Abstract
The role of macrophages in homeostatic conditions and the immune system range from clearing debris to recognizing and killing pathogens. While classically activated macrophages (CAMacs) are induced by T helper type 1 (Th1) cytokines and exhibit microbicidal properties, Th2 cytokines promote alternative activation of macrophages (AAMacs). AAMacs contribute to the killing of helminth parasites and mediate additional host-protective processes such as regulating inflammation and wound healing. Yet, other parasites susceptible to Th1 type responses can exploit alternative activation of macrophages to diminish Th1 immune responses and prolong infection. In this review, we will delineate the factors that mediate alternative activation (e.g. Th2 cytokines and chitin) and the resulting downstream signaling events (e.g. STAT6 signaling). Next, the specific AAMac-derived factors (e.g. Arginase1) that contribute to resistance or susceptibility to parasitic infections will be summarized. Finally, we will conclude with the discussion of additional AAMac functions beyond immunity to parasites, including the regulation of inflammation, wound healing and the regulation of metabolic disorders.
Keywords: Alternatively activated macrophage, helminth, metabolism, parasite, protozoan, Th2 inflammation
Introduction
Parasites afflict billions of people worldwide; helminths infect 1.5 billion people, malaria affects over 200 million people, and over 1 billion people are infected with Toxoplasma sp. [1]. In addition to morbidity associated with infection, parasites contribute to over 1.6 million deaths a year. Understanding how the host eliminates these pathogens and develops long-lasting immunity could offer new treatment strategies to eradicate parasitic disease. Macrophages are a major component of the innate immune response to parasitic infections. Originally discovered by Elie Metchnikoff [2], macrophages are a heterogeneous family of phagocytic immune cells capable of a battery of homeostatic, housekeeping, and infection-induced functions. These responsibilities include responding to and destroying pathogens, clearing debris caused by apoptotic cells, and regulating the host immune response. Following parasite infection, infection-induced signals, including pathogen associated molecular patterns (PAMPs) and cytokines, instruct macrophage activation. In response to T helper type 1 (Th1) cytokines such as IFNγ, classically activated macrophages (CAMac) provide protection against intracellular parasites via phagocytosis and elimination by the production of microbicidal products [3]. As a counterpart to CAMacs, alternatively activated macrophages (AAMac) are elicited by Th2 cytokines, including IL-4, IL-13, IL-21 and IL-33, which are highly produced following helminth infection [4–7].
Our understanding of the multiple functions of parasite-induced AAMacs has benefited from animal models of parasite infection and the use of transgenic mice that are deficient in macrophages or macrophage-specific factors (Table 1). Additionally, patient studies investigating areas where parasite infections are endemic have revealed increased helminth-induced AAMac responses [8]. AAMacs express several signature proteins including the mannose receptor, Arginase1, RELMα, and chitinases [4]. Together, they act to promote immunity to helminth parasites, regulate inflammation or mediate wound healing. Although AAMacs can be host-protective in helminth infection, AAMac activation can impede protective immunity to protozoan parasites [9]. These contrasting functions have important implications for understanding how the host generates an ideal immune response when faced with multiple infections. Given the high incidence of co-infection [1], a better understanding of the specific AAMac-derived proteins that mediate helminth clearance and/or prevent protozoan parasite killing, may allow the design of more targeted treatment strategies for multiple infections. In addition to potent effects in regulating the immune response to parasites, AAMacs are extensively studied in metabolic disorders such as diabetes. The effect of parasite-induced AAMacs in regulating metabolic function is a new and active area of research [10].
Table 1.
Mouse models to Investigate AAMacs
| Strategy | Target | Description |
|---|---|---|
| Reporters | LysM-GFP or YFP | Lysozyme promoter found on monocytes/macrophages and neutrophils drive reporter expression [106]. |
| YARG (Arginase-YFP) | Arginase1 promoter drives YFP expression allowing visualization of AAMacs [57]. | |
| CCR2-RFP/GFP | CCR2 is the receptor for monocyte chemotactic protein-1 (MCP-1) on migrating monocytes [107]. | |
| CX3CR1-GFP | This fractalkine receptor is present on monocytes/macrophages, but can also be found on natural killer cells and dendritic cells [108]. | |
| Knockouts | LysMCre; Tie2Cre; CD11bCre | Macrophage-specific promoters to drive Cre recombinase expression. These can be used in combination with floxed genes for cell-specific deletion. Caveats include promoters that are active in neutrophils (LysM), endothelial cells (Tie2), or granulocytes (CD11b) [77, 109, 110]. |
| IL-4Rα−/−, STAT6−/− | Cells are unable to respond to the Th2 cytokines IL-4/IL-13 and their downstream signaling [16, 77]. | |
| CD11b-DTR | Inducible depletion of macrophages (and granulocytes) following diphtheria toxin treatment [78]. | |
| Inhibitors | Clodronate liposomes | Phagocytosis of liposomes by macrophages induces apoptosis [40]. |
| nor-NOHA | Chemical inhibitor of arginase activity [111]. |
In this review, we will explore AAMac activation and function following parasitic infections. We will first summarize the factors (i.e. cytokines and antigens) and resulting downstream signaling events that trigger AAMac activation and expansion. Second, we will describe the main AAMac-derived proteins, how they affect immunity to helminths or protozoan parasites, and the implication for co-infection studies. Third, we will discuss the emerging evidence supporting additional functions for AAMacs beyond immunity to parasites. These include dampening inflammation, promoting wound healing and regulating metabolism.
AAMac activation and expansion
AAMac activation
Alternative activation of macrophages is a prominent immune response observed in helminth infections and many chronic protozoan parasite infections [11, 12]. CD4+ Th2 cells and innate cells such as eosinophils, basophils and mast cells secrete the cytokines IL-4 and IL-13, which induce alternative activation of macrophages (Fig. 1.1). Additional cytokine/receptor pathways that contribute to AAMac activation include IL-21/IL-21R and IL-33/ST2 (Fig. 1.2). For example, IL-21 promotes the host Th2 immune response to helminth Nippostrongylus brasiliensis [13] and protozoan parasite Toxoplasma gondii [14]. Here, IL-21 may promote AAMac activation indirectly by inducing macrophage expression of IL-4Rα and IL-13Rα1 expression, thereby promoting responsiveness to these Th2 cytokines [6]. IL-33 can directly induce AAMac gene expression and also activates innate lymphoid cells and CD4+ Th2 cells to promote the type 2 cytokine environment allowing the control of helminths including gastrointestinal nematode Trichuris muris [7, 15].
