclosed oocytes were isolated by mechanical perforation of the ovaries with a 27-gauge needle. The cumulus cells were removed by repeated mouth pipetting using narrow-bore glass Pasteur pipettes. For longer term incubation, the oocytes were cultured in M16 in a 5% CO2 humidified incubator at 37°C.
Treatments
For treatments, oocytes were incubated in culture media (M2 or M16) containing the following drugs: 50 µM roscovitine (EMD Millipore), 50 µM UO126 (Promega), 50 µM MG132 (EMD Millipore), 1 µM okadaic acid (EMD Millipore), or 2 µM AZ3146 (Santa Cruz Biotechnology, Inc.). Because roscovitine is oil soluble, no mineral oil was added to the media, and the culture dish was covered with a lid to prevent evaporation. All drug treatments were performed between 3 and 5 h after release from IBMX, unless otherwise stated.
Microinjection and imaging
All microinjections of GV stage oocytes were performed in M2 medium on the stage of an inverted microscope (DM IRB; Leica). In brief, fabricated micropipettes were inserted into cells using the negative capacitance overcompensation facility on an electrophysiological amplifier (World Precision Instruments), whereas cells were immobilized with a holding pipette (Hunter Scientific). A precise injection volume (2–5% of the total egg volume) was achieved using a pressure regulator (Pneumatic PicoPump; World Precision Instruments). Epifluorescence images of oocytes incubated in M2 medium at 37°C were recorded using an objective (20×, 0.75 NA) on an inverted microscope (Axiovert 100; Carl Zeiss) equipped with an interline cooled charge-coupled device camera (Princeton Instruments MicroMAX; Roper Scientific). GFP-tagged securin and geminin were imaged using an FITC filter set at band pass 450–490 nm for excitation, dichroic mirror 510 nm, and band pass 520 nm for emission. Oocytes were imaged every 10 min to minimize photobleaching and photodamage. MetaMorph and MetaFluor software 6.1 (Molecular Devices) were used for image capture and data analysis.
cRNA
The cRNA for GFP-tagged securin and GFP-tagged geminin were prepared from the T3 promoter of a pRN3-GFP vector, using a transcription kit (T3 mMESSAGE mMACHINE; Ambion). The cRNA was then polyadenylated and purified in nuclease-free water to a concentration of ∼1 µg/µl before microinjection.
Western blotting
Meiosis I oocytes, at 5 h after release from IBMX, unless otherwise stated, were washed in PBS with 1% polyvinylpyrrolidone solution and then heated at 95°C for 5 min with 5× sample buffer. Proteins were fractionated at 200 mV for 50 min on an X Cell II Blot Module (Invitrogen) using a 4–12% NuPAGE Bis-Tris precast gel (Invitrogen) and MOPS running buffer. Proteins were blotted onto polyvinylidene fluoride membranes for 1.5 h at 100 mV. Mouse anti-securin (ab3305; 1:1,000; Abcam), mouse anti–cyclin B1 (ab72; 1:400; Abcam), and mouse anti–β-actin (ab3280; 1:400; Abcam) were used for Western blotting. For primary antibody detection, we used a goat HRP-conjugated anti–mouse secondary antibody (Sigma-Aldrich). Standard ECL techniques (GE Healthcare) were used for secondary antibody detection according to the manufacturer’s instructions. The densitometric analysis of the blots involved the measurement of the intensity of each band using Photoshop CS2 (Adobe), which was then normalized against the relevant actin loading control.
Immunofluorescence
Meiosis I oocytes, at 5 h after release from IBMX, unless otherwise stated, were fixed and permeabilized in PHEM buffer (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, and 2 mM MgCl2) containing 4% paraformaldehyde and 0.5% Triton X-100 and then labeled with calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia (CREST) serum, a human centromere antiserum, (1:300; a gift from G. Fitzharris, University College London, London, England, UK), rabbit anti-Mad2 (PRB-452C; 1:300; Covance), mouse anti–β-tubulin (T4026; 1:1,000; Sigma-Aldrich), and 10 µg/ml Hoechst 33342 (Sigma-Aldrich). Serial z sections of fixed oocytes in PBS were acquired at room temperature using a Plan Apochromat 63×, 1.4 NA oil differential interference contrast objective and a laser-scanning confocal microscope imaging system (LSM 510 META; Carl Zeiss) with the following band pass emission filters in nm 385–470 (Hoechst 33342), 505–530 (Alexa Fluor 488), and 585–615 (Alexa Fluor 546). Z sections were analyzed and projected into one picture using the LSM image browser (Carl Zeiss). The images were then assembled by Illustrator CS2 (Adobe).
