Abstract
Background and Objectives
The purpose of this study was to examine the effect of hepatitis C virus (HCV) infection on buprenorphine pharmacokinetics in opioid-dependent, buprenorphine/naloxone-maintained adults.
Methods
A retrospective analysis of buprenorphine pharmacokinetics in HCV seropositive and seronegative buprenorphine/naloxone-maintained individuals (N = 49) was undertaken.
Results
Relative to HCV seronegative subjects, HCV seropositive subjects had higher buprenorphine exposure, as demonstrated by elevated buprenorphine AUC and Cmax values (p = .03 and .02, respectively) and corresponding elevations in the metabolites, buprenorphine-3-glucuronide AUC values (p = .03) and norbuprenorphine-3-glucuronide AUC and C24 values (p = .05 and .03, respectively).
Discussion and Conclusions
HCV infection was associated with higher plasma concentrations of buprenorphine and buprenorphine metabolites.
Scientific Significance and Future Directions
Findings suggest the potential for opioid toxicity among HCV-infected patients treated with buprenorphine/naloxone, and possible hepatotoxic effects related to increased buprenorphine exposure. HCV-infected patients receiving buprenorphine may need lower doses to maintain therapeutic plasma concentrations.
Background
Buprenorphine and buprenorphine/naloxone have demonstrated efficacy and safety in the treatment of opioid dependence.1 The availability of buprenorphine treatment in office-based medical practice settings in the U.S. offers opioid-addicted patients more medical options, and increases access to evidence-based pharmacotherapies. Wider availability of buprenorphine treatment for opioid-dependent patients may attract patients who present with serious complications of injection drug use including hepatic disease. However, buprenorphine's pharmacokinetic properties have not been evaluated in patients with liver disease. Given the mainly hepatic elimination of buprenorphine, there is the potential risk of its accumulation in individuals with liver disease. This is of particular concern among opioid-dependent individuals who self-administer opiates through the injection route because of the high prevalence of hepatitis C virus (HCV) infection in this population. Furthermore, buprenorphine has been associated with elevations in liver tests, mainly in those with HCV.2,3 Should buprenorphine accumulate in those with HCV infection, the risk for hepatotoxicity might be increased.
The prevalence of HCV infection in injection drug users in the U.S. varies in published data, but rates of 32% to 91% have been reported.4-9 HCV infection can be acquired rapidly by injection drug users with 65% positive for anti-HCV after 1 year or less of injection drug use.4 Chronic HCV infection occurs in 70% to 80% of cases, and of those, up to 20% will develop liver disease, including cirrhosis, liver failure or hepatocellular carcinoma.10 Given the high rates of chronic HCV infection among - individuals dependent on illicit opioids, knowledge regarding the effect of HCV infection on buprenorphine pharmacokinetics and the potential for liver toxicity would be important in the clinical care and management of these patients.
Few studies have examined the potential toxic effects of buprenorphine treatment on the liver. The available evidence suggests that buprenorphine may be associated with liver toxicity even at therapeutic doses in patients who are susceptible, such as those with HCV infection. For example, Petry et al.3 found elevated liver enzyme aspartate aminotransferase (AST) and alanine aminotransferse (ALT) levels in patients with a history of liver disease who were treated with therapeutic doses of sublingual buprenorphine. However, additional data are needed to better understand the potential hepatotoxic effects of buprenorphine in HCV-infected individuals.
The purpose of this study was to examine differences in buprenorphine pharmacokinetic parameters in HCV seropositive and seronegative opioid-dependent, buprenorphine/naloxone-maintained subjects with otherwise normal liver tests. Findings from this study may inform clinical practice guidelines for the use of buprenorphine in the treatment of opioid dependence and HCV infection.