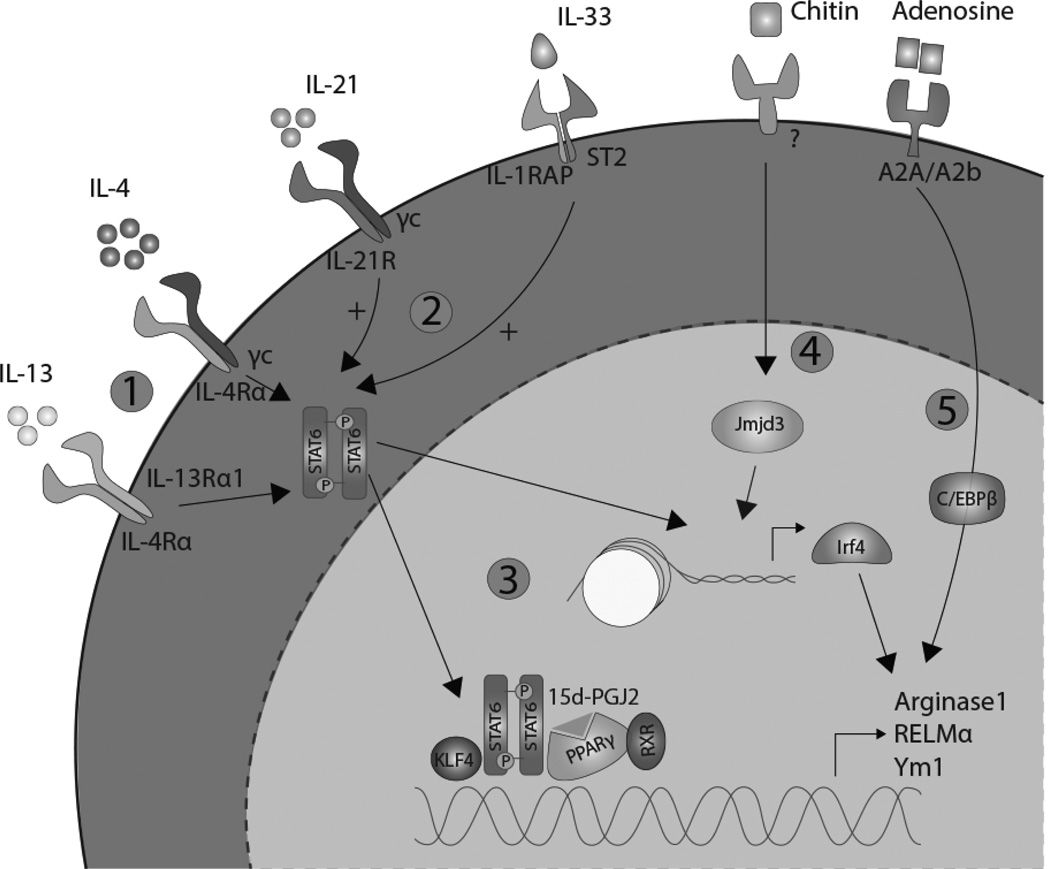
Figure 1. Polarization of alternatively activated macrophages.
1. IL-4 and IL-13, upon binding to their common receptor IL-4Rα phosphorylate STAT6 to begin the induction of AAMac polarization. 2. IL-21 and IL-33 promote this signaling pathway. 3. STAT6 mediates expression of AAMac signature genes and activates other transcription factors such as PPARγ. 4. Helminths and chitin promote alternative activation via Jmjd3-mediated histone modification and expression of the transcription factor Irf4. 5. Adenosine promotes macrophage alternative activation through a C/EBPβ-dependent mechanism.
Both IL-4 and IL-13 bind to the common receptor IL-4Rα, activating the Signal Transducer and Activator of Transcription (STAT) 6 signaling pathway for AAMac specific gene expression and proliferation [16]. STAT6−/− mice exhibit defective Th2-mediated immune responses and AAMac expansion in numerous helminth infections including N. brasiliensis and Heligmosomoides polygyrus [17, 18]. While STAT6 directly promotes expression of AAMac signature genes, such as Arginase1 and Resistin-like molecule (RELM) α, it also augments AAMac polarization indirectly, by binding to the promoter region of other transcription factors that induce AAMac gene expression (Fig. 1.3). These include Peroxisome Proliferator-Activated Receptor (PPAR) γ, Krüppel-like Factor (KLF) 4, and Interferon regulatory factor (Irf) 4 [19–21].
The PPAR family contains nuclear receptors found on many leukocytes, including macrophages and T cells, and binds to fatty acids and eicosanoids [22]. In macrophages, PPARγ can be activated by IL-4/IL-13 [23], or indirectly via prostaglandins (e.g. 15d-PGJ2) or eicosanoids (e.g. PGI2). As transcription factors, PPARs form heterodimers with the retinoid X receptor (RXR) [24] to promote AAMac gene expression (e.g. Arginase1) [23]. In infection with the filarial nematode Brugia malayi, arachidonic acid metabolism was induced in macrophages, resulting in increased levels of PGI2 and PPARγ-mediated AAMac activation [25]. PPARγ also acts synergistically with STAT6 to promote macrophage alternative activation. Indeed, the binding ability of PPARγ to promoter sites is improved when it interacts with STAT6 [19]. PPARγ activity is also regulated by 12/15 lipoxygenase (12/15 LOX), an enzyme that catalyzes the oxygenation of fatty acids. During chronic infection with Taenia crassiceps, AAMac-derived 12/15 LOX catalyzes the production of 13-hydroxyoctadecadienoic acid, which acts as a ligand to PPARγ [26]. In this chronic infection setting, the 12/15 LOX effects on PPARγ are anti-inflammatory, and inhibit T cell proliferation by blocking the transcription factors NFAT and NFκB, which mediate expression of the mitogenic cytokine IL-2 [27].
Phosphorylated STAT6 also induces expression of KLF4, a member of the Krüppel-like family of transcription factors that regulates cellular differentiation and growth. In LysMCre/KLF4Flox mice, there were significant increases in CAMacs and delayed wound healing, characteristic of a deficiency in AAMacs [20]. Similar to PPARγ, 15d-PGJ2 can activate KLF4, although this pathway is independent of PPAR but instead signals through MAPK and ERK [28]. Maximal Arginase1 expression is reliant on the binding of the STAT6-KLF4 heterodimer to the Arginase1 promoter region [20]. Although the synergistic effect of KLF4 and STAT6 in AAMac activation and function is clear, the significance of KLF4 in parasitic infections is unknown.
