Abstract
Radiation dermatitis occurs in approximately 95% of patients receiving radiotherapy (RT) for breast cancer. We conducted a randomized, double-blind, placebo-controlled clinical trial to assess the ability of curcumin to reduce radiation dermatitis severity in 30 breast cancer patients. Eligible patients were adult females with noninflammatory breast cancer or carcinoma in situ prescribed RT without concurrent chemotherapy. Randomized patients took 2.0 grams of curcumin or placebo orally three times per day (i.e., 6.0 grams daily) throughout their course of RT. Weekly assessments included Radiation Dermatitis Severity (RDS) score, presence of moist desquamation, redness measurement, McGill Pain Questionnaire-Short Form and Symptom Inventory questionnaire. The 30 evaluable patients were primarily white (90%) and had a mean age of 58.1 years. Standard pooled variances t test showed that curcumin reduced RDS at end of treatment compared to placebo (mean RDS =2.6 vs. 3.4; P =0.008). Fisher’s exact test revealed that fewer curcumin-treated patients had moist desquamation (28.6% vs. 87.5%; P =0.002). No significant differences were observed between arms for demographics, compliance, radiation skin dose, redness, pain or symptoms. In conclusion, oral curcumin, 6.0 g daily during radiotherapy, reduced the severity of radiation dermatitis in breast cancer patients.
INTRODUCTION
Radiation dermatitis is one of the most common side effects experienced by patients receiving radiotherapy (RT) for sarcoma, breast, lung, and head and neck cancer. Approximately, 95% of these patients will have radiation-induced skin reactions, with 10% being severe (1, 2). Although innovative medical technology, such as intensity-modulated radiotherapy (IMRT), has reduced the incidence of severe radiation dermatitis, radiation-induced skin reactions are still a significant complication (3). Currently, there is no standard treatment for radiation dermatitis with demonstrated effectiveness.
The skin is susceptible to damage by ionizing radiation because it is a highly proliferative and self-renewing organ. The doses and timing employed in standard fractionation regimens lead to an accumulated loss of basal keratinocytes and impairment of the epidermal skin barrier (1, 4, 5). Radiation dermatitis ranges in severity from faint or definite erythema to dry or moist desquamation and ulceration (1, 2, 4, 5). The current clinical guidelines for management of radiation-induced skin reactions include: (1) washing with lukewarm water and mild soap; (2) applying unscented, lanolin-free, water-based moisturizers; and (3) IMRT (2, 5, 6). However, there is no consensus for an agent that effectively mitigates or prevents radiation dermatitis.
Curcumin, a component of turmeric, is a potent antioxidant and anti-inflammatory agent that has been used to treat skin ailments, such as scabies, acne, eczema, wrinkled skin and wound healing (7–11). Due to its low toxicity and a broad spectrum of applications, curcumin has been studied in a variety of fields, including anti-cancer (12–15) and cancer prevention (16, 17). Previously, we published that administration of curcumin to mice significantly reduced their radiation-induced skin toxicity by 50% (18). Its low toxicity profile in normal tissues combined with antitumor effects suggested that curcumin would be a good candidate for a clinical trial to treat radiation dermatitis. This randomized, double-blind, placebo-controlled study extended our preclinical findings by examining the ability of oral curcumin to reduce radiation dermatitis severity in 30 patients receiving RT alone for breast cancer.
PATIENTS AND METHODS
Patients and Study Design
Eligible patients were adult (≥18 years of age), able to read and understand English, who were diagnosed with noninflammatory breast cancer or carcinoma in situ and prescribed RT without concurrent chemotherapy at the University of Rochester Cancer Center. Exclusion criteria included patients who: had bilateral breast cancer; previous radiation to the chest or breast area; diagnosis of inflammatory breast cancer; breast reconstruction and/or expanders prior to RT; were taking anti-coagulant therapy (warfarin, coumadin or heparin) or anti-epidermal growth factor receptor (EGFR) therapy or were receiving partial breast irradiations. All patients received standard fractionated RT (~1.8–2.4 Gy per session) for four to seven weeks with or without boost for a total radiation dose of ≥42 Gy.
This study was a randomized, double-blind, placebo-controlled trial conducted under FDA IND no. 75,444 for Curcumin C3 Complex, approved by the University of Rochester Institutional Review Board, and registered on ClinicalTrials.gov Identifier: NCT01042938 (http://clinicaltrials.gov/ct2/show/results/NCT01042938). All patients provided written informed consent for participation. Patients were randomly assigned to curcumin or placebo capsules using a 1:1 ratio with block size of 10. The primary outcome measures included severity of radiation dermatitis at the end of RT and the proportion of patients with moist desquamation at the end of RT in each treatment arm. Secondary outcome measures included redness and pain at the RT site, as well as the severity of various other treatments of cancer related symptoms.
Study Medication
Curcumin (Curcumin C3 Complex®) and placebo capsules were manufactured by Sabinsa Corporation (Payson, UT). The curcumin capsules contained yellow granular powder, shown by spectroscopic and chromatographic fingerprint to contain a minimum of ≥95% curcuminoids (approximately 390 mg curcumin, 75 mg demethoxycurcumin, 12.5 mg bisdemethoxycurcumin) plus excipients (>20 mg microcrystalline cellulose, magnesium stearate, silicone dioxide) in 500 mg size “0”, red opaque, hard gelatin capsules. Matching placebo capsules contained 500 mg of dicalcium phosphate, excipients and a yellow food coloring. Patients were dispensed one 84-count bottle (i.e., 7 day supply of capsules) of study medication every seven days throughout their course of RT. Compliance was measured by weekly pill counts.