Methods
A secondary analysis of data from individuals participating in drug interaction studies between buprenorphine and either HIV antiretroviral medications or anti-tuberculosis medications was conducted to examine the effect of evidence of HCV infection as determined by HCV seropositive status on the pharmacokinetics of buprenorphine. HCV seropositivity was determined using an enzyme immunoassay for the qualitative detection of antibody to HCV available either from Abbott Laboratories or Bayer, Inc. with a specificity of 99.79% or 97.5% respectively. Of the 49 patients enrolled in these studies, 20 were seropositive for HCV antibody at baseline. Methods and procedures for the drug interaction studies from which the buprenorphine pharmacokinetics data was obtained have been published elsewhere.11-15 Briefly, subjects in these studies were opioid-dependent individuals who were stable for 14-24 days on buprenorphine/naloxone sublingual tablets (range 8/2-16/4 mg daily). The drug interaction studies from which this sample was drawn were conducted in inpatient university-based research units and occurred over seven years. During this time, some study subjects applied to participate in several drug interaction studies. Only the initial buprenorphine pharmacokinetics study data for each unique subject was included for this analysis. Our previous retroactive study on gender differences in the same population showed there was minimal difference between use of first session only and use of mean results for all sessions.16 Steady-state buprenorphine concentrations appear to be approximately dose-proportional.17 Accordingly, buprenorphine and metabolite concentration data were normalized to a standard dose of buprenorphine 16 mg daily before pharmacokinetics analysis. Each study subject initially underwent a baseline pharmacokinetics study in which blood samples were collected immediately before buprenorphine/naloxone dosing and at pre-determined subsequent time points following buprenorphine/naloxone administration over a 24- hour dosing interval. This study compared the baseline pharmacokinetics of buprenorphine and buprenorphine metabolites (i.e., before administration of any antiretroviral or anti-tuberculosis medications) in subjects who were either seropositive or seronegative for HCV. The study also compared baseline liver enzyme aspartate aminotransferase (AST) and alanine aminotransferase (ALT) values between HCV seropositive and negative subjects, and differences in AST and ALT values between these groups after at least 2 weeks of administration of buprenorphine/naloxone (at the time of the pharmacokinetics study). Hepatic fibrosis was evaluated using the APRI (AST/platelet count ratio) with a score of < 0.5 being indicative of a lack of clinically significant hepatic fibrosis.18 A within subjects analysis was also conducted to examine changes in AST and ALT values before and after administration of buprenorphine/naloxone. All subjects provided voluntary, written, informed consent prior to enrollment and were compensated for their participation in the study. Protocols were reviewed and approved by appropriate institutional review boards. A Certificate of Confidentiality was obtained from the National Institutes of Health.
Biochemical Assays
Buprenorphine Assay
Buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucronide concentrations were determined using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS).19 Buprenorphine-d4 and norbuprenorphine-d3were added to samples as the internal standards. The pH of the matrix was adjusted to 9.3 with ammonium carbonate buffer and samples were extracted using C18 solid-phase extraction columns. The eluate was evaporated and reconstituted with 0.1% formic acid in water and analyzed by LC-MS/MS using electrospray ionization. During validation, inter-run precision was within 10.7, 5.2, and 8.2% at 0.25, 25, and 40 ng/mL, respectively.
Pharmacokinetics Analysis
The pharmacokinetic parameters of buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucronide were determined for each subject following sublingual administration. Area under the concentration-time curve (AUC), 24-hour plasma concentration (C24), maximum plasma concentration (Cmax), time of Cmax (Tmax), and sublingual buprenorphine clearance adjusted for bioavailability (Cl/F) were determined using the noncompartmental analysis module of WinNonLin Professional Version 5.2 (Pharsight Corp., Mountain View, CA). For metabolites, Cl/F was calculated based on the administered dose of buprenorphine. The F term thereby represents the fraction of parent drug ultimately converted to the metabolite. Values for Cmax, C24, and Tmax were determined by inspection of the raw data. For purposes of noncompartmental analysis, drug concentrations that were less than the limit of quantification were expressed as one half of the limit.
The independent samples t-test was used to test the significance of the differences in continuous demographic and clinical characteristics between HCV-positive and HCV-negative subjects. Comparisons between HCV-positive and HCV-negative subjects for dichotomous demographic and clinical characteristics were conducted using the Fisher's exact test. Pharmacokinetic parameters for HCV-positive and negative-subjects were compared using the independent samples t-test or, for Tmax, the Mann-Whitney U test. For the analysis of liver chemistries, the independent samples t-test was used to compare differences in AST and ALT values between HCV-positive and HCV-negative subjects (i.e., between subjects comparison). Paired t-tests were used to examine changes in AST and ALT values prior to and following administration of buprenorphine/naloxone separately for subjects with and without HCV infection (i.e., within subjects analysis). Results were considered statistically significant at p < .05 (two-tailed).