Chromosome counts
Chromosome counts were performed as previously described (Duncan et al., 2009; Illingworth et al., 2010). In brief, spindles were collapsed using a 90-min pulse of 200 µM monastrol (EMD Millipore). Oocytes were fixed and permeabilized in PHEM buffer, labeled with CREST serum and Hoechst, and mounted on slides in PBS. Serial z sections were acquired at 0.5-µm intervals at room temperature using a Plan Apochromat 63×, 1.4 NA oil differential interference contrast objective and a laser-scanning confocal microscope imaging system (LSM 510 META) with the following band pass emission filters (nm): 385–470 (Hoechst 33342) and 585–615 (Alexa Fluor 546). Z sections were analyzed and projected into one picture using the LSM image browser. The images were then assembled by Illustrator CS2. All chromosome counts were scored by two independent people. To measure the interkinetochore distance, we used ImageJ software (National Institutes of Health). In brief, a linescan was drawn across each pair of CREST-labeled sister kinetochores. The interkinetochore distance was then measured as the distance between the two peaks of fluorescence, which represent the center of each kinetochore. However, when the sister kinetochores were not in the same plane of focus, we used the Pythagorean theorem to calculate the actual interkinetochore distance.
Statistics and data analysis
Data analysis was performed using appropriate statistical tests in Prism software (GraphPad Software). Analysis was performed for at least three replicate experiments and represented as means and SDs, unless otherwise stated. The level of significance is indicated in the figures and figure legends by NS, P > 0.05; *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Online supplemental material
Fig. S1 shows that flavopiridol and Mos morpholino cause a similar decrease in securin levels as seen with roscovitine and UO126 in early prometaphase I. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201305049/DC1.
Supplementary Material
Acknowledgments
We thank Katarzyna Kuleszewicz for assistance with APC2−/− mice.
This work was supported by a Medical Research Council program grant to J. Carroll. Research in the N.R. Kudo laboratory was funded by the Medical Research Council, the Genesis Research Trust, and the Royal Society of London. Research in the P. Marangos laboratory was funded by John S. Latsis Public Benefit Foundation, Greece.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- APC/C
- anaphase-promoting complex/cyclosome
- CREST
- calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia
- GV
- germinal vesicle
- GVBD
- GV breakdown
- IBMX
- 3-isobutyl-1-methylxanthine
- SAC
- spindle assembly checkpoint
References
- Angell R.R. 1991. Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum. Genet. 86:383–387 10.1007/BF00201839 [DOI] [PubMed] [Google Scholar]
- Angell R. 1997. First-meiotic-division nondisjunction in human oocytes. Am. J. Hum. Genet. 61:23–32 10.1086/513890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon J.P., Touati S.A., Berneau S., Cladière D., Hebras C., Groeme R., McDougall A., Wassmann K. 2013. The PP2A inhibitor I2PP2A is essential for sister chromatid segregation in oocyte meiosis II. Curr. Biol. 23:485–490 10.1016/j.cub.2013.02.004 [DOI] [PubMed] [Google Scholar]
- Chung E.N., Chen R.H. 2003. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 5:748–753 10.1038/ncb1022 [DOI] [PubMed] [Google Scholar]
- Ciosk R., Zachariae W., Michaelis C., Shevchenko A., Mann M., Nasmyth K. 1998. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 93:1067–1076 10.1016/S0092-8674(00)81211-8 [DOI] [PubMed] [Google Scholar]
- Clute P., Pines J. 1999. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1:82–87 10.1038/10049 [DOI] [PubMed] [Google Scholar]