Study Procedures and Measures
Patients were screened for eligibility and informed consent was obtained prior to the start of RT. They were assessed at baseline, weekly after every fifth RT session, at the end of RT (EndRT), and at 2 post-RT appointments (1-month and 6-months post-RT). All patients took four 500 mg curcumin or placebo capsules three times daily throughout their prescribed course of RT. “Standard care” topical agents for radiation dermatitis (i.e., Radiaplex® gel, silvadine, hydrocortisone cream) were allowed. Patient assessments involved a radiation dermatitis severity score, redness measurement, digital imaging of skin changes and completion of two self-report questionnaires: Symptom Inventory (SI) and McGill Pain Questionnaire-Short Form (MPQ-SF) (19–23). Digital images of radiation-induced skin changes were obtained using a Canon PowerShot SD790IS camera. Radiation dermatitis severity was measured using the Radiation Dermatitis Severity (RDS) scoring scale (Fig. 1). This scoring system was adapted from the Radiation Treatment Oncology Group (RTOG) and NIH Common Toxicity Criteria-Adverse Event (CTCAE) scales for acute radiation skin toxicity (1, 2). The irradiated skin of each patient was given a rating by two study personnel (i.e., Radiation Oncologist and Dermatologist). Redness at the RT site was measured using a colorimeter (CR-200, Konica Minolta), which is a noninvasive device that assigns numerical values to color using the L*a*b* color scale. A normal skin color measurement was taken each time on the nonirradiated breast. A 19-item SI, adapted from the MD Anderson Symptom Inventory (MDASI), was used to assess potential side effects of curcumin and RT (19, 20, 22). Patients were asked to rate the severity of potential symptoms (pain at treatment site, other pain, nausea, vomiting, upset, shortness of breath, memory, lack of appetite, diarrhea, change in urination, skin problems in treatment area, disturbed sleep, fatigue, general activity, mood, work, relationships, walking and quality of life) using an 11-point scale anchored by 0 (“not present”) and 10 (“as bad as you can imagine”). The MPQ-SF was used to monitor the severity and type of pain (i.e., sensory pain, affective pain, and perceived pain intensity) experienced by patients at the radiation treatment site (23, 24). The MPQ-SF contained 11 sensory pain items (i.e., descriptors), 4 affective pain items (i.e., descriptors) and 1 perceived pain item. The sensory and affective pain items were rated on a 4-point scale anchored by 0 (“none”) to 3 (“severe”), whereas the perceived pain item was rated on a 6-point scale anchored by 0 (“no pain”) to 5 (“excruciating”). Radiation skin dose (maximum and average) was calculated by radiation physicists using two millimeter skin thickness and the AAA algorithm in Varian dosimetry planning software.
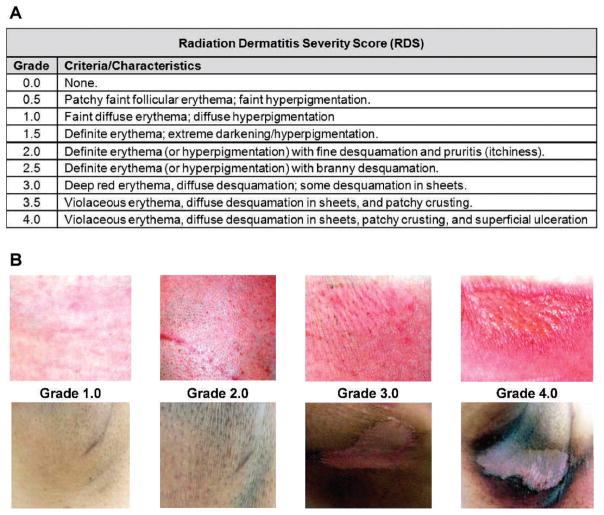
FIG. 1.
Radiation Dermatitis Severity (RDS) scoring. Panel A: The severity of radiation dermatitis was measured using this scoring system, range 0.0 to 4.0 at increments of 0.5. The RDS score incorporates changes in redness, pigment, texture and integrity of the skin. Panel B: Digital images of portraying the appearance of various RDS scores (1.0, 2.0, 3.0 and 4.0 from left to right) in fair (top) and darkly pigmented (bottom) skin.
Statistical Analyses
The sample size of 30 evaluable patients was based upon the expectation that data generated from this number of patients would adequately determine patient receptivity of study medication and generate an effect size estimate for a subsequent larger study. All statistical tests were performed at the two-tailed 5% level of significance. The pooled variances t test was used to examine the differences in mean RDS at EndRT between the treatments arms. Longitudinal repeated measures model identified differences in trajectories between treatment arms in mean RDS over time. Fisher’s exact test was used to test differences in the proportion of moist desquamation in each treatment group. Analysis of covariance (ANCOVA) adjusting for baseline was used to examine differences in pain at the RT site between the two treatment arms as measured by the SI and MPQ-SF questionnaires. Repeated measures analyses were used to compare redness (i.e., a* values) at RT site and over time between treatment arms. Additionally, regression analyses were used to explore relationships between RDS and redness or radiation skin dose. The assumptions of the pooled t test were checked (i.e., distributions were not grossly skewed and no significant difference in variance) and there were no major concerns. Moreover, for the longitudinal models, the distribution of the residuals (using model RDS = Arm + Residual) were well behaved and normally distributed (Shapiro-Wilk test, P = 0.266). Computations were performed using SAS version 9.2 and/or JMP Version 9.