Results
Study Subjects
Table 1 summarizes demographic and clinical features of the 49 study subjects by HCV infection status. HCV seropositive subjects were older (p = .04), and were more likely to have a history of injection drug use (p = .0001) than HCV negative subjects. HCV negative subjects were more likely to be African-American. HCV seropositive subjects were less likely to show evidence of recent cocaine use (p = .019), but the groups did not differ on other demographic and substance use characteristics. With regard to liver enzyme tests, HCV seropositive subjects had higher baseline AST (34.1(13.3) mean (SD)) relative to those without evidence of HCV infection 22.3 (7.8) (p = .001) and higher ALT levels (36.3 (24.3) vs. 23.1 (9.7), p = .03). Following buprenorphine/naloxone treatment for at least two weeks at a constant dose within the recommended therapeutic range (8/2-16/4 mg daily), AST was modestly increased in both groups (HCV seropositive: 38.4 (17.7) and HCV seronegative: 25.4 (8.5)). These increases were not statistically significant. The differences between the groups, however, remained statistically significant (p = .01). Modest, but statistically significant increases in ALT were observed after at least two weeks of buprenorphine/naloxone treatment in HCV seropositive individuals, with the mean rising from a baseline of 36.3 (24.3) to 40.8 (23.7), p = .05. The HCV seronegative group also showed a modest increase in mean ALT following buprenorphine/naloxone treatment to 27.7 (18.9) relative to the baseline value of 23.1 (9.7), but the difference did not reach statistical significance. APRI scores for HCV seropositive participants (0.14 (0.6)) were significantly greater (p = .0009) than those for HCV seronegative participants (0.08 (0.04)), but results for both groups indicated a lack of clinically significant hepatic fibrosis (i.e., APRI score of < 0.5). Results for bilibrubin, alkaline phosphatase, and total protein at baseline were within normal limits and did not differ significantly between groups.
Table 1.
Demographic and clinical characteristics of HCV seropositive and HCV seronegative opioid-dependent adults.
| Characteristic | HCV Seropositive n = 20), No. (%) or Mean ± SD | HCV Seronegative (n = 29), No. (%) or Mean ± SD | P-value |
|---|---|---|---|
| Female | 6 (30%) | 10 (34%) | NS |
| Race | |||
| African-American | 7 (35%) | 22 (76%) | .01 |
| Caucasian | 10 (50%) | 4 (14%) | |
| Other | 3 (15%) | 3 (10%) | |
| Substance use disorders | |||
| Opioid dependence | 20 (100%) | 29 (100%) | |
| Cocaine abuse | 4 (20%) | 4 (14%) | |
| Marijuana abuse | 3 (15%) | 1 (3%) | |
| Amphetamine abuse | 1 (5%) | 0 (0%) | |
| Alcohol abuse | 0 (0%) | 2 (<1%) | |
| IDU | 18 (90%) | 4 (14%) | .0001 |
| Urine toxicology screen | |||
| Opiates | 20 (100%) | 29 (100%) | |
| Marijuana | 7 (35%) | 8 (28%) | |
| Benzodiazepines | 0 (0%) | 1 (3%) | |
| Cocaine | 5 (25%) | 18 (62%) | .019 |
| Nicotine use (packs/day) | 0.9 (0.5) | 0.7 (0.5) | * NS |
| Age (years) | 44.2 (8.7) | 35.0 (8.7) | .001 |
| Weight (kg) | 75.8 (16.3) | 79.9 (21.0) | NS |
| AST pre/post Normal range: 10-41 U/L | 34.1 (13.3)/38.3 (60.3) | 22.3 (7.8)/25.4 (8.5) | .001/.01 |
| ALT pre/post Normal range: 10-40 U/L | 36.3 (24.3)/40.8 (113.5) | 23.1 (9.7)/27.7 (18.9) | .03/.05 |
| APRI (AST/platelet count) | 0.5 (0.2) | 0.5 (0.2) | NS |
| Total bilirubin Normal range: 0.1-1.2 mg/dL | 0.14 (0.06) | 0.08 (0.04) | .0009 |
| Alkaline phosphatase Normal range: 53-128 U/L | 70.3 (18.6) | 78.1 (16.8) | NS |
| Total protein Normal range: 6.4-8.3 g/dL | 7.0 (0.5) | 6.7 (0.5) | NS |
Note: Other race/ethnicity category includes Hispanic, Native American, Pacific Islander, and mixed race/ethnicity individuals.