- Conti M., Hsieh M., Zamah A.M., Oh J.S. 2012. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 356:65–73 10.1016/j.mce.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod S.A., Bolling L.C., Wright P.W., Visconti P.E., Herr J.C. 2001. A morpholino phenocopy of the mouse mos mutation. Genesis. 30:198–200 10.1002/gene.1065 [DOI] [PubMed] [Google Scholar]
- D’Angiolella V., Mari C., Nocera D., Rametti L., Grieco D. 2003. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 17:2520–2525 10.1101/gad.267603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydenko O., Schultz R.M., Lampson M.A. 2013. Increased CDK1 activity determines the timing of kinetochore-microtubule attachments in meiosis I. J. Cell Biol. 202:221–229 10.1083/jcb.201303019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan F.E., Chiang T., Schultz R.M., Lampson M.A. 2009. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol. Reprod. 81:768–776 10.1095/biolreprod.109.077909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata M.F., Horiuchi K.Y., Manos E.J., Daulerio A.J., Stradley D.A., Feeser W.S., Van Dyk D.E., Pitts W.J., Earl R.A., Hobbs F., et al. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623–18632 10.1074/jbc.273.29.18623 [DOI] [PubMed] [Google Scholar]
- Gavet O., Pines J. 2010. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell. 18:533–543 10.1016/j.devcel.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr I.H., Reis A., Boos D., Wühr M., Madgwick S., Jones K.T., Stemmann O. 2006. Essential CDK1-inhibitory role for separase during meiosis I in vertebrate oocytes. Nat. Cell Biol. 8:1035–1037 10.1038/ncb1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hached K., Xie S.Z., Buffin E., Cladière D., Rachez C., Sacras M., Sorger P.K., Wassmann K. 2011. Mps1 at kinetochores is essential for female mouse meiosis I. Development. 138:2261–2271 10.1242/dev.061317 [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Kishimoto T. 1988. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev. Biol. 126:242–252 10.1016/0012-1606(88)90135-2 [DOI] [PubMed] [Google Scholar]
- Herbert M., Levasseur M., Homer H., Yallop K., Murdoch A., McDougall A. 2003. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat. Cell Biol. 5:1023–1025 10.1038/ncb1062 [DOI] [PubMed] [Google Scholar]
- Hershko A., Ganoth D., Sudakin V., Dahan A., Cohen L.H., Luca F.C., Ruderman J.V., Eytan E. 1994. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J. Biol. Chem. 269:4940–4946 [PubMed] [Google Scholar]
- Hewitt L., Tighe A., Santaguida S., White A.M., Jones C.D., Musacchio A., Green S., Taylor S.S. 2010. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1–C-Mad2 core complex. J. Cell Biol. 190:25–34 10.1083/jcb.201002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Maro B., Kubiak J.Z., Polanski Z. 2011. A single bivalent efficiently inhibits cyclin B1 degradation and polar body extrusion in mouse oocytes indicating robust SAC during female meiosis I. PLoS ONE. 6:e27143 10.1371/journal.pone.0027143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J.E., Lane S.I.R., Jennings P., García-Higuera I., Moreno S., Jones K.T. 2012. APC(FZR1) prevents nondisjunction in mouse oocytes by controlling meiotic spindle assembly timing. Mol. Biol. Cell. 23:3970–3981 10.1091/mbc.E12-05-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer H. 2013. The APC/C in female mammalian meiosis I. Reproduction. 146:R61–R71 10.1530/REP-13-0163 [DOI] [PubMed] [Google Scholar]
- Homer H.A., McDougall A., Levasseur M., Murdoch A.P., Herbert M. 2005a. Mad2 is required for inhibiting securin and cyclin B degradation following spindle depolymerisation in meiosis I mouse oocytes. Reproduction. 130:829–843 10.1530/rep.1.00856 [DOI] [PubMed] [Google Scholar]