Irf4, another target of STAT6, can also mediate alternative macrophage activation by direct binding to the promoter region of AAMac signature genes. In Irf4−/− mice, expression of Arginase1, Ym1, and RELMα was significantly lower compared to wild-type mice [29] in response to allergen challenge. Irf4 gene expression is also epigenetically regulated by Jumonji domain containing-3 (Jmjd3), a H3K27 demethylation enzyme. Following N. brasiliensis infection or treatment with the allergen chitin, Jmjd3−/− mice exhibited defective AAMac responses [29]. Mechanistically, Jmjd3 demethylates the histone region in the Irf4 promoter, allowing access for binding to phosphorylated STAT6 and subsequent Irf4 expression [21] (Fig. 1.4). Epigenetic regulation of AAMac markers is not limited to histone demethylation. For example, employing mice with a macrophage-specific deletion in histone deacetylase 3 (HDAC3), we observed that cell-intrinsic expression of HDAC3 inhibited AAMac responses following challenge with helminth Schistosoma mansoni ova [30].
AAMac activation can also occur independently of STAT6, and is mediated instead by phosphorylation of the transcription factor, CCAAT-enhancer-binding protein β (C/EBPβ). For example, adenosine, a purine nucleoside that is typically upregulated during hypoxia and tissue injury, can augment the polarization of AAMac via a C/EBPβ-dependent mechanism [31] (Fig. 1.5).
In addition to parasitic infection, Th2 cytokines are produced in situations where there is physiologic stress to the body, potentially in an attempt to maintain the body’s homeostatic condition. The upregulation of these Th2 cytokines polarize AAMacs to perform several different homeostatic functions (e.g. glucose homeostasis or response to thermal stress) [32], along with host protective functions following injury (e.g. wound healing). This heterogeneity in AAMac function and its relevance in helminth infection will be discussed later.
AAMac expansion
Increased macrophage frequency at the site of infection is observed in several protozoan and helminth infections [11, 12]. However, whether these macrophages arise from blood monocytes or from proliferation at the site of infection has been the subject of several new studies [33–36]. As part of the mononuclear phagocyte system, hematopoietic stem cells in the bone marrow give rise to macrophage precursors called monocytes. These monocytes are released into the circulation system, where they are able to travel to all parts of the body to replenish the body’s macrophages and dendritic cells. During an acute Th1 inflammatory response, monocytes are recruited from the blood followed by differentiation into macrophages (CAMac) [33, 34]. This recruitment and differentiation process is dependent on the macrophage colony stimulating factor (CSF)-1. In contrast, two recent studies showed Th2 cytokine-induced AAMac responses resulted from local proliferation at the site of infection as an alternative mechanism to recruitment from the blood [35, 36]. Using intravenous injection of clodronate liposomes to deplete macrophages from the blood, Allen and colleagues observed that CAMac, but not AAMac, expansion was impaired, suggesting that AAMac expansion was independent of monocyte precursors from the bloodstream. Instead, AAMac expansion resulted from IL-4 induced proliferation of resident tissue macrophages, which occurred independently of CSF-1. Following acute inflammatory stimuli however, studies by Davies et al. showed that tissue resident macrophages also proliferated in the tissue, but independently of IL-4 [33]. Together, these studies reveal complexity in the regulation of tissue macrophage expansion, and suggest that AAMacs arise from IL-4 dependent tissue resident macrophage proliferation, while CAMac expansion occurs via CSF-1 mediated macrophage proliferation and bone marrow precursors. A previous study identified a distinct population of yolk sac macrophages that pre-dates and operates outside the mononuclear phagocyte system and gives rise to certain tissue-specific macrophages [37]. It is plausible that AAMacs may derive, in part, from this newly identified macrophage population.
AAMac function in helminth infection
Helminths elicit a canonical Th2 signaling cascade leading to eventual expulsion or killing of worms. Amongst many other Th2 cytokine-mediated effector mechanisms, including epithelial cell activation and turnover [38, 39], AAMacs also contribute to immunity to certain helminths. In a secondary infection with H. polygyrus, a model used to study vaccination strategies against helminths, or following infection with N. brasiliensis, AAMacs were critical in mediating worm expulsion [40, 41]. Another study examined the human parasite Strongyloides stercoralis, where both humans and mouse AAMacs acted in concert with neutrophils to kill S. stercoralis larvae [42]. Following T. muris infection, macrophage-specific deletion in SH2-containing inositol 5’-phosphatase 1 (Ship1) resulted in impaired worm expulsion due to excessive CAMac activation [43], suggesting that the balance between classical and alternative activation is critically involved in immunity to helminth parasites.
AAMac-derived proteins
AAMacs are characterized by the expression of several signature proteins including the mannose receptor, Arginase1, chitinases and RELMα, all of which are critically dependent on Th2 cytokines [4, 44, 45]. The contributions of these AAMac-derived factors to the host immune response to parasites are summarized below.
The macrophage mannose receptor (MMR) is a pattern recognition receptor of the innate immune system that binds to mannose, a sugar found on many pathogens including Candida albicans and HIV. Following helminth infection, MMR is involved in the immune recognition of S. mansoni larvae and T. muris [46, 47]. MMR exposure to S. mansoni excretory/secretory material increases antigen presentation and dampens pro-inflammatory Th1 cytokine expression. For instance, in the absence of the mannose receptor, there is an increase in CD4+ T cell expression of IFN-γ and a decrease in IL-4 [46]. Following infection with intestinal nematode T. muris, MMR−/− mice were equally capable of worm expulsion compared to their wild-type counterparts, suggesting that MMR may instead regulate parasite-induced inflammatory responses in certain heminth infections.
While there are two arginase enzymes found in the body, Arginase1 is the main enzyme associated with AAMac and Th2 cytokines. Arginase expression in the liver is constitutive and essential, as it catalyzes the formation of urea to help the body expel ammonia. In AAMacs, Arginase1 catalyzes the breakdown of arginine to prolines and polyamines. NOS2 and Arginase1 both compete for the substrate arginine to create nitric oxide (NOS2) or ornithine/urea (Arginase1), respectively. In the context of helminth infection, studies employing Arginase1-deficient bone marrow chimeras or macrophage-specific Arginase1 deficient mice support the hypothesis that Arginase1 is anti-inflammatory and host-protective. Following S. mansoni infection, Arginase1 suppressed the helminth egg-induced acute intestinal inflammation and hemorrhaging, potentially via inhibition of proinflammatory cytokines such as IL-12/IL-23p40. Further, employing macrophage-specific Arginase1 knockout mice (LysMCre/Arg1Flox or Tie2Cre/Arg1Flox), Wynn and colleagues demonstrated an important function for macrophage-derived Arginase1 in dampening S. mansoni granulomatous pathology in the liver and Th2 immune responses [48]. The importance of Arginase1 in the immune response to helminths may depend on the helminth. For instance, macrophage-specific Arginase1 expression was dispensable for T. muris expulsion [49]. In contrast, both AAMac and arginase-depleted mice are unable to expel H. polygyrus [40], and during N. brasiliensis infection, AAMacs promote intestinal smooth muscle contractility, thereby aiding worm expulsion, via a mechanism that is partly dependent on Arginase1 [41].