NS = non-significant
Effect of HCV Infection on Buprenorphine Pharmacokinetics
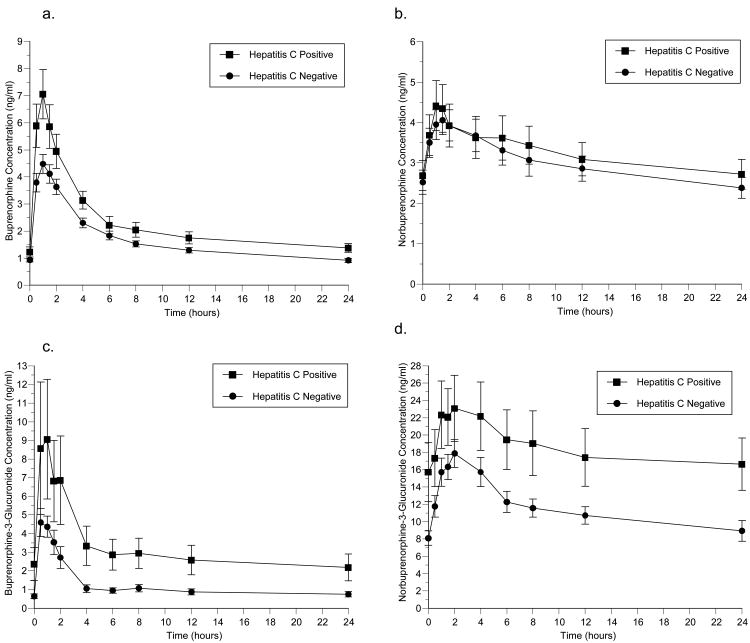
Figure 1 and Table 2 compare the pharmacokinetics of buprenorphine and its metabolites (norbuprenoprhine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide) for HCV seropositive and HCV seronegative subjects. HCV seropositive subjects had greater exposure buprenorphine as shown by higher plasma concentrations over the 24-hour dosing interval and significantly higher AUC and Cmax (p = .03 and .02, respectively). No statistically significant differences were found between groups in norbuprenorphine concentrations or pharmacokinetics. HCV seropositive subjects had significantly higher buprenorphine-3-glucuronide AUC values (p = .03), but the buprenorphine-3-glucuronide C24 was lower (p = .07). For norbuprenorphine-3-glucuronide, AUC and C24 values were significantly higher for HCV seropositive subjects (p = .05 and .03, respectively).
Figure 1.
Effect of HCV serostatus on the pharmacokinetics of (a) buprenorphine, (b) norbuprenorphine, (c) buprenorphine-3-glucuronide, and (d) norbuprenorphine-3-glucuronide.
Table 2.
Effect of HCV serostatus on buprenorphine and buprenorphine metabolite pharmacokinetic parameters.
| Pharmacokinetic Parameter | HCV Seropositive (n = 20) | HCV Seronegative (n = 29) | P-value |
|---|---|---|---|
| Buprenorphine | |||
| AUC0-24 (ng-h/ml) | 53.4 (5.9) | 40.5 (2.4) | .03 |
| Cl/F (L/h) | 425 (86) | 460 (45) | .70 |
| Cmax (ng/ml) | 7.2 (0.8) | 5.3 (0.4) | .02 |
| Tmax (h) | 1.0 (0.5–2.0) | 1.0 (0.5–2.0)) | .36 |
| C24 | 1.22 (0.17) | 0.94 (0.08) | .11 |
| Half-life (h) | 32.0 (3.7) | 27.7 (3.5) | .40 |
| Norbuprenorphine | |||
| AUC0-24 (ng*h/ml) | 72 (9.7) | 73 (7.3) | .95 |
| Cl/F (L/h) | 418 (120) | 274 (23) | .17 |
| Cmax (ng/ml) | 4.5 (0.5) | 4.7 (0.4) | .76 |
| Tmax (h) | 1.25 (0.5 – 6) | 1.5 (0.5 – 6) | .36 |
| C24 | 2.5 (0.3) | 2.4 (0.3) | .83 |
| Half-life (h) | 69.4 (8.8) | 68.6 (9.2) | .95 |
| Buprenorphine-3-Glucuronide | |||
| AUC0-24 (ng*h/ml) | 67 (20) | 29 (4.1) | .03 |
| Cl/F (L/h) | 799 (221) | 903 (139) | .68 |
| Cmax (ng/ml) | 9.5 (2.8) | 6.74 (0.9) | .29 |
| Tmax (h) | 1.0 (0.5–2) | 1.0 (0.5–2) | .39 |
| C24 | 0.49 (0.16) | 1.41 (0.57) | .07 |
| Norbuprenorphine-3-Glucuronide | |||
| AUC0-24 (ng*h/ml) | 426 (78) | 281 (24) | .05 |
| Cl/F (L/h) | 96 (36) | 69 (6) | .39 |
| Cmax (ng/ml) | 25.1 (4.1) | 21.1 (1.7) | .32 |
| Tmax (h) | 2 (1–12) | 2 (0.5–8) | .71 |
| C24 | 15.4 (3.0) | 9.1 (1.2) | .03 |
Note: Concentration data were normalized to a standard dose of 16 mg buprenorphine before analysis. Values are the mean (standard error of the mean), except that Tmax is given as median (range). The unpaired t-test was used to determine P-values for all parameters except Tmax, where the Mann-Whitney U test was used.