- Homer H.A., McDougall A., Levasseur M., Yallop K., Murdoch A.P., Herbert M. 2005b. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 19:202–207 10.1101/gad.328105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer H., Gui L., Carroll J. 2009. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science. 326:991–994 10.1126/science.1175326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth C., Pirmadjid N., Serhal P., Howe K., Fitzharris G. 2010. MCAK regulates chromosome alignment but is not necessary for preventing aneuploidy in mouse oocyte meiosis I. Development. 137:2133–2138 10.1242/dev.048306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D., Ohe M., Kanemori Y., Nobui T., Sagata N. 2007. A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature. 446:1100–1104 10.1038/nature05688 [DOI] [PubMed] [Google Scholar]
- Jaspersen S.L., Charles J.F., Morgan D.O. 1999. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9:227–236 10.1016/S0960-9822(99)80111-0 [DOI] [PubMed] [Google Scholar]
- Jones K.T. 2008. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum. Reprod. Update. 14:143–158 10.1093/humupd/dmm043 [DOI] [PubMed] [Google Scholar]
- Kim S., Sun H.B., Ball H.L., Wassmann K., Luo X.L., Yu H.T. 2010. Phosphorylation of the spindle checkpoint protein Mad2 regulates its conformational transition. Proc. Natl. Acad. Sci. USA. 107:19772–19777 10.1073/pnas.1009000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Tugendreich S., Fujii M., Jorgensen P.M., Watanabe N., Hoog C., Hieter P., Todokoro K. 1998. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell. 1:371–380 10.1016/S1097-2765(00)80037-4 [DOI] [PubMed] [Google Scholar]
- Kraft C., Herzog F., Gieffers C., Mechtler K., Hagting A., Pines J., Peters J.M. 2003. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22:6598–6609 10.1093/emboj/cdg627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledan E., Polanski Z., Terret M.E., Maro B. 2001. Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev. Biol. 232:400–413 10.1006/dbio.2001.0188 [DOI] [PubMed] [Google Scholar]
- Madgwick S., Nixon V.L., Chang H.Y., Herbert M., Levasseur M., Jones K.T. 2004. Maintenance of sister chromatid attachment in mouse eggs through maturation-promoting factor activity. Dev. Biol. 275:68–81 10.1016/j.ydbio.2004.07.024 [DOI] [PubMed] [Google Scholar]
- McGarry T.J., Kirschner M.W. 1998. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 93:1043–1053 10.1016/S0092-8674(00)81209-X [DOI] [PubMed] [Google Scholar]
- McGuinness B.E., Anger M., Kouznetsova A., Gil-Bernabé A.M., Helmhart W., Kudo N.R., Wuensche A., Taylor S., Hoog C., Novak B., Nasmyth K. 2009. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr. Biol. 19:369–380 10.1016/j.cub.2009.01.064 [DOI] [PubMed] [Google Scholar]
- Meijer L., Borgne A., Mulner O., Chong J.P., Blow J.J., Inagaki N., Inagaki M., Delcros J.G., Moulinoux J.P. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527–536 10.1111/j.1432-1033.1997.t01-2-00527.x [DOI] [PubMed] [Google Scholar]
- Morin V., Prieto S., Melines S., Hem S., Rossignol M., Lorca T., Espeut J., Morin N., Abrieu A. 2012. CDK-dependent potentiation of MPS1 kinase activity is essential to the mitotic checkpoint. Curr. Biol. 22:289–295 10.1016/j.cub.2011.12.048 [DOI] [PubMed] [Google Scholar]
- Musacchio A., Salmon E.D. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- Nabti I., Reis A., Levasseur M., Stemmann O., Jones K.T. 2008. Securin and not CDK1/cyclin B1 regulates sister chromatid disjunction during meiosis II in mouse eggs. Dev. Biol. 321:379–386 10.1016/j.ydbio.2008.06.036 [DOI] [PubMed] [Google Scholar]
- Nagaoka S.I., Hassold T.J., Hunt P.A. 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13:493–504 10.1038/nrg3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T., Ohsumi K., Kishimoto T. 2007. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature. 446:1096–1099 10.1038/nature05696 [DOI] [PubMed] [Google Scholar]
- Peters J.M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 9:931–943 10.1016/S1097-2765(02)00540-3 [DOI] [PubMed] [Google Scholar]
- Peters J.M. 2006. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7:644–656 10.1038/nrm1988 [DOI] [PubMed] [Google Scholar]