Chitinase, an enzyme that cleaves and breaks down the chitin found on fungi, worms, and other organisms, has been implicated in Th2 immune responses during allergic reactions and parasitic infections. Since chitin is present in many parasites, chitinase may have evolved as a way to control these parasites [50]. Two chitinase family members, Ym1, a chitin-binding protein without chitinase activity, and acidic mammalian chitinase (AMCase), are expressed by AAMacs. Ym1 was originally discovered as an eosinophil chemotactic factor [51], and is also chemotactic for T cells and other bone marrow cells. Functionally, Ym1 may promote host effector responses to parasites by mediating eosinophil accumulation in the infected tissue. For example, eosinophils are a dominant cell type in the Schistosoma sp. induced granulomas, where they produce Th2 cytokines that contribute to the granulomatous tissue [52, 53]. In addition, in vitro studies have shown that eosinophils can mediate helminth killing by binding to and releasing toxic granules for killing of the S. mansoni [54], B. pahangi, and B. malayi larvae [55]. Ym1 can also augment Th2 cytokine signaling through inhibition of 12/15 LOX [56]. Despite these studies showing that Ym1 promotes protective Th2 immune responses following helminth infection, there is currently no evidence that AAMacs mediate immunity to helminths via Ym1. Instead, it is likely that AAMac-derived Ym1 contributes to the regulation of helminth-induced inflammation and pathology rather than worm killing. Similarly, AMCase, which is a functional chitinase, is highly upregulated in helminth infection and acts to breakdown chitin, thereby reducing the inflammatory effects of this allergen [57]. Although the importance of AMCase in immunity to helminths is unknown, in protozoan parasite infections, AMCase can promote T. gondii killing (discussed in the next section).
The RELM family of proteins was originally identified in allergen-induced pulmonary inflammation [58]. Originally called FIZZ (found in inflammatory zone), this family of cysteine-rich proteins contains RELMα, RELMβ, and RELMγ. RELMα is expressed by AAMacs, eosinophils and epithelial cells, and is predominantly found in the lungs in response to allergens and helminth infections. Studies from our lab and others employing RELM−/− mice revealed that RELMα inhibited type 2 inflammation in multiple helminth infection models [59, 60]. Rather than promoting immunity to helminths, RELMα-mediated regulation of Th2 immune responses may instead impede worm clearance, and RELMα−/− mice exhibited more rapid expulsion of N. brasiliensis [59]. Consistent with the hypothesis that parasites may modulate host proteins to regulate host protective immunity, S. mansoni-derived hemozoin downregulated RELMα expression in macrophages, which could have inhibitory effects on the subsequent Th2 immune response [61].
AAMacs in humans
Functional studies using mice are models to set a framework for helminth infections in humans. Similar to mouse models, CD14+ blood monocytes from filaria-infected patients had increased AAMac gene expression compared to uninfected individuals. This included expression of Mannose receptor C type 1, macrophage Galactose type C lectin, Resistin and Arginase1 [62]. With well over 1.5 billion people infected with helminths, elucidating the immune response to these parasites and the importance of AAMacs could have significant medical implications.
AAMac function in protozoan parasite infection
Not all infections rely on AAMacs for clearance and AAMacs are detrimental to the host in several infections. Protozoan parasites, such as Trypanosoma cruzi, Leishmania sp., and Plasmodium sp., elicit a strong type 1 immune response during the acute stages of infection, characterized by the phagocytosis of parasites by macrophages, reactive oxygen species (ROS) production and heavy pro-inflammatory signaling. However, if the infection becomes chronic, the immune response shifts toward a Th2 type response [63]. In these cases, a Th2 response is necessary to counterbalance the inflammatory type 1 cytokines and prevent excessive inflammation and collateral damage to the host. Alternatively activated macrophages - also known as regulatory or M2 macrophages in this context - can mediate these regulatory effects through the production of IL-10 and transforming growth factor (TGF)-β. This balance is temporally sensitive, as early expression of type 2 cytokines increases susceptibility to infection by counteracting the parasite-killing type 1 immune response. For example IL-10 promotes Leishmania sp. growth by inhibiting classical activation of macrophages and subsequent intracellular parasite killing [64]. Likewise, early TGF-β expression by macrophages results in an increase in malaria parasite growth [65]. In Leishmania infection, mice in which macrophages had defective PPARγ signaling exhibited reduced AAMac responses and increased resistance to L. major. Further, in a hamster model of visceral leishmaniasis with L. donovani, STAT6 dependent AAMacs impaired parasite clearance via production of Arginase [66]. Arginase is also a susceptibility factor for T. gondii and Trypanosoma sp., where it acts twofold to prevent production of microbicidal nitric oxide while generating polyamines, which are essential nutrients for the parasite [67–69]. Protozoan parasites may even directly promote AAMac differentiation through the secretion of host modulatory proteins. For example, T. gondii secretes effector proteins from organelles called rhoptries that can determine the virulence of the parasite. ROP16 is one such effector protein that directly phosphorylates STAT6 [70] to shift the activation of macrophages from CAMac to AAMac, thereby aiding parasite survival [71]. On the other hand, AAMacs can help protozoan pathogens killing in certain circumstances. During chronic T. gondii infection in the brain, alternatively activated macrophages combat this infection by producing AMCase to lyse the chitin-rich cysts, exposing the parasites to allow immune-mediated killing [72]. In L. infantum infection, expression of MMR and Dectin-1, another pathogen recognition receptor that is upregulated by Th2 cytokines, promoted parasite killing [73]. Dectin-1 and MMR–dependent immunity was associated with the production of ROS and IL-1β, through activation of the caspase-1 dependent inflammasome.
Infectious diseases often have overlapping geographical ranges, where several individuals are co-infected with different parasites. Co-infection studies allow us to study the dichotomy between Th1 and Th2 type signaling and their interactions with each other, with the ultimate goal of identifying the ideal immune response that promotes host survival and fitness. In N. brasiliensis and Mycobacteria tuberculosis (Mtb) co-infection studies, mice exhibited increased susceptibility to Mtb that was mediated by AAMacs [74]. This impaired resistance to Mtb was dependent on IL-4Rα signaling, as IL-4Rα−/− co-infected mice were equally capable of controlling infection compared to Mtb alone-infected mice. Similar results were found in T. crassiceps and Leishmania sp. co-infected mice, where increased AAMac responses correlated with more severe Leishmania-induced lesions [75]. However, not all co-infections lead to detrimental outcomes; in some instances, one infection may protect the host from a second infection. For instance, mice co-infected with N. brasiliensis and P. chabaudi exhibited a slight amelioration in malaria-induced anemia [76].