Discussion and Conclusions
This study demonstrates that among individuals receiving equivalent doses of buprenorphine/naloxone, HCV seropositive subjects had higher concentrations of circulating parent drug and glucuronide metabolites than those found to be HCV seronegative. These findings suggest that bioavailability of buprenorphine is increased and/or that metabolism of buprenorphine is decreased in HCV seropositive individuals, including those without evidence of active liver disease. There was no evidence for hepatic fibrosis in any study participant, however modest increases in liver enzyme tests were observed in those who were HCV seropositive. While differences from those without evidence of HCV infection were statistically significant; those differences are unlikely to be of clinical significance.
Buprenorphine is extensively metabolized by N-dealkylation to norbuprenorphine. Both buprenorphine and norbuprenorphine undergo glucuronidation.20 Buprenorphine N-dealkylation is primarily mediated by cytochrome P450 (CYP) 3A4 and CYP2C8,21 and it has been shown that expression of CYP3A may be significantly decreased in patients with severe chronic liver disease.22 In this study higher buprenorphine concentrations were observed in HCV seropositive individuals, but these individuals had similar plasma concentrations of norbuprenorphine relative to HCV seronegative subjects. This could be explained by a combination of increased bioavailability of buprenorphine (e.g., due to increased absorption and/or decreased first pass metabolism of swallowed or enterohepatically recirculated buprenorphine) and decreased metabolism to norbuprenorphine. These effects would be additive in increasing buprenorphine concentration, but offsetting in terms of changes in norbuprenorphine. Progressive HCV decreases drug metabolizing enzymes.23 Whether decreases in first pass metabolism or systemic metabolism occurs in earlier stages of the disease (without obvious hepatic compromise) is unknown, but this study may provide some support for the hypothesis that HCV has more effect in early stages than has been appreciated to date. While buprenorphine and norbuprenorphine have been reported to inhibit CYP 3A4, this occurred at concentrations substantially higher than those observed in standard clinical use of this medication;24 even the elevated concentrations observed in the present study, and thus is unlikely to be a factor. The observed significant increases in buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide in HCV seropositive individuals may be indicative of impaired biliary clearance of the glucuronides. Of note, no study participant had a history of or evidence for pre-existing biliary disease. Further, no study subject had renal disease or evidence of renal impairment; thus impaired clearance of glucuronides is likely to be mediated by the liver.
One consequence of reduced drug metabolism is the risk of accumulation of the drug and its metabolites, especially with repeated administration. Findings from this study are significant because differences in liver enzyme tests between HCV seropositive and seronegative subjects, while statistically significant, were not of a magnitude that would be considered clinically significant. However, buprenorphine concentrations in HCV seropositive patients were increased. Thus, results of liver tests alone may not offer adequate guidance in determining initial buprenorphine dose for patients with chronic HCV infection. It may be advisable to evaluate all potential medical complications of injection drug use prior to initiating buprenorphine therapy, including routine testing for evidence of HCV infection and obtaining baseline liver chemistries. Clinicians should also consider monitoring liver tests periodically in HCV seropositive individuals, including those with normal liver test results prior to initiation of buprenorphine. Buprenorphine treatment has been associated with increases in liver enzyme tests in those with HCV infection and the results of this study are consistent with those previously reported.2,3 There should also be ongoing evaluation of response to buprenorphine, including evaluation for evidence of opioid excess. This should include careful clinical evaluation of high-risk individuals, such as those with HCV infection.25 If clinical concern arises, one option might be to obtain buprenorphine levels which could be measured during the first few weeks of treatment or once steady state has been reached after a dose increase (after approximately 5-7 days on the new dose). The use of lower maintenance doses of buprenorphine should also be considered for those with HCV seropositivity should evidence of opioid excess or significant opioid-related side effects occur.