RESULTS
Patient Characteristics
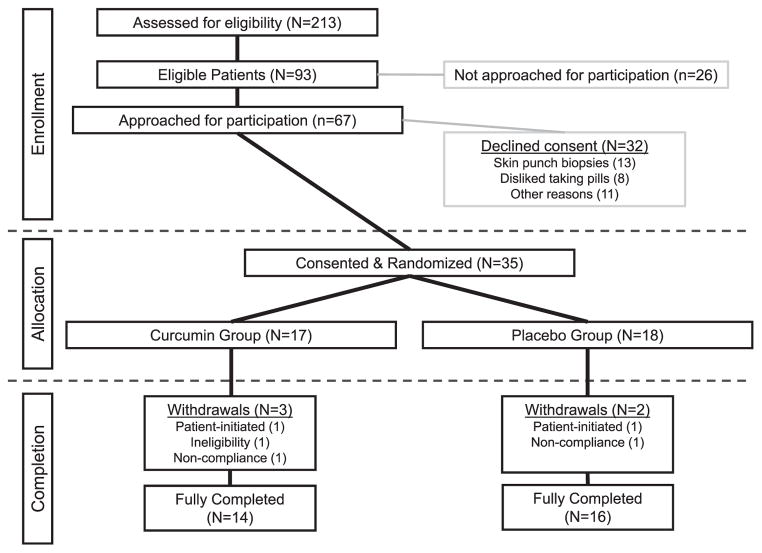
From January 2008 to January 2010, a total of 213 patients with breast cancer were prescribed radiation therapy at the University of Rochester Wilmot Cancer Center (Fig. 2). Of these 213 patients, 93 patients were eligible to participate in this clinical trial. Out of the 67 patients approached for participation in the trial, 35 (52%) patients consented. Patients declined participation because of the skin punch biopsies (13/32; 41%) or the consumption of pills (8/32; 25%). There were five withdrawals from the study: patient-reported hot flashes (1/5); undisclosed personal reasons (1/5); ineligibility due to concurrent participation in another RT intervention trial (1/5); and noncompliance with study procedures (2/5). Analyses reported herein were conducted on the 30 patients who fully completed the clinical trial.
FIG. 2.
Consort diagram. This diagram documents the patient flow for the clinical trial. Only 93/213 (43.7%) of patients prescribed radiation therapy for breast cancer were eligible for this trial. Of the patients approached for participation, 35/67 (52.2%) consented. The withdrawal rate for the study was 5/35 (14.2%).
The baseline demographics were equivalent between curcumin and placebo treatment groups (Table 1). The majority of patients were white females (90%) with mean age of 58.1 years and ER+/PR+ tumors (76.7%) who did not have total mastectomy or chemotherapy prior to RT. The majority of patients had a bra cup size of B (31%) or C (37.9%) and a mean bra size of 37.5 (SE = 0.62). No significant differences were observed between curcumin and placebo groups for bra cup size (% A, B, C, D = 7.7%. 38.5%, 38.5%, 15.4% vs. 6.3%, 25%, 37.5%, 31.3%, respectively; Fisher’s exact, P = 0.774) or bra size (mean = 36.8 vs. 38, respectively; ANOVA, P = 0.332). At baseline, 80% of patients expected to have skin reactions but only 26.7% of patients expected to have pain from RT. The majority of patients did not report pain at baseline and no significant difference was observed between treatment arms (P ≥ 0.359). Overall, the total prescribed radiation dose to patients ranged from 42.6–50.4 Gy and the total number of RT sessions ranged from 16–33 sessions. There were no significant differences between the curcumin and placebo groups in mean total prescribed radiation dose or total number of RT sessions (P = 0.343 and P = 0.162, respectively). Additionally, the radiation skin dose (maximum or average) were similar between treatment arms (P = 0.895 and P = 0.628, respectively). The majority of patients were compliant to both curcumin and placebo capsules [mean (SD) = 96.6% (6.6%) vs. 98.4% (3.2%), respectively; P = 0.344]. To evaluate patient blinding, all patients were asked, “What study medication did you think you were taking during this clinical trial?” 5/14 (35.7%) of patients randomized to the curcumin arm guessed that they were taking curcumin, whereas only 2/16 (12.5%) of patients randomized to the placebo arm guessed that they were taking placebo.
TABLE 1.
Patient Demographic
| All N = 30 |
Curcumin N = 14 |
Placebo N = 16 |
|
|---|---|---|---|
| Age | |||
| Mean (SE) | 58.1 (2.2) | 54.6 (3.3) | 61.1 (2.8) |
| Race | |||
| White/Caucasian | 27 (90%) | 13 (92.8%) | 14 (87.5%) |
| Black/African American | 2 (6.7%) | 0 (0%) | 2 (12.5%) |
| Multiracial | 1 (3.3%) | 1 (7.1%) | 0 (0%) |
| Chemotherapy before radiation | |||
| Yes | 13 (43.3%) | 7 (50%) | 6 (37.5%) |
| No | 17 (56.7%) | 7 (50%) | 10 (62.5%) |
| Surgery type | |||
| Lumpectomy | 27 (90%) | 12 (85.7%) | 15 (93.8%) |
| Mastectomy | 3 (10%) | 2 (14.3%) | 1 (6.25%) |
| ER/PR status | |||
| ER−/PR− | 4 (13.3%) | 2 (14.3%) | 2 (12.5%) |
| ER+/PR− | 3 (10%) | 1 (7.1%) | 2 (12.5%) |
| ER+/PR+ | 23 (76.7%) | 11 (78.6%) | 12 (75%) |
| Staging | |||
| 0 | 4 (13.3%) | 0 (0%) | 4 (25%) |
| I | 14 (46.7%) | 6 (42.8%) | 8 (50%) |
| II | 8 (26.7%) | 5 (35.7%) | 3 (18.8%) |
| III | 6 (10%) | 3 (21.4%) | 0 (0%) |