AAMac function in regulating inflammation
In any type of infection, controlling the rate and amount of inflammation is instrumental for host survival. Rather than mediating parasite killing, the most critical function of AAMacs might be to protect the host from parasite-induced inflammation and damage. Following S. mansoni infection, macrophage-specific IL-4Rα−/− mice (LysMCre/IL-4RαFlox) succumbed to acute infection associated with increased inflammation, including increased Th1 cytokine production, NOS2 activity and sepsis [77]. These studies support an essential role for AAMacs in protection against lethal infection-induced inflammation. In N. brasiliensis infection, AAMacs were essential to dampen the infection-induced lung inflammatory response; both IL-4R−/− mice and CD11b-DTR transgenic infected mice suffered from exacerbated lung hemorrhaging that was alleviated with the transfer of IL-4R responsive macrophages [78]. Finally, in a mouse model of colitis induced by the chemical dextran sodium sulfate, macrophages from S. mansoni infected mice ameliorated intestinal inflammation [79]. Harnessing the anti-inflammatory potential of helminths could provide new strategies to treat inflammatory diseases. In particular, a filarial nematode-derived protein – chitohexaose – may have therapeutic properties in limiting septic shock and endotoxemia by blocking TLR4 signaling and instead promoting AAMac activation [80].
Mechanistically, AAMacs dampen inflammation through the production of several immunoregulatory mediators including cytokines, receptors and enzymes. In N. brasiliensis infection, AAMac-induced IL-10 and Insulin-like Growth Factor (IGF) 1 dampened inflammatory Th17 cell and neutrophil responses [78].
AAMac-derived Arginase1 acts to inhibit inflammatory responses both by inhibiting proinflammatory cytokine expression and by blocking T cell proliferation. For instance, arginase catalyzes the production of polyamines, which can antagonize Th1 associated inflammatory genes [81]. In S.mansoni infection, arginase inhibited inflammation by blocking IL-12 and IL-23p40 production associated with neutrophil inflammation [82]. As excessive Th2 associated inflammation can also be detrimental to the host, the Th2 immune response can be negatively regulated by AAMac production of RELMα [59, 60]. Inhibition of Th2 type immunity was mediated through direct binding of RELMα to macrophages, dendritic cells and Th2 cells, followed by induction of Bruton’s tyrosine kinase (BTK) signaling [60]. As RELMα can also bind to B7-H3 [83], an inhibitor of T cells [84], it is also possible that the anti-inflammatory function of RELMα is dependent on the B7-H3 pathway. Surprisingly, in other inflammatory settings such as allergen-induced airway inflammation or intestinal inflammation, RELMα is proinflammatory, suggesting that the immunoregulatory effects of RELMα may depend on the tissue site and the infectious agent [85, 86].
In addition to secretion of immunoregulatory cytokines, Brugia malayi-induced AAMacs inhibited T cell proliferation via a cell-to-cell contact-dependent mechanism [87]. One mechanism of AAMac-mediated suppression may include expression of the inhibitory surface molecule Programmed Death Ligand 2 (PD-L2) [88]. PD-L1 and PD-L2 bind to the same receptor (PD-1) and are differentially expressed by CAMacs and AAMacs respectively [89]. STAT6-dependent expression of PD-L2 in AAMacs is observed in multiple helminth infection models including S. mansoni, T. crassiceps, B. malayi, and N. brasiliensis [87, 88, 90, 91]. Another AAMac dependent mechanism for regulating inflammation is through the induction of regulatory T cells; in S. mansoni infection, AAMacs synthesize retinoic acid, which acts in conjunction with TGF-β to promote Foxp3+ regulatory T cells [92].
AAMac function in wound healing
As helminths burrow into tissue, they can cause significant tissue damage and hemorrhaging. As a result, the type 2 immune response may have evolved to mediate wound repair caused by helminth infection [93]. AAMacs express a variety of proteins that contribute to wound healing. These factors mediate wound repair directly, or indirectly, via angiogenesis or activation of fibroblasts. Angiogenesis, the formation of blood vessels, is an essential wound healing step, allowing vascularization of the new tissue for access to nutrients and oxygen [94]. Fibroblast activation is also critical for wound healing, and initiates the scaffolding of the collagen fibers to build the extracellular matrix (ECM) foundation for the new tissue [95]. The main AAMac-derived factors that contribute to wound healing are summarized below and include Arginase, RELMα, metalloproteinases and growth factors.
Arginase catalyzes the production of prolines and polyamines to resolve damage by formation of collagen and by induction of cell proliferation. RELMα induces collagen production and differentiation of myofibroblasts, both of which contribute to formation of the ECM [96]. Additionally, the expression of a number of matrix metalloproteinases and tissue inhibitors of metalloproteinases helps build up and break down ECM as needed. Perhaps the most important contribution of AAMacs in wound healing is the expression of growth factors, including IGF-1, vascular endothelial growth factor (VEGF) and TGF-β. These factors promote angiogenesis and activate fibroblasts. Although AAMacs and Th2 cytokines mediate wound healing, this host immune response can cause detrimental fibrosis if dysregulated. Fibrosis, a condition characterized by formation of scar tissue, causes an estimated 45% of deaths throughout the world [1]. Dependent on the tissue, fibrosis causes cirrhosis, idiopathic pulmonary fibrosis, and other serious health problems in the event where the hardened scars impair normal tissue function.
AAMac function in metabolism
Obesity, a growing epidemic, affects over 1.5 billion people worldwide, with 100 million in the United States alone [1]. Among several debilitating diseases associated with obesity, the presence of excessive fat tissue promotes insulin resistance, a key contributor to type 2 diabetes. Obesity has been linked to a low level, chronic state of inflammation, of which the outcome depends on the macrophage activation status [97]. CAMacs in adipose tissue are detrimental and produce inflammatory cytokines such as TNFα that contribute to insulin resistance. In fact, inflammatory cytokines and saturated fatty acids impair insulin sensitivity by inhibiting insulin receptor signaling pathways such as PI3K, while further inducing inflammatory gene expression in a positive feedback loop. These effects are mediated in part through JNK1 and IKKβ signaling [98, 99]. In contrast, increased AAMac frequency in the fat is linked to reduced inflammation and better prognosis for insulin resistance [100].