Limitations of this study should be noted. The study examined the short-term effects of buprenorphine in a secondary analysis using data not generated with the objective of determining the direct effect of HCV infection on buprenorphine exposure. Replication of these results in well-controlled prospective studies with a longer period of administration of buprenorphine treatment is necessary. Because this data analysis compares 22 pharmacokinetic parameters across buprenorphine and its metabolites, it is likely that we would observe at least one apparently significant difference that is actually due to chance alone. However, the likelihood of a series of internally consistent differences occurring by chance is very low. For example, the mean buprenorphine concentration at each and every time point is higher in the hepatitis C positive group (Figure 1). The conjoint probability of finding consistently higher means at every time point is <3 × 10−10. Finally, chronic HCV infection was not verified by HCV viral load; therefore it is possible that some characterized as HCV seropositive did not actually have active HCV infection. However, although this study tested individuals for HCV exposure (HCV antibody positive) rather than chronic infection, based on estimates obtained in global samples of injection drug users, only a minority (∼20%) of those infected with HCV would be likely to spontaneously have cleared the virus.26 It is also possible that a participant could have been recently infected with HCV such that the HCV antibody test was not positive at the time of study participation, and these individuals could have been included in the HCV seronegative group. Thus, the observed differences between HCV seropositive and seronegative participants may have been attenuated. In this sample, no subject had ever received treatment for HCV. Moreover, HCV-infected subjects had higher mean baseline AST and ALT levels as compared to HCV negative subjects; however, the differences were not clinically significant. Nonetheless, findings from this study indicate that HCV-infected patients had higher buprenorphine and buprenorphine metabolite levels even with normal liver function tests.
Scientific Significance and Future Directions
In summary, the present study is the first to show that HCV seropositive individuals treated with standard maintenance doses of buprenorphine/naloxone are exposed to higher concentrations of buprenorphine and its metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide relative to opioid dependent individuals without HCV infection. These findings are important in that they show that even in those with evidence of mild HCV infection, where liver enzyme tests are within normal range or only mildly elevated, the metabolism and clearance of buprenorphine is likely to be delayed. Such individuals may be at greater risk for drug-drug interactions and associated toxicities. Based on these findings and previous evidence demonstrating a risk for elevations in liver enzymes with therapeutic doses of buprenorphine among HCV-infected patients,3 as well as data from this study showing some evidence for modest increases in liver enzymes with buprenorphine/naloxone treatment, such individuals should be monitored for response to buprenorphine treatment, and adjustment of maintenance dose considered, if clinically indicated.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse, Bethesda, MD: R01 DA13004 (Elinore F. McCance-Katz, University of California, San Francisco), K24 DA023359 (Elinore F. McCance-Katz, University of California, San Francisco), R01 DA10100 (David E. Moody, University of Utah), and P50 DA009253 (Joseph R. Guydish, University of California, San Francisco).
The authors thank Jan Vincent Samson, B.A. for his assistance with data coding.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.McCance-Katz E. Office based treatment of opioid dependence with buprenorphine. Harv Rev Psychiat. 2004;12(6):321–338. doi: 10.1080/10673220490905688. [DOI] [PubMed] [Google Scholar]
- 2.Lange WR, Fudala PJ, Dax EM, Johnson RE. Safety and side effects of buprenorphine in the clinical management of heroin addiction. Drug Alcohol Depend. 1990;26(1):19–28. doi: 10.1016/0376-8716(90)90078-s. [DOI] [PubMed] [Google Scholar]
- 3.Petry NM, Bickel WK, Piasecki D, Marsch LA, Badger GJ. Elevated liver enzyme levels in opioid-dependent patients with hepatitis treated with buprenorphine. Am J Addict. 2000;9(3):265–269. doi: 10.1080/10550490050148099. [DOI] [PubMed] [Google Scholar]