| IV | 1 (3.3%) | 0 (0%) | 1 (6.2%) |
| Mean radiation dose (Gy) | |||
| Total prescribed dose (SE) | 46.51 (3.48) | 46.15 (5.79) | 46.83 (4.14) |
| Maximum skin dose (SE) | 55.48 (1.76) | 55.73 (2.79) | 55.25 (2.29) |
| Average skin dose (SE) | 31.27 (1.68) | 32.16 (2.53) | 30.49 (2.30) |
| Total number of RT sessions (SE) | 30.90 (0.79) | 29.71 (1.61) | 31.94 (0.36) |
| Expected RT skin problems | |||
| Yes | 24 (80%) | 12 (85.7%) | 12 (75%) |
| No | 6 (20%) | 2 (14.3%) | 4 (25%) |
| Expected RT pain | |||
| Yes | 8 (26.7%) | 3 (21.4%) | 5 (31.3%) |
| No | 22 (73.3%) | 11 (78.6%) | 11 (68.7%) |
| Mean baseline pain (SE) | |||
| SI pain at treatment site | 0.300 (0.137) | 0.214 (0.155) | 0.375 (0.221) |
| SI other pain | 0.733 (0.318) | 0.571 (0.500) | 0.875 (0.417) |
| Total MPQ score | 0.830 (0.369) | 0.860 (0.455) | 0.810 (0.579) |
| Perceive pain intensity | 0.170 (0.084) | 0.210 (0.155) | 0.130 (0.085) |
| Sensory pain subscale score | 0.633 (0.282) | 0.643 (0.341) | 0.625 (0.446) |
| Affective pain subscale score | 0.033 (0.183) | 0.000 (0.000) | 0.063 (0.063) |
Curcumin Reduced the Severity of Radiation Dermatitis, But Not Redness
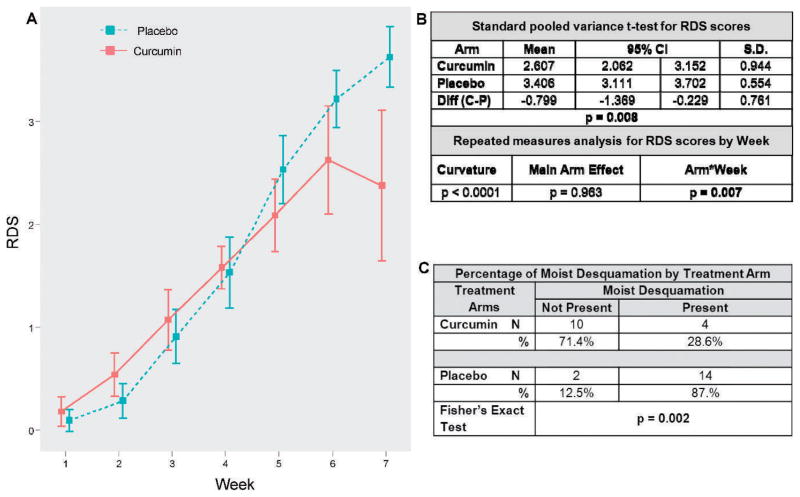
Skin assessments were performed on each patient weekly after the fifth RT session to rate the severity of worst dermatitis using the RDS scale (Fig. 1). The pooled variances t test (Fig. 3A and B) showed that curcumin reduced RDS at EndRT compared to placebo (P = 0.008). The mean RDS scores for curcumin-treated patients were 0.8 lower than the placebo-treated patients, which was more than one increment on the RDS scale. Additionally, repeated measures analyses demonstrated that RDS scores diverged between curcumin and placebo arms after week 4 (Fig. 3A and B). The crucial test of Arm*Week interaction was significant (P = 0.007), showing the weekly profiles differed between the curcumin and placebo arms. The main Arm effect was insignificant (P =0.963), but the interaction was significant (P = 0.007) reflecting the divergence of the time trajectories between arms. Furthermore, Fisher’s exact test (Fig. 3C) showed that fewer patients in the curcumin arm had moist desquamation compared to the placebo arm (28.6% vs. 87.5%, respectively; P = 0.002).
FIG. 3.
Curcumin reduced radiation dermatitis severity and presence of moist desquamation. Panel A: Line graph plotting the mean RDS scores by week of RT for curcumin and placebo arms. The two arms are similar for week 1 to week 4, but then diverge from week 5 to week 7. Panel B: Standard pooled variances t test showed that the curcumin arm had significantly lower RDS scores at the end of RT compared to the placebo. Repeated measures analyses demonstrated that the Arm*Week interaction and the total Arm effect (Arm + Arm*Week) were highly significant. Panel C: Fisher’s exact test showed that fewer patients in the curcumin arm had the presence of moist desquamation at the end of RT compared to the placebo arm.
Even though the RDS scale incorporates redness as a characteristic of each severity score, we examined redness as its own separate variable using a colorimeter, which measures color using the L*a*b* colorimetric scale. L* values represent white color levels, a* values represent red color levels and b* values represent blue/black color levels. All L*a*b* values for the irradiated skin site were normalized to L*a*b* values for the nonirradiated skin prior to analysis (Table 2) (25, 26). Repeated measures analyses showed insignificant Arm*Week interaction for all 3 color values. Interestingly, the overall Week effect was highly significant for all color values, suggesting that skin color changes significantly over the course of RT. Furthermore, regression analysis for the correlation of a* values (i.e., redness) with RDS scores did not explain the variance in RDS scores over time (Table 2). Although curcumin appeared to reduce radiation dermatitis severity, it did not alter skin redness.
TABLE 2.