With both obesity and helminth infections linked to AAMacs, it is natural to question how helminths might affect obesity. To determine the helminth’s role in obesity, glucose tolerance, and insulin sensitivity, mice fed a high fat diet were later infected with N. brasiliensis [10]. N. brasiliensis infection resulted in sustained insulin sensitivity and glucose tolerance for up to 35 days post infection. This beneficial effect was dependent on IL-4/IL-13-induced AAMacs. The ability to increase insulin sensitivity may be regulated by AAMac expression of IL-10 to modulate inflammatory macrophage populations and inflammatory cytokines such as TNFα and IFNγ [100]. Additionally, AAMacs mediate the more efficient fatty acid oxidation and oxidative metabolic pathway, while CAMacs undergo glycolysis for energy production [101]. Not only do CAMacs fail to breakdown fatty acid as a source of energy, they also generate acetyl-coA, an important intermediate in the synthesis of fatty acids. Thus, CAMacs add to the total fat by increasing fatty acid production while decreasing fatty acid catabolism.
The polarization of AAMac in adipose tissue is partially dependent on Trib1, a scaffolding protein involved in protein degradation. Trib1−/− mice have altered macrophage differentiation because of an abnormal C/EBPα expression [102]. In addition, PPARδ and PPARγ, are activated through adipocyte-derived IL-4/13 and are necessary to control inflammation caused by metabolic dysfunction [103]. When using adoptive transfer of PPARδ−/− bone marrow into wild-type mice, AAMac responses and insulin sensitivity were impaired, and there was a higher incidence of hepatic inflammation and necrosis [104], suggesting that Kupffer cells (liver-specific macrophages) are safeguards against type 2 diabetes. In macrophage-specific PPARγ−/− mice, impaired AAMac responses led to diet-induced obesity and insulin resistance [23]. In addition, genes encoding fatty acid oxidation and oxidative metabolism were significantly reduced. Mechanistically, PPARs control many aspects of obesity and metabolism in various tissues and cells through mediating the metabolism of fatty acids. For instance, PPARδ increases oxidative lipid catabolism in tissues such as skeletal tissue, adipose tissue, and the liver, improves insulin sensitivity and as a result, controls weight gain [105]. PPARγ, on the other hand, regulates long-term storage of fatty acids and has been used for many years for as a therapeutic to promote insulin sensitivity. As the incidence of obesity increases, AAMac-derived therapeutics to suppress inflammation and CAMacs may provide new avenues to treat obesity-associated diseases.
Conclusion
Macrophage activation is a fundamental component of the immune response to parasitic infection. While classically activated macrophages control small intracellular pathogens, immunity to large extracellular parasites such as helminths that cannot be phagocytosed, is a challenge. AAMacs contribute to host protective immunity to these prevalent infections via several mechanisms (Fig. 2). These include promoting protective Th2 immune responses and producing factors that mediate worm expulsion. In addition to anti-helminth properties, AAMacs are critical effectors in dampening inflammation, mediating wound healing, and even regulating metabolic diseases. AAMacs express a number of genes to contain the pro-inflammatory effects of CAMacs and inflammatory T cells, thereby limiting inflammation. AAMacs also mediate phagocytosis and clearance of cellular debris while producing factors to promote wound healing following tissue injury. Finally, AAMac frequency is positively correlated with improved outcomes in several metabolic disorders. With the ability of AAMacs to participate in many aspects of immunity and homeostasis, understanding AAMac function in parasitic infections may have significant implications in the medical field.
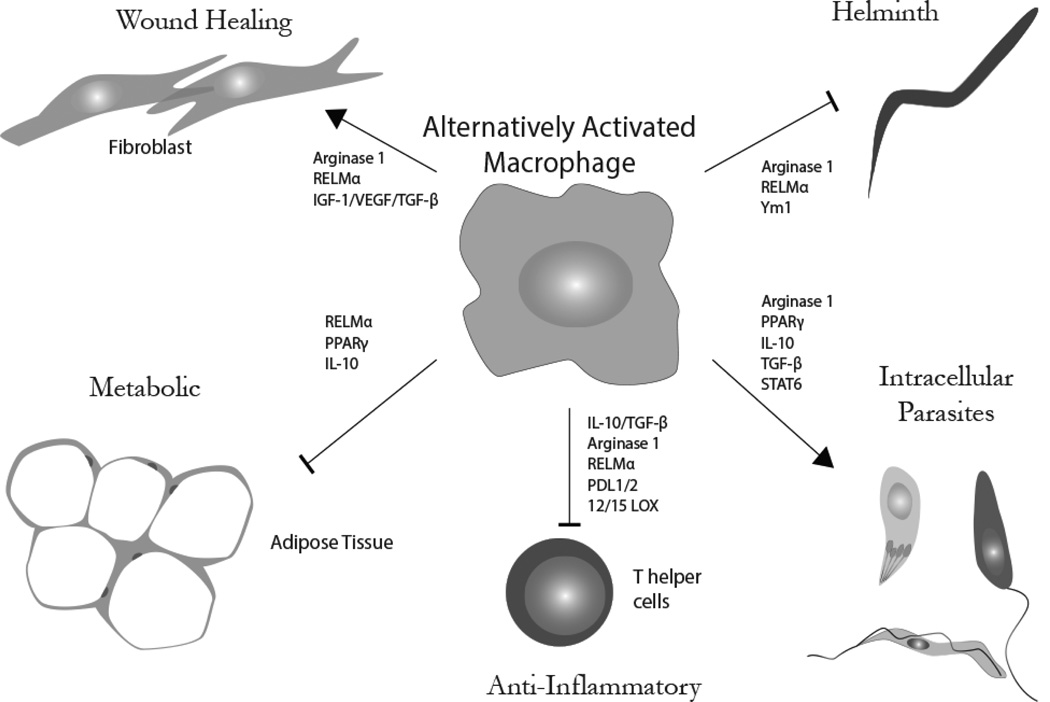
Figure 2. Functional diversity of alternatively activated macrophages.
AAMacs have multifunctional capabilities in host response to parasite infection that include helminth killing, susceptibility to intracellular parasites, dampening inflammation, regulating metabolism and wound healing.
Acknowledgements
The authors would like to thank Josiah Chung, Alex Chan and Spencer Wang for critical reading of this manuscript. The Nair lab is supported by the National Institute of Health (R01AI091759 awarded to MGN).
