Abstract
Human epidermal growth factor receptor-2 (HER2) is a tyrosine kinase family protein receptor that is known to undergo heterodimerization with other members of the family of epidermal growth factor receptors (EGFR) for cell signaling. Overexpression of HER2 and deregulation of signaling has implications in breast, ovarian, and lung cancers. We have designed several peptidomimetics to block the HER2-mediated dimerization, resulting in antiproliferative activity for cancer cells. In the present work we have investigated the structure-activity relationships of peptidomimetic analogs of compound 5. Compound 5 was conformationally constrained by N- and C-terminal modification and cyclization as well as by substitution with D-amino acids at the N-and C-termini. Among the compounds studied in this work, a peptidomimetic compound 21 with D-amino acid substitution and its N- and C-termini capped with acetyl and amide functional groups and a reversed sequence compared to that of compound 5 exhibited better antiproliferative activity in HER2-overexpressed breast, ovarian, and lung cancer cell lines. Compound 21 was further evaluated for its protein-protein interaction (PPI) inhibition ability using enzyme fragment complementation (EFC) assay, proximity ligation assay (PLA), and Western blot analysis. Results suggested that compound 21 is able to block HER2:HER3 interaction and inhibit phosphorylation of the kinase domain of HER2. The mode of binding of compound 21 to HER2 protein was modeled using a docking method. Compound 21 seems to bind to domain IV of HER2 near the PPI site of EGFR:HER2 and HER:HER3 and inhibit PPI.
Keywords: breast cancer, docking, enzyme fragment complementation assay, HER2, lung cancer, peptidomimetic, protein-protein interaction
INTRODUCTION
In a biological system, signaling pathways involve the interaction between two or more proteins and play a vital role in physiological functions. Also, it is well known that deregulation of these interactions is a key driving force for malignancies such as cancer. Modulation or inhibition of protein-protein interactions (PPI) can lead to therapeutic effects that have benefits in treating many human diseases.1 However, inhibiting PPI has been a big challenge to researchers for a long time.2–4 The epidermal growth factor receptor (EGFR) family of tyrosine kinase receptors is involved in signal transduction pathways.5,6 Interaction of EGFR leads to their homo- and heterodimerization, resulting in signals for cell growth, differentiation, and motility. It is well established that deregulation of EGFR dimerization or mutation leads to cancer and, hence, EGFR are attractive targets for developing therapeutic agents for many types of cancers.7–10 The EGFR family consists of four members—EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3, and HER4/ErbB4. Each HER receptor is a transmembrane receptor that comprises an extracellular membrane, a transmembrane, a juxtamembrane, and intracellular tyrosine kinase domains. The extracellular domain in turn can be divided into four domains—domains I, II, III and IV.11–13 In spite of some controversial results, there are studies that indicate that domains II and IV are involved in interactions between different dimerization partners and the stabilization of dimers.14,15
Small molecules and antibodies as well as peptides and peptidomimetics are known to inhibit PPI.2,16,17 Using peptides and peptidomimetics as inhibitors of PPI is reasonable since PPI surfaces have peptide epitopes. Our interest is in using peptides and peptidomimetics with conformational constraints to modulate PPI. We have targeted HER2 protein with peptidomimetics to inhibit PPI of EGFR.18–21 HER2 positiveness is associated with disease aggressiveness in about 30% of all breast cancer cases.22,23 Many targeted therapies are now available for HER2-overexpressing breast cancers. Among these is trastuzumab, an antibody that binds