- 4.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human Immunodeficiency, and human t-lymphotropic viruses. Am J Public Health. 1996;86(5):655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiede H, Hagan H, Murrill CS. Methadone treatment and HIV and hepatitis B and C risk reduction among injectors in the Seattle area. J Urban Health. 2000;77(3):331–345. doi: 10.1007/BF02386744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier VM, Zhao M, Friedman SR, et al. Injecting and sexual risk correlates of HBV and HCV seroprevalence among new injectors. Drug Alcohol Depend. 2007;89(2-3):234–243. doi: 10.1016/j.drugalcdep.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng F, O'Brien TR, Zhang M, Kral AH, Ortiz-Conde BA, Lorvick J, et al. Seroprevalence of hepatitis C virus and hepatitis B virus among San Francisco injection drug users. Hepatology. 2007;46(3):666–671. doi: 10.1002/hep.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amon JJ, Garfein RS, Ahdieh-Grant L, Armstrong GL, Ouellet LJ, Latka MH, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States. Clin Infect Dis. 2008;46(12):1852–1858. doi: 10.1086/588297. [DOI] [PubMed] [Google Scholar]
- 9.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200(8):1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses-a model for viral persistence. Immunol Res. 2010;47(1-3):216–227. doi: 10.1007/s12026-009-8152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker J, Rainey PM, Moody DE, Morse GD, Ma Q, McCance-Katz EF. Interactions between buprenorphine and antiretrovirals: nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine, lamivudinand tenofovir. Am J Addict. 2010;19(1):17–29. doi: 10.1111/j.1521-0391.2009.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCance-Katz EF, Moody DE, Morse GD, Friedland G, Pade P, Baker J, et al. Interactions between buprenorphine and antiretrovirals. I. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis. 2006;43(suppl 4):S224–S234. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- 13.McCance-Katz EF, Moody DE, Smith PF, Morse GD, Friedland G, Pade P, et al. Interactions between buprenorphine and antiretrovirals. II. The protease inhibitors nelfinavir, lopinavir/ritonavir, and ritonavir. Clin Infect Dis. 2006;43(suppl 4):S235–S246. doi: 10.1086/508188. [DOI] [PubMed] [Google Scholar]
- 14.McCance-Katz EF, Moody DE, Morse GD, Ma Q, DiFrancesco R, Friedland G, et al. Interaction between buprenorphine and atazanavir or atazanavir/ritonavir. Drug Alcohol Depend. 2007;91(2-3):269–278. doi: 10.1016/j.drugalcdep.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCance-Katz EF, Moody DE, Morse GD, Ma Q, Rainey PM. Lack of clinically significant drug interactions between nevirapine and buprenorphine. Am J Addict. 2010;19(1):30–37. doi: 10.1111/j.1521-0391.2009.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody DE, Fang WB, Morrison J, McCance-Katz E. Gender differences in pharmacokinetics of maintenance-dosed buprenorphine. Drug Alcohol Depend. 2011;118(2-3):479–483. doi: 10.1016/j.drugalcdep.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend. 2003;70:S39–S47. doi: 10.1016/s0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok A. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Moody DE, McCance-Katz EF. The invivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-eletrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28(2):245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 20.Cone EJ, Gorodetzky CW, Yousefnejad D, Buchwald WF, Johnson RE. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577–581. [PubMed] [Google Scholar]
- 21.Chang Y, Moody DE, McCance-Katz EF. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos. 2006;34(3):440–448. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 22.Elkader A, Sproule B. Buprenorphine: Clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44(7):661–680. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
- 23.Nakai K, Tanaka H, Hanada K, Ogata H, Suzuki F, Kumada H, et al. Decreased expression of cytochromes P450 1A2, 2E1, and 3A4 and drug transporters Na+-taurocholate-cotransporting polypeptide, organic cation transporter 1, and organic anion-transporting peptide-C correlates with the progression of liver fibrosis in chronic hepatitis C patients. Drug Metab Dispos. 2008;36(9):1786–1793. doi: 10.1124/dmd.107.020073. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Ramamoorthy Y, Tyndale RF, Sellers EM. Interaction of buprenorphine and its metabolite norbuprenorphine with cytochromes p450 in vitro. Drug Metab Dispos. 2003;31(6):768–772. doi: 10.1124/dmd.31.6.768. [DOI] [PubMed] [Google Scholar]
- 25.Zuin M, Giorgini A, Selmi C, Battezzati PM, Cocchi CA, Crosignani A, et al. Acute liver and renal failure during treatment with buprenorphine at therapeutic dose. Digest Liver Dis. 2009;41(7):e8–e10. doi: 10.1016/j.dld.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Te HS, Jensen DM. Epidemiology of hepatitis B and C virus: a global overview. Clin Liver Dis. 2010;14(1):1–21. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]