Abbreviations
- AAMac
Alternatively Activated Macrophage
- CAMac
Classically Activated Macrophage
- C/EBP
CCAAT-enhancer-binding protein
- CSF
Colony Stimulating Factor
- HDAC
Histone Deacetylase
- IFN
Interferon
- IL
Interleukin
- Irf
Interferon Regulatory Factor
- KLF
Krüppel-like Factor
- MMR
Macrophage Mannose Receptor
- PAMP
Pathogen Associated Molecular Pattern
- PPAR
Peroxisome Proliferator-Activated Receptor
- PD-L
Programmed Death Ligand
- RELM
Resistin-like Molecule
- RXR
Retinoid X Receptor
- STAT
Signal Transducer and Activator of Transcription
- TGF
Transforming Growth Factor
- TNF
Tumor Necrosis Factor
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
References
- 1.WHO. World Health Statistics. 2013
- 2.Metchnikoff E, Binnie FG. Immunity in Infective Diseases. University Press; 1905. [Google Scholar]
- 3.van Zandbergen G, Klinger M, Mueller A, et al. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 4.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle AG, Herbein G, Montaner LJ, et al. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur J Immunol. 1994;24:1441–1445. doi: 10.1002/eji.1830240630. [DOI] [PubMed] [Google Scholar]
- 6.Pesce J, Kaviratne M, Ramalingam TR, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurowska-Stolarska M, Stolarski B, Kewin P, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 8.Semnani RT, Mahapatra L, Moore V, Sanprasert V, Nutman TB. Functional and phenotypic characteristics of alternative activation induced in human monocytes by interleukin-4 or the parasitic nematode Brugia malayi. Infect Immun. 2011;79:3957–3965. doi: 10.1128/IAI.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723–1733. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raes G, Beschin A, Ghassabeh GH, De Baetselier P. Alternatively activated macrophages in protozoan infections. Curr Opin Immunol. 2007;19:454–459. doi: 10.1016/j.coi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Frohlich A, Marsland BJ, Sonderegger I, et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 14.Stumhofer JS, Silver JS, Hunter CA. IL-21 Is Required for Optimal Antibody Production and T Cell Responses during Chronic <italic>Toxoplasma gondii</italic>Infection. PLoS ONE. 2013;8:e62889. doi: 10.1371/journal.pone.0062889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a Potent Inducer of Adaptive Immunity to Intestinal Nematodes. The Journal of Immunology. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Tanaka T, Shi W, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 17.Weng M, Huntley D, Huang IF, et al. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reece JJ, Siracusa MC, Southard TL, Brayton CF, Urban JF, Jr, Scott AL. Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun. 2008;76:3511–3524. doi: 10.1128/IAI.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szanto A, Balint BL, Nagy ZS, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao X, Sharma N, Kapadia F, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Chartouni C, Schwarzfischer L, Rehli M. Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology. 2010;215:821–825. doi: 10.1016/j.imbio.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Issemann I, Prince RA, Tugwood JD, Green S. The peroxisome proliferator-activated receptor:retinoid X receptor heterodimer is activated by fatty acids and fibrate hypolipidaemic drugs. J Mol Endocrinol. 1993;11:37–47. doi: 10.1677/jme.0.0110037. [DOI] [PubMed] [Google Scholar]
- 23.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A IJ, Jeannin E, Wahli W, Desvergne B. Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J Biol Chem. 1997;272:20108–20117. doi: 10.1074/jbc.272.32.20108. [DOI] [PubMed] [Google Scholar]
- 25.Thomas GD, Ruckerl D, Maskrey BH, Whitfield PD, Blaxter ML, Allen JE. The biology of nematode- and IL4Ralpha-dependent murine macrophage polarization in vivo as defined by RNA-Seq and targeted lipidomics. Blood. 2012;120:e93–e104. doi: 10.1182/blood-2012-07-442640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brys L, Beschin A, Raes G, et al. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol. 2005;174:6095–6104. doi: 10.4049/jimmunol.174.10.6095. [DOI] [PubMed] [Google Scholar]
- 27.Yang XY, Wang LH, Mihalic K, et al. Interleukin (IL)-4 indirectly suppresses IL-2 production by human T lymphocytes via peroxisome proliferator-activated receptor gamma activated by macrophage-derived 12/15-lipoxygenase ligands. J Biol Chem. 2002;277:3973–3978. doi: 10.1074/jbc.M105619200. [DOI] [PubMed] [Google Scholar]
- 28.Chen ZY, Tseng C-C. 15-Deoxy-Δ12,14 Prostaglandin J2 Up-Regulates Krüppel-Like Factor 4 Expression Independently of Peroxisome Proliferator-Activated Receptor γ by Activating the Mitogen-Activated Protein Kinase Kinase/Extracellular Signal-Regulated Kinase Signal Transduction Pathway in HT-29 Colon Cancer Cells. Molecular Pharmacology. 2005;68:1203–1213. doi: 10.1124/mol.105.014944. [DOI] [PubMed] [Google Scholar]
- 29.Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 30.Mullican SE, Gaddis CA, Alenghat T, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csoka B, Selmeczy Z, Koscso B, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. Faseb j. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies LC, Rosas M, Jenkins SJ, et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins SJ, Ruckerl D, Thomas GD, et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginhoux F, Greter M, Leboeuf M, et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbert DR, Yang JQ, Hogan SP, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 40.Anthony RM, Urban JF, Jr, Alem F, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature medicine. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao A, Urban JF, Jr, Anthony RM, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135:217–225. e1. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonne-Annee S, Kerepesi LA, Hess JA, et al. Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun. 2013 doi: 10.1128/IAI.00625-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadidi S, Antignano F, Hughes MR, et al. Myeloid cell-specific expression of Ship1 regulates IL-12 production and immunity to helminth infection. Mucosal Immunol. 2012;5:535–543. doi: 10.1038/mi.2012.29. [DOI] [PubMed] [Google Scholar]
- 44.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 46.Paveley RA, Aynsley SA, Turner JD, et al. The Mannose Receptor (CD206) is an important pattern recognition receptor (PRR) in the detection of the infective stage of the helminth Schistosoma mansoni and modulates IFNgamma production. Int J Parasitol. 2011;41:1335–1345. doi: 10.1016/j.ijpara.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 47.deSchoolmeester ML, Martinez-Pomares L, Gordon S, Else KJ. The mannose receptor binds Trichuris muris excretory/secretory proteins but is not essential for protective immunity. Immunology. 2009;126:246–255. doi: 10.1111/j.1365-2567.2008.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pesce JT, Ramalingam TR, Mentink-Kane MM, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS pathogens. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowcutt R, Bell LV, Little M, et al. Arginase-1-expressing macrophages are dispensable for resistance to infection with the gastrointestinal helminth Trichuris muris. Parasite Immunol. 2011;33:411–420. doi: 10.1111/j.1365-3024.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shuhui L, Mok YK, Wong WS. Role of mammalian chitinases in asthma. Int Arch Allergy Immunol. 2009;149:369–377. doi: 10.1159/000205583. [DOI] [PubMed] [Google Scholar]
- 51.Owhashi M, Arita H, Hayai N. Identification of a novel eosinophil chemotactic cytokine (ECF-L) as a chitinase family protein. J Biol Chem. 2000;275:1279–1286. doi: 10.1074/jbc.275.2.1279. [DOI] [PubMed] [Google Scholar]
- 52.Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol. 1999;162:1003–1009. [PubMed] [Google Scholar]