Statistical Analyses for L*a*b* Colorimetric Values
| Repeated measures analyses on normalized L*a*b* values
| |||
|---|---|---|---|
| Response | Curvature | Main arm effect | Arm*Week |
| L*-baseline | P = 0.080 | P = 0.746 | P = 0.387 |
| a*-baseline | P = 0.001 | P = 0.328 | P = 0.588 |
| b*-baseline | P = 0.484 | P = 0.059 | P = 0.093 |
| Regression model: correlation of a* value (redness) with RDS
| ||||
|---|---|---|---|---|
| Week | R2 | RSME | Estimate | P value |
| 1 | 0.299 | 0.199 | 0.053 | 0.267 |
| 2 | 0.122 | 0.354 | 0.052 | 0.253 |
| 3 | 0.546 | 0.355 | 0.145 | <0.0001 |
| 4 | 0.238 | 0.484 | 0.076 | 0.067 |
| 5 | 0.168 | 0.604 | 0.006 | 0.873 |
| 6 | 0.333 | 0.636 | 0.030 | 0.545 |
| 7 | 0.172 | 0.929 | 0.133 | 0.189 |
Patient-Reported Pain and Adverse Events
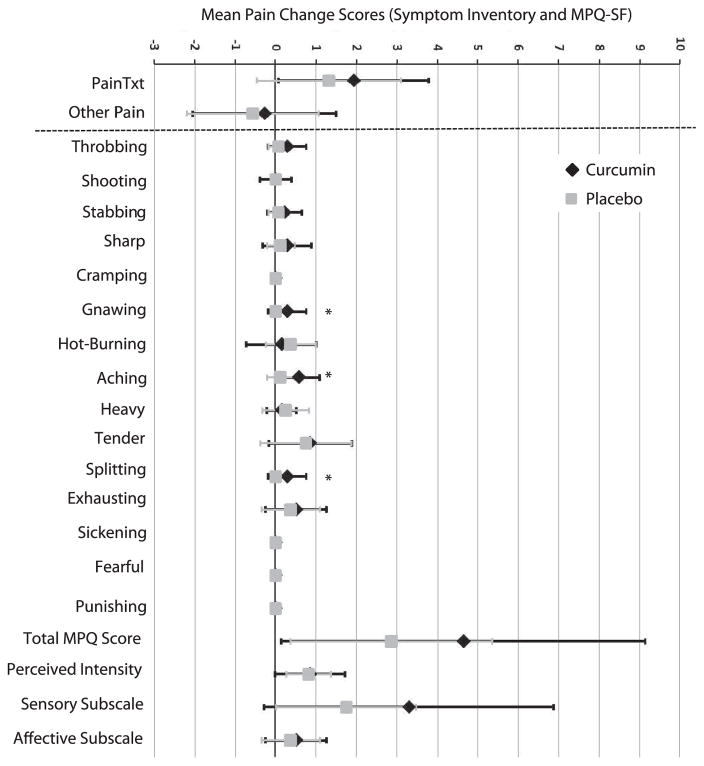
The severity of pain and other various symptoms were monitored using two self-report questionnaires, the SI and the MPQ-SF. ANCOVA analyses were used to examine significant changes in patient-reported pain from baseline to EndRT (Table 3). The mean change scores for pain for each treatment group are plotted in Fig. 4. No significant differences between the two treatment arm for the two SI pain items, “pain at treatment site” and “other pain”. Similarly, no significant differences were revealed between treatment arms for the total MPQ or perceived pain intensity, sensory pain and affective pain subscale scores. However, examination of each individual pain descriptor showed significant differences between the curcumin and placebo arms for the sensory pain descriptors, “gnawing,” “aching” and “splitting” (P ≤ 0.021) (Fig. 4).
Table 3.
Statistical Analyses for Pain Items (MPQ-SF and SI)
| Mean change (95% CI)
|
P value
|
|||
|---|---|---|---|---|
| Curcumin | Placebo | t test | ANCOVA | |
| Total MPQ | 4.643 (2.045, 7.241) | 2.875 (1.543, 4.207) | 0.187 | 0.144 |
| Perceived pain | 0.857 (0.358, 1.356) | 0.813 (0.523, 1.102) | 0.865 | 0.644 |
| Sensory pain | 3.286 (1.217, 5.354) | 1.750 (0.827, 2.673) | 0.138 | 0.081 |
| Affective pain | 0.500 (0.061, 0.939) | 0.375 (−0.008, 0.758) | 0.647 | 0.550 |
| Pain at RT site | 1.929 (0.855, 3.002) | 1.313 (0.365, 2.260) | 0.362 | 0.504 |
| Other pain | −0.286 (−1.309, 0.738) | −0.563 (−1.432, 0.307) | 0.660 | 0.967 |
FIG. 4.
Mean change scores for pain items from Symptom Inventory (SI) and MPQ-SF. The forest plot presents the mean change scores (EndRT-Baseline) for the 2 pain items on the SI (“pain at RT site” and “other pain”) and the 15 different pain items, total MPQ, and Subscale scores on the MPQ-SF. Error bars represent standard deviation. Individual MPQ-SF pain items, “gnawing,” “aching” and “splitting” showed slightly higher pain at EndRT in curcumin patients compared to placebo. However, no differences were observed in total or subscale MPQ-SF scores or in the 2 SI items.
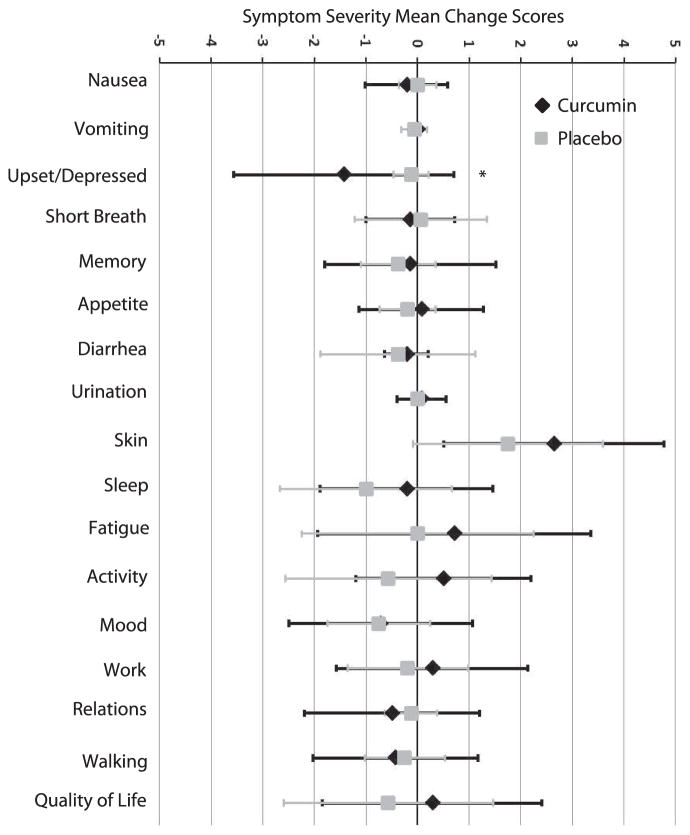
The remaining 17 various symptoms on the SI were also evaluated for differences between treatment arms (Fig. 5). ANCOVA analyses did not reveal any significant findings (all P values ≥0.100). However, t test on mean change scores did show a difference in “upset/depressed” between curcumin and placebo arms [mean change (95% CI) = −1.429 (−2.663, −0.194) vs. −0.125 (−0.307, 0.057); P = 0.023]. These data resulted from the higher baseline mean value for “upset/depressed” in the curcumin group compared to the placebo group (1.7 vs. 0.19; P = 0.011). Although other research studies reported mild to moderate diarrhea with curcumin consumption (7), no significant increase in severity of diarrhea was observed with curcumin compared to placebo [mean change (95% CI) = −0.214 (−0.460, 0.032) vs. −0.375 (−1.174, 0.424); P =0.702]. No significant adverse events were reported during the course of the study. Monitoring clinical blood tests did not demonstrate any significant clinical concerns with curcumin consumption (data not shown). Overall, our data suggest that oral curcumin was well tolerated by patients.