- Phillips K.P., Petrunewich M.A.F., Collins J.L., Booth R.A., Liu X.J., Baltz J.M. 2002. Inhibition of MEK or cdc2 kinase parthenogenetically activates mouse eggs and yields the same phenotypes as Mos−/− parthenogenotes. Dev. Biol. 247:210–223 10.1006/dbio.2002.0680 [DOI] [PubMed] [Google Scholar]
- Pines J. 2011. Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 12:427–438 10.1038/nrm3132 [DOI] [PubMed] [Google Scholar]
- Polanski Z., Ledan E., Brunet S., Louvet S., Verlhac M.H., Kubiak J.Z., Maro B. 1998. Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development. 125:4989–4997 [DOI] [PubMed] [Google Scholar]
- Potapova T.A., Daum J.R., Pittman B.D., Hudson J.R., Jones T.N., Satinover D.L., Stukenberg P.T., Gorbsky G.J. 2006. The reversibility of mitotic exit in vertebrate cells. Nature. 440:954–958 10.1038/nature04652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A., Madgwick S., Chang H.Y., Nabti I., Levasseur M., Jones K.T. 2007. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat. Cell Biol. 9:1192–1198 10.1038/ncb1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M.S., Roberts B.T., Gross S.D., Tunquist B.J., Taieb F.E., Lewellyn A.L., Maller J.L. 2001. Bub1 is activated by the protein kinase p90(Rsk) during Xenopus oocyte maturation. Curr. Biol. 11:141–150 10.1016/S0960-9822(01)00045-8 [DOI] [PubMed] [Google Scholar]
- Shoji S., Yoshida N., Amanai M., Ohgishi M., Fukui T., Fujimoto S., Nakano Y., Kajikawa E., Perry A.C.F. 2006. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 25:834–845 10.1038/sj.emboj.7600953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terret M.E., Wassmann K., Waizenegger I., Maro B., Peters J.M., Verlhac M.H. 2003. The meiosis I-to-meiosis II transition in mouse oocytes requires separase activity. Curr. Biol. 13:1797–1802 10.1016/j.cub.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Tung J.J., Hansen D.V., Ban K.H., Loktev A.V., Summers M.K., Adler J.R., III, Jackson P.K. 2005. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA. 102:4318–4323 10.1073/pnas.0501108102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R., Prinz S., Amon A. 1997. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 278:460–463 10.1126/science.278.5337.460 [DOI] [PubMed] [Google Scholar]
- Wassmann K., Liberal V., Benezra R. 2003a. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 22:797–806 10.1093/emboj/cdg071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann K., Niault T., Maro B. 2003b. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr. Biol. 13:1596–1608 10.1016/j.cub.2003.08.052 [DOI] [PubMed] [Google Scholar]
- Waters J.C., Chen R.H., Murray A.W., Salmon E.D. 1998. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141:1181–1191 10.1083/jcb.141.5.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.Q., Hansen D.V., Guo Y.X., Wang M.Z., Tang W.L., Freel C.D., Tung J.J., Jackson P.K., Kornbluth S. 2007. Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc. Natl. Acad. Sci. USA. 104:16564–16569 10.1073/pnas.0707537104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Guacci V., Koshland D. 1996. Pds1p is required for faithful execution of anaphase in the yeast, Saccharomyces cerevisiae. J. Cell Biol. 133:85–97 10.1083/jcb.133.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.Z., Xiong B., Gao W.X., Wang C.M., Zhong Z.S., Huo L.J., Wang Q., Hou Y., Liu K., Liu X.J., et al. 2007. MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle. 6:330–338 10.4161/cc.6.3.3805 [DOI] [PubMed] [Google Scholar]
- Zachariae W., Schwab M., Nasmyth K., Seufert W. 1998. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 282:1721–1724 10.1126/science.282.5394.1721 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen R.H. 2006. Mps1 phosphorylation by MAP kinase is required for kinetochore localization of spindle-checkpoint proteins. Curr. Biol. 16:1764–1769 10.1016/j.cub.2006.07.058 [DOI] [PubMed] [Google Scholar]
- Zur A., Brandeis M. 2001. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 20:792–801 10.1093/emboj/20.4.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.