to domain IV of HER2 and is therapeutically useful for HER2-positive breast cancer patients.9,23–25 Due to the heterogeneity of the disease and its resistance to the available targeted therapies, there is a need to develop new and novel molecules to inhibit EGFR-mediated signaling for cancer. In an earlier study, we showed that a peptidomimetic (compound 5) is able to bind to domain IV of HER2 protein and inhibit the PPI of EGFR-HER2 as well as HER2–HER3. The antiproliferative activity of compound 5 was in the range of lower micromolar concentration in HER2-overexpressing breast cancer cell lines such as SKBR-3 and BT-474.18,19 However, the peptidomimetic has free N- and C-termini, which makes it viable for enzymatic degradation. Linear peptides/peptidomimetics have poor biological activity due to in vivo enzymatic cleavage. There are a variety of strategies to modify the structure of peptides to achieve enzymatic stability.26,27 We have used backbone cyclization strategy and incorporation of D-amino acids in the peptide sequence to improve the stability and activity of peptidomimetic compound 5.28 Changes in chirality of amino acids in the peptide/peptidomimetic sequence can have an influence on the orientation of side chains of amino acids and the way they are presented to the receptors with respect to the backbone structure.29 Hence, we also reversed the sequence in the designed peptidomimetics compared to that in the parent compound 5. The structure-activity relationships of the peptidomimetics were evaluated using antiproliferative activity in HER2-overexpressing breast cancer cell lines, ovarian cancer cell lines, and lung cancer cell lines. The peptidomimetics with D-amino acids exhibited better activity than those with L-amino acids with conformational constraints. The ability of the compounds to inhibit PPI and signaling was investigated by enzyme fragment complementation (EFC) assay and Western blot. Results indicated that compound 21 exhibited PPI of HER2–HER3 and inhibited phosphorylation of the kinase domain of HER2. To provide a model of interaction of peptidomimetics with HER2 protein, docking studies of compound 21 with domain IV of HER2 were performed. Compound 21 docked near the PPI interface of EGFR as proposed in the crystal structure of the homodimer of EGFR. A possible model for PPI inhibition was proposed based on these studies.
MATERIALS AND METHODS
Materials
Fmoc-protected amino acids were purchased from AAPPTEC (Louisville, KY) and EMD Biosciences (San Diego, CA). Resins were obtained from Chem-Implex (Wood Dale, IL), N-methyl-2-pyrrolidinone (NMP) from Advanced ChemTech (Louisville, KY), and 4-methylmorpholine (NMM) from Sigma-Aldrich (St. Louis, MO); all were used without further purification. Acetic anhydride (Ac2O) was purchased from Fisher Scientific (Pittsburgh, PA). All the cancer cell lines and media were obtained from American Type Culture Collection (ATCC, Manassas, VA). PathHunter assay kit was from DiscoverX technologies (Fremont, CA). For Western blot experiment, Novex® 4–20% tris-glycine gels and cell lysis buffer were ordered from Life Technologies (Grand Island, NY) and antibodies from Abcam, Inc. (Cambridge, MA). The antibodies for the housekeeping protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX).
Synthesis of Peptidomimetics
Peptidomimetics were synthesized by manual microwave synthesis procedures adapted from Gorske et al.30 Compounds 9 and 21–28 were synthesized on a solid support in a CEM MARS 5 multimodal microwave reactor equipped with a magnetic stirrer. In the case of compounds with C-terminal amides, couplings were performed using Rink amide AM resin (0.46 mmol/g, Matrix Innovation, Quebec City, Quebec, Canada). In the case of the C-terminal acids, couplings were performed using Fmoc-Asp(OtBu)-Wang resin (0.57 mmol/g, AAPPTEC), Fmoc-dPhe-Wang resin (0.80 mmol/g, Chem-Impex) and Fmoc-dAsp(OtBu)-Wang resin (0.53 mmol/g, Chem-Impex).
Standard peptide synthesis protocols were modified for use with microwaves. All syntheses were carried out with sufficient resin for 50 micromole scale syntheses. The resin for each synthesis was swollen in NMP for 30 min. Fmoc-protecting groups were removed with 2 mL of 20% piperidine in NMP. Microwave deprotection conditions were a 30 s ramp to 75°C, hold at 75°C for 30 s. The deprotection solution was drained, and fresh deprotection solution was added followed by a 1-min ramp to 75°C, hold at 75°C for 5 min. The resin was then drained and washed with NMP (2 mL, 5 × 30 s). Next, 4 eq. of Fmoc-protected amino acid and 4 eq of DIC/HOBT were dissolved in 2 mL of NMP and allowed to preactivate for 2 min. The activated amino acid was added to the resin and coupling was then carried out in the microwave at 75°C (1 min ramp to 75°C, hold for 5 min). Acetylation reactions were carried out at room temperature for 15 min in NMP: NMM: Ac2O (3:1:1). After removal of final Fmoc-protecting groups or N-terminal acetylations, the resins were washed with NMP (2 mL, 5 × 30 s) followed by DCM (2 mL, 5 × 30 s). The peptides were then cleaved from the resin using 2 mL of TFA:TIPS:water (95:2.5:2.5) for 2 h at room temperature. The peptides were isolated by precipitation from the cleavage cocktail with diethyl ether at 4°C then dissolved in 0.1% TFA in water and lyophilized. Analytical HPLC was done on an AAPPTEC Spirit C18 column (5 µm, 4.6 × 250 mm) with a Spirit Peptide guard column (3.2 × 10 mm) at a flow rate of 1 mL/min with gradient elution by 5% to 55% acetonitrile in 0.1% TFA over 50 min. All chromatograms except for the chromatogram of peptide 26 showed the crude peptides to be >95% pure. Peptide 26 was purified by preparatory HPLC on an AAPPTEC Spirit C18 column (5 µm, 2.12 × 25cm) with a Spirit Peptide guard column (2.12 × 1cm) at a flow rate of 20 mL/min with gradient elution by 5% to 55% acetonitrile in 0.1% TFA over 50 min. All peptides were characterized by MALDI-TOF mass spectrometry (Bruker Corporation, Billerica, MA) or LC/MS Finnigan MAT-LC/Q. Sequences of the peptidomimetics, their purity, and mass spectrometry data are provided in Table 1.
Table 1.
Analytical data for the compounds studied
| Code | Peptide Sequence | Purity % | Calcd mass | Expt mass |
|---|---|---|---|---|
| 5 | H2N-R(Anapa)F-OH | >95 | 518.60 | 519.22 (M+1) |
| 9 | H2N-R(Anapa)FD-OH | >95 | 633.69 | 635 (M+H) |
| 16 | H2N-W(Anapa)F-OH | >95 | 548.63 | 549 (M+H) |
| 17 | H2N-R(Anapa)W-OH | >95 | 557.64 | 558 (M+H) |
| 19 | p1P2G3W4L5T6 | >95 | 651.75 | 652.3 (M+H)+ |
| 20 | G1P2R3(Anapa)4F5D6E7F8W9R10 | 95% | 1388.52 | 1389.25 (M+H)+ |
| 21 | Ac-f(Anapa)r-NH2 | >95 | 559.65 | 560.24 |
| 22 | Ac-df(Anapa)r-NH2 | >95 | 674.74 | 675.32 |
| 23 | Ac-r(Anapa)fd-NH2 | >90 | 674.74 | 675.35 |
| 24 | Ac-r(Anapa)f-NH2 | >90 | 559.65 | 560.13 |
| 25 | H2N-r(Anapa)f-OH | 94 | 518.60 | 518.56 |
| 26 | H2N-r(Anapa)fd-OH | >95 | 633.69 | 633.83 |
| 27 | H2N-r(Anapa)f-NH2 | >95 | 517.62 | 518.28 |
| 28 | H2N-r(Anapa)fd-NH2 | >95 | 632.71 | 633.37 |
| Control CP | H2N-K[3-amino 3-(biphenyl)-propionic acid]-Phe-OH | >95 | 516.63 | 517.28 (M+H)+ |
| FITC-5 | FITC-aminocaproic acid -R(Anapa)F-OH | >90 | 1021.14 | 1020.88 |
Anapa, [3-amino-3(napthyl)]-propionic acid. Capital letters represent L-amino acid in the sequence and small letters refer to D-amino acids in the sequence.
For cyclic peptides 19 and 20, cyclization is indicated by underline suggesting the link between first and last amino acid in the sequence.
Cell Lines
Human breast cancer cell line SKBR-3 and ovarian cancer cell line SKOV-3 were cultured using McCoy’s medium. The basal medium for maintaining human breast cancer cell lines BT-474 and MCF-7 is RPMI-1640 medium. Calu-3, a human lung cancer cell line was cultured in Eagle’s minimum essential medium (EMEM). The complete medium for all the cell lines was prepared from respective basal media supplemented with 10% fetal bovine serum (FBS), 1% penicillinstreptomycin, and 1% insulin. Cells were maintained in incubator at 37°C and 5% CO2.
Antiproliferative Assay
To determine the antiproliferative activity of the compounds, a Cell Titer-Glo® Luminescent cell viability assay (Promega Corporation, Fitchburg, WI)31 was performed. In this cell-based assay, the number of viable cells was analyzed by quantitation of ATP in the cells. In a 96-well cell culture plate, approximately 104 cells/well were coated using complete medium and were incubated overnight at 37°C and 5% CO2. The dilutions of compounds were prepared in serum-free medium, and triplicates of each concentration were used. The cell viability experiments were conducted using a wide range of concentrations (100 nM to 200 µM) in the first step; compounds that exhibited antiproliferative activity less than 50 µM were then screened in the second stage in the concentration range of 0.001 to 100 µM. Cells treated with 1% sodium dodecyl sulfate (SDS) and 1% dimethyl sulfoxide (DMSO) were used as negative and positive controls, respectively. After incubation for 72 h, 100 µL/well of CellTitre-Glo® reagent was added and luminescence was measured using a luminometer. A log dose-response curve was plotted using Prism® (GraphPad software, La Jolla, CA) from which IC50 was determined. Experiments were repeated at least three times and standard deviation was reported.
Competitive Binding Assay
The competitive binding of compound 21 to the HER2 binding site was demonstrated using FITC-labeled compound 5, which showed a desired binding to the domain IV of HER2 in previous studies.21 In this florescence based assay, BT-474 was coated in a 96-well tissue culture plate using complete medium at 104 cells/well and incubated overnight at 37°C and 5% CO2. Then the medium was removed and cells were treated with a constant concentration of FITC-labeled compound 5 (50 µM) and various concentrations of compound 21 ranging from 0.1 to 50 µM. Treatment of cells with phosphate-buffered saline (PBS) and control compound (CP) (Supporting Information) was employed as a negative control. All the dilutions were made in PBS. The plate was covered with aluminum foil and incubated for 45 min at 37°C and 5% CO2. After that, the cells were washed twice with 100 µL PBS/well, and fluorescence was determined with a microplate reader using 485 nm and 528 nm as excitation and emission wavelengths, respectively. A relative fluorescence was obtained from each well by subtracting the fluorescence of negative control wells, and a graph with concentration versus relative fluorescence was plotted.
PathHunter® Functional Assay
PathHunter® is a patented technique that uses U2OS cells expressing only HER2 and HER3 receptors on the membrane. EFC technology is used in this assay.32 The manufacturer’s protocol was followed for performing the assay. In brief, the U2OS cells were plated in the 96-well tissue culture plate provided with the kit and incubated at 37°C and 5% CO2 for 24 h. After this period, serial dilutions of the compound 21 and lapatinib were made in PBS and added to the wells. The plate was incubated at 37°C and 5% CO2 for 60 min. Then, the concentration corresponding to the EC80 value of agonist/heregulin (neuregulin-1) was added to all the wells except the control and was allowed to remain at room temperature for 3 h. Detection reagent was prepared with the detection solutions in the kit as per the protocol and added to the wells. After incubation for 60 min, luminescence was read using a Bioteck plate reader. A dose-response curve was plotted using GraphPad software and compared to that of lapatinib,33 which was used as a positive control in this experiment. A control compound (CP) was used as negative control.
Western Blot
BT-474 cells were treated with compound 21 (1 µM) and lapatinib (positive control at 0.07 µM). Cells without any treatment and cells treated with control compound were employed as negative controls. After 36 h, cells were trypsinized and cell lysate was prepared using cell lysis buffer containing protease inhibitor and phosphatase inhibitor. The protein concentration in each sample was analyzed using Bradford’s assay. 40 µg of protein from each sample was loaded on Novex® 4–20% tris-glycine gels. The gels were run at 30 mA, 125 V, and then transferred at 30 V for 12–16 h at 4°C onto a nitrocellulose membrane (GE Healthcare, Buckinghamshire, UK); 2% bovine serum albumin (BSA) in TBST was used for blocking the membrane. Antibodies for