FIG. 5.
Symptom severity mean change scores. The forest plot presents the mean change severity scores for the other 17 symptoms on the Symptom Inventory. Error bars represent standard deviation. ANCOVA analyses did not reveal any significant findings. However, t test on mean change scores did show a difference in “upset/depressed” between curcumin and placebo arms. These results were due to the high baseline level of “upset/depressed” in the curcumin arm compared to placebo, which was accounted for in the ANCOVA.
DISCUSSION
Radiation dermatitis is a common side effect of RT with few, if any, effective treatment options available. The severity of the skin reaction ranges by individual, body area, as well as type and dose of radiation (1, 2, 4, 5, 27, 28). Since 2006, the guidelines for management of skin during RT recommends “washing with lukewarm water and mild soap” (2, 5, 29). Various research studies have yielded conflicting results on the use of corticosteroids, hyaluronate and other creams. For example, corticosteroid cream was shown to be significantly more effective than an emollient cream in reducing the occurrence of acute radiation dermatitis in two small randomized studies (30). But, research also showed that the use of corticosteroids can worsen dermatitis (4) and the superiority of hylauronate cream remains unclear (31). For example, Pinnix et al. published that topical sodium hyaluronate did not reduce the development of ≥grade 2.0 radiation dermatitis severity, whereas Ravo et al. published that patients using sodium hyaluronate did not develop dermatitis >grade 2.0 severity (32, 33). Clearly, identification of effective topical or systemic treatment for radiation dermatitis is still necessary.
Over the past several years, curcumin has been investigated as a potential anticancer agent in clinical trials for the treatment or prevention of various cancers, including pancreatic cancer, colorectal cancer and osteosarcoma. It is usually recommended that patients avoid antioxidants during RT due to the concern that antioxidants will reduce the efficacy of RT by protecting tumor cells from radiation-induced cell death. Therefore, patients would be at higher risk for recurrence of cancer after RT (34). Published studies have shown that curcumin actually sensitized to radiation or inhibit growth of colorectal cancer, cervical cancer, and head and neck cancer (35–39). Additionally, in vitro and in vivo studies have demonstrated that curcumin does not protect breast cancer cells (40–44).2 Overall, curcumin may be a promising anti-cancer treatment with and without radiation treatment. Our clinical trial protocol underwent thorough and comprehensive review by the FDA (i.e., IND 75,444) to ensure that patients were not being put at undue risk for participation in this trial. An interesting follow-up study could potentially examine the five to ten year post-RT recurrence rate of the participants in this study, however, this type of study may be futile given the low five- to ten-year post-RT recurrence rate for breast cancer, the published literature and the transient curcumin exposure (i.e., 4 to 7 weeks of exposure).
This clinical trial demonstrated that oral curcumin, at 6.0 grams daily, significantly reduced the severity of radiation dermatitis and moist desquamation, though not erythema. Curcumin did not appear effective at reducing the severity of radiation dermatitis in patients who had total mastectomy prior to radiation therapy. The three patients that had total mastectomy (2 in curcumin arm and 1 in placebo arm) had the most severe skin reaction (RDS = 4.0), which may suggest a threshold of radiation dose to the skin above which curcumin is ineffective. The nondose-finding design of this clinical trial could be seen as a limitation because the “real effective dose” of curcumin for severe radiation dermatitis cannot be discerned. However, the 6.0 grams daily dose of curcumin ensured minimal adverse reactions and detection of curcumin in plasma, while minimizing total daily number of capsules. Despite this reasoning for the 6.0 grams dose, it is possible that a higher oral dose of curcumin, such as ≥8.0 grams daily, may be more effective against radiation dermatitis severity including erythema and skin reactions in patients with total mastectomy. Furthermore, pharmacokintetic studies have demonstrated ways to increase curcumin’s bioavailability without compromising its biological activity through combined formulation or synthetic analog development (45). For example, piperine given at 20 mg combined with 2 grams of curcumin increased curcumin’s total bioavailability by 20-fold and allowed detection in the serum of healthy volunteers (45). Future clinical trials should investigate the potential effectiveness of curcumin at a higher oral dose and/or a readily bioavailable combined formulation or analog of curcumin. Overall, although curcumin did not completely prevent radiation dermatitis in this trial, the reduction in moist desquamation is clinically significant and suggests improved quality of life during RT.
This study incorporated the “standard care” for radiation dermatitis used in the clinic with the implementation of curcumin or placebo for study subjects. Radiaplex® gel, silvadine and/or hydrocortisone cream were provided to the patients as needed for radiation dermatitis in both treatment arms upon visible skin changes (i.e., ~week 3 of RT). The main strength of the study was the longitudinal and multiple measures for assessing radiation dermatitis severity. Radiation-induced skin changes were documented each week throughout the course of radiation therapy for breast cancer, which enabled the use of repeated measures analyses. Our study incorporated multiple, separate measures to accurately assess patient and treatment-related factors, skin redness and texture, moist desquamation and pain. Other clinical studies used the Skin Toxicity Assessment Tool (STAT) for assessment of radiation dermatitis (46). The STAT consists of three components: (1) patient and treatment characteristics; (2) objective scoring of skin reaction (i.e., intact skin, mild erythema, dry or moist desquamation); and (3) patient-reported severity of symptoms, such as burning, itchiness and tenderness (46). Although our study design did not use the STAT, our measures did encompass the three STAT component areas.
The limitations of the study included the small sample size and the large quantity of capsules. Although the sample size was small, the proportion of patients with radiation-induced moist desquamation was relatively large. It is possible that the incidence of moist desquamation will vary between institutions and patient subsets due to variations in the RT dosimetry, RT machine, RT session number and individual factors. Overall, the data suggest that a larger confirmatory trial is warranted given the large curcumin effect. To have an effect on the skin, this study required an oral dose of curcumin that would enter the blood. Due to the low bioavailability of curcumin, an oral dose less than 4.0 grams is not detectable in blood (47). Dose escalations studies have shown that the incidence of diarrhea and gastrointestinal upset increases at doses greater than 8.0 grams daily (48, 49). Therefore, 6.0 grams daily was used to ensure that curcumin would enter the blood with minimal side effects. Unfortunately, this dose required patients to consume twelve capsules daily. Synthetic forms of curcumin exist that appear to have increased bioavailability, which could potentially decrease the curcumin dosage and number of capsules (50–53). Additional clinical trials are warranted to determine if a synthetic curcumin would provide a similar reduction in radiation dermatitis severity. Alternatively, a topical formulation of curcumin may serve as another viable treatment option. For example, patients with head and neck cancer have extensive radiation-induced skin reactions, but are unable to swallow capsules due to radiation-induced esophagitis and mucositis. Overall, oral curcumin successfully reduced radiation dermatitis severity and moist desquamation; however a larger study is required to further confirm its effectiveness.
Acknowledgments
This study was supported by the FDA IND no. 75,444; NCI PHS grant 1R25CA10618; Dermatology Foundation Research Career Development Award; and URMC CTSI KL2-RR024136. A special thanks to all of the patients with breast cancer who participated in this clinical trial. We thank the Radiation Oncology Red and Yellow Service team members and the Departments of Radiation Oncology and Dermatology for assistance and support of this study.
Footnotes
Curcumin: A Treatment For Breast Cancer and Radiation-induced Dermatitis. American Association of Cancer Research Annual Meeting 2008. The manuscript of these results is under review for publication.
References
- 1.Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 Pt 2):985–93. doi: 10.1038/jid.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, Breen D, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. 2010;17(4):94–112. doi: 10.3747/co.v17i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MF, Chen WC, Lai CH, Hung CH, Liu KC, Cheng YH. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer. 2010;10:508. doi: 10.1186/1471-2407-10-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 5.McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs. 2011;27(2):e1–17. doi: 10.1016/j.soncn.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Bolderston A, Lloyd NS, Wong RK, Holden L, Robb-Blenderman L. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer. 2006 Aug;14(8):802–17. doi: 10.1007/s00520-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 8.Bhagavathula N, Warner RL, DaSilva M, McClintock SD, Barron A, Aslam MN, et al. A combination of curcumin and ginger extract improves abrasion wound healing in corticosteroid-impaired hairless rat skin. Wound Repair Regen. 2009;17(3):360–6. doi: 10.1111/j.1524-475X.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25(10):1911–7. doi: 10.1016/s0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- 10.Jagetia GC, Rajanikant GK. Role of curcumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wound, in mice whole-body exposed to various doses of gamma-radiation. J Surg Res. 2004;120(1):127–38. doi: 10.1016/j.jss.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Phan TA, Halliday GM, Barnetson RS, Damian DL. Melanin differentially protects from the initiation and progression of threshold UV-induced erythema depending on UV waveband. Photodermatol Photoimmunol Photomed. 2006;22(4):174–80. doi: 10.1111/j.1600-0781.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Current Medicinal Chem. 2010;17(3):190–7. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- 13.Gao W, Chan JY, Wei WI, Wong TS. Anti-Cancer Effects of Curcumin On Head And Neck Cancers. Anticancer Agents Med Chem. 2012;12(9):1110–6. doi: 10.2174/187152012803529736. [DOI] [PubMed] [Google Scholar]
- 14.Rajasekaran SA. Therapeutic potential of curcumin in gastrointestinal diseases. World J Gastrointest Pathophysiol. 2011;2(1):1–14. doi: 10.4291/wjgp.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Molecular Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaefer CM, Milner JA. Herbs and spices in cancer prevention and treatment. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2. Boca Raton: 2011. [Google Scholar]
- 17.Shi HS, Gao X, Li D, Zhang QW, Wang YS, Zheng Y, et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int J Nanomedicine. 2012;7:2601–11. doi: 10.2147/IJN.S31439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, et al. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006;65(3):890–8. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Anderson KO. NCI-OCAM, editor. Expert opinions on methodology: Development of cancer CAM symptom research. Bethesda, MD: National Cancer Institute; 2003. Assessment of cancer-related symptoms: relevance for CAM research methodology; pp. 67–90. [Google Scholar]
- 20.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Lerman SF, Rudich Z, Shahar G. Distinguishing affective and somatic dimensions of pain and depression: a confirmatory factor analytic study. J Clin Psychol. 2010;66(4):456–65. doi: 10.1002/jclp.20674. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal DI, Mendoza TR, Chambers MS, Asper JA, Gning I, Kies MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the MD Anderson symptom inventory, head and neck module. Head Neck. 2007;29(10):923–31. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 23.Zinke JL, Lam CS, Harden RN, Fogg L. Examining the cross-cultural validity of the english short-form McGill Pain Questionnaire using the matched moderated regression methodology. Clin J Pain. 2010;26(2):153–62. doi: 10.1097/AJP.0b013e3181b99f56. [DOI] [PubMed] [Google Scholar]
- 24.Lerman SF, Rudich Z, Shahar G. Distinguishing affective and somatic dimensions of pain and depression: a confirmatory factor analytic study. J Clin Psychol. 2010;66(4):456–65. doi: 10.1002/jclp.20674. [DOI] [PubMed] [Google Scholar]
- 25.Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: comparison between three instruments: the Chromameter(R), the DermaSpectrometer(R) and the Mexameter(R) Skin Res Technol. 2000;6(4):230–8. doi: 10.1034/j.1600-0846.2000.006004230.x. [DOI] [PubMed] [Google Scholar]
- 26.Yun IS, Lee WJ, Rah DK, Kim YO, Park BY. Skin color analysis using a spectrophotometer in Asians. Skin Res Technol. 2010;16(3):311–5. doi: 10.1111/j.1600-0846.2010.00434.x. [DOI] [PubMed] [Google Scholar]
- 27.Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(1 Suppl):15S–21S. doi: 10.1016/j.jvs.2010.06.175. [DOI] [PubMed] [Google Scholar]
- 28.Burris HA, 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15(11):1227–37. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McQuestion M. Evidence-based skin care management in radiation therapy. Semin Oncol Nurs. 2006;22(3):163–73. doi: 10.1016/j.soncn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Schmuth M, Wimmer MA, Hofer S, Sztankay A, Weinlich G, Linder DM, et al. Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. Br J Dermatol. 2002;146(6):983–91. doi: 10.1046/j.1365-2133.2002.04751.x. [DOI] [PubMed] [Google Scholar]
- 31.Liguori V, Guillemin C, Pesce GF, Mirimanoff RO, Bernier J. Double-blind, randomized clinical study comparing hyaluronic acid cream to placebo in patients treated with radiotherapy. Radiother Oncol. 1997;42(2):155–61. doi: 10.1016/s0167-8140(96)01882-8. [DOI] [PubMed] [Google Scholar]
- 32.Pinnix C, Perkins GH, Strom EA, Tereffe W, Woodward W, Oh JL, et al. Topical hyaluronic acid vs.standard of care for the prevention of radiation dermatitis after adjuvant radiotherapy for breast cancer: single-blind randomized phase III clinical trial. Int J Radiat Oncol Biol Phys. 2012;83(4):1089–94. doi: 10.1016/j.ijrobp.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravo V, Calvanese MG, Di Franco R, Crisci V, Murino P, Manzo R, et al. Prevention of cutaneous damages induced by radiotherapy in breast cancer: an institutional experience. Tumori. 2011;97(6):732–6. doi: 10.1177/030089161109700609. [DOI] [PubMed] [Google Scholar]
- 34.Bairati I, Meyer F, Gelinas M, Fortin A, Nabid A, Brochet F, et al. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J Clin Oncol. 2005;23(24):5805–13. doi: 10.1200/JCO.2005.05.514. [DOI] [PubMed] [Google Scholar]
- 35.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73(5):1491–501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khafif A, Hurst R, Kyker K, Fliss DM, Gil Z, Medina JE. Curcumin: a new radio-sensitizer of squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132(2):317–21. doi: 10.1016/j.otohns.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Khafif A, Lev-Ari S, Vexler A, Barnea I, Starr A, Karaush V, et al. Curcumin: a potential radio-enhancer in head and neck cancer. Laryngoscope. 2009;119(10):2019–26. doi: 10.1002/lary.20582. [DOI] [PubMed] [Google Scholar]
- 38.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269(2):199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14(7):2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 40.Hutzen B, Friedman L, Sobo M, Lin L, Cen L, De Angelis S, et al. Curcumin analogue GO-Y030 inhibits STAT3 activity and cell growth in breast and pancreatic carcinomas. Int J Oncol. 2009;35(4):867–72. doi: 10.3892/ijo_00000401. [DOI] [PubMed] [Google Scholar]
- 41.Kang HJ, Lee SH, Price JE, Kim LS. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB in breast cancer cells and potentiates the growth inhibitory effect of paclitaxel in a breast cancer nude mice model. Breast J. 2009;15(3):223–9. doi: 10.1111/j.1524-4741.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 42.Labbozzetta M, Notarbartolo M, Poma P, Maurici A, Inguglia L, Marchetti P, et al. Curcumin as a possible lead compound against hormone-independent, multidrug-resistant breast cancer. Ann N Y Acad Sci. 2009;1155:278–83. doi: 10.1111/j.1749-6632.2009.03699.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu Q, Loo WT, Sze SC, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine. 2009;16(10):916–22. doi: 10.1016/j.phymed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/beta-catenin signaling. Chem Biol Interact. 2009;181(2):263–71. doi: 10.1016/j.cbi.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Grynkiewicz G, Slifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta biochimica Polonica. 2012;59(2):201–12. [PubMed] [Google Scholar]
- 46.Berthelet E, Truong PT, Musso K, Grant V, Kwan W, Moravan V, et al. Preliminary reliability and validity testing of a new Skin Toxicity Assessment Tool (STAT) in breast cancer patients undergoing radiotherapy. Am J Clin Oncol. 2004;27(6):626–31. doi: 10.1097/01.coc.0000138965.97476.0f. [DOI] [PubMed] [Google Scholar]
- 47.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 48.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer research. 2001;21(4B):2895–900. [PubMed] [Google Scholar]
- 49.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mimeault M, Batra SK. Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Chinese Medicine. 2011;6:31. doi: 10.1186/1749-8546-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar FH, Li Y, Wang Z, Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Current pharmaceutical design. 2010;16(16):1801–12. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res. 2007;13(4):1269–77. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 53.Tham CL, Liew CY, Lam KW, Mohamad AS, Kim MK, Cheah YK, et al. A synthetic curcuminoid derivative inhibits nitric oxide and proinflammatory cytokine synthesis. European J Pharmacol. 2010;628(1–3):247–54. doi: 10.1016/j.ejphar.2009.11.053. [DOI] [PubMed] [Google Scholar]