- 53.Owhashi M, Maruyama H, Nawa Y. Kinetic study of eosinophil chemotactic factor production with reference to eosinophilia and granuloma formation in mice infected with Schistosoma japonicum. Int J Parasitol. 1996;26:705–711. doi: 10.1016/0020-7519(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 54.Ramalho-Pinto FJ, McLaren DJ, Smithers SR. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J Exp Med. 1978;147:147–156. doi: 10.1084/jem.147.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990;144:3166–3173. [PubMed] [Google Scholar]
- 56.Cai Y, Kumar RK, Zhou J, Foster PS, Webb DC. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: identification of a novel pathway for regulating allergic inflammation. J Immunol. 2009;182:5393–5399. doi: 10.4049/jimmunol.0803874. [DOI] [PubMed] [Google Scholar]
- 57.Reese TA, Liang HE, Tager AM, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. Embo j. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pesce JT, Ramalingam TR, Wilson MS, et al. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS pathogens. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nair MG, Du Y, Perrigoue JG, et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truscott M, Evans DA, Gunn M, Hoffmann KF. Schistosoma mansoni Hemozoin Modulates Alternative Activation of Macrophages via Specific Suppression of Retnla Expression and Secretion. Infection and Immunity. 2013;81:133–142. doi: 10.1128/IAI.00701-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009;199:1827–1837. doi: 10.1086/599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noel W, Hassanzadeh G, Raes G, et al. Infection stage-dependent modulation of macrophage activation in Trypanosoma congolense-resistant and -susceptible mice. Infect Immun. 2002;70:6180–6187. doi: 10.1128/IAI.70.11.6180-6187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 65.Walther M, Tongren JE, Andrews L, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Osorio EY, Zhao W, Espitia C, et al. Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase 1 expression. PLoS pathogens. 2012;8:e1002417. doi: 10.1371/journal.ppat.1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kropf P, Fuentes JM, Fahnrich E, et al. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. Faseb j. 2005;19:1000–1002. doi: 10.1096/fj.04-3416fje. [DOI] [PubMed] [Google Scholar]
- 68.Stempin CC, Tanos TB, Coso OA, Cerban FM. Arginase induction promotes Trypanosoma cruzi intracellular replication in Cruzipain-treated J774 cells through the activation of multiple signaling pathways. Eur J Immunol. 2004;34:200–209. doi: 10.1002/eji.200324313. [DOI] [PubMed] [Google Scholar]
- 69.El Kasmi KC, Qualls JE, Pesce JT, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ong YC, Reese ML, Boothroyd JC. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem. 2010;285:28731–28740. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jensen KD, Wang Y, Wojno ED, et al. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe. 2011;9:472–483. doi: 10.1016/j.chom.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nance JP, Vannella KM, Worth D, et al. Chitinase dependent control of protozoan cyst burden in the brain. PLoS pathogens. 2012;8:e1002990. doi: 10.1371/journal.ppat.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lefevre L, Lugo-Villarino G, Meunier E, et al. The C-type Lectin Receptors Dectin-1, MR, and SIGNR3 Contribute Both Positively and Negatively to the Macrophage Response to Leishmania infantum. Immunity. 2013;38:1038–1049. doi: 10.1016/j.immuni.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Sosa M, Rivera-Montoya I, Espinoza A, et al. Acute cysticercosis favours rapid and more severe lesions caused by Leishmania major and Leishmania mexicana infection, a role for alternatively activated macrophages. Cell Immunol. 2006;242:61–71. doi: 10.1016/j.cellimm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Hoeve MA, Mylonas KJ, Fairlie-Clarke KJ, Mahajan SM, Allen JE, Graham AL. Plasmodium chabaudi limits early Nippostrongylus brasiliensis-induced pulmonary immune activation and Th2 polarization in co-infected mice. BMC Immunol. 2009;10:60. doi: 10.1186/1471-2172-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herbert DR, Holscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 78.Chen F, Liu Z, Wu W, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nature medicine. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith P, Mangan NE, Walsh CM, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 80.Panda SK, Kumar S, Tupperwar NC, et al. Chitohexaose activates macrophages by alternate pathway through TLR4 and blocks endotoxemia. PLoS pathogens. 2012;8:e1002717. doi: 10.1371/journal.ppat.1002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang M, Caragine T, Wang H, et al. Spermine Inhibits Proinflammatory Cytokine Synthesis in Human Mononuclear Cells: A Counterregulatory Mechanism that Restrains the Immune Response. The Journal of Experimental Medicine. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herbert DR, Orekov T, Roloson A, et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu L, Zhang G, Zhou Y, et al. Stimulation of B7-H3 (CD276) directs the differentiation of human marrow stromal cells to osteoblasts. Immunobiology. 2011;216:1311–1317. doi: 10.1016/j.imbio.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Leitner J, Klauser C, Pickl WF, et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182:2357–2363. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osborne LC, Joyce KL, Alenghat T, et al. Resistin-like molecule alpha promotes pathogenic Th17 cell responses and bacterial-induced intestinal inflammation. J Immunol. 2013;190:2292–2300. doi: 10.4049/jimmunol.1200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loke P, MacDonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 88.Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodriguez-Sosa M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int J Parasitol. 2005;35:1349–1358. doi: 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith P, Walsh CM, Mangan NE, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173:1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 91.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–3320. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 92.Broadhurst MJ, Leung JM, Lim KC, et al. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS pathogens. 2012;8:e1002883. doi: 10.1371/journal.ppat.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nature reviews Immunology. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willenborg S, Lucas T, van Loo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 95.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 96.Liu T, Dhanasekaran SM, Jin H, et al. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lehrke M, Lazar MA. Inflamed about obesity. Nature medicine. 2004;10:126–127. doi: 10.1038/nm0204-126. [DOI] [PubMed] [Google Scholar]
- 98.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nature medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 99.Solinas G, Vilcu C, Neels JG, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 100.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Satoh T, Kidoya H, Naito H, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524–528. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 103.Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Research. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 107.Saederup N, Cardona AE, Croft K, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 110.Ferron M, Vacher J. Targeted expression of Cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis. 2005;41:138–145. doi: 10.1002/gene.20108. [DOI] [PubMed] [Google Scholar]
- 111.Tenu JP, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher JL. Effects of the new arginase inhibitor N(omega)-hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide. 1999;3:427–438. doi: 10.1006/niox.1999.0255. [DOI] [PubMed] [Google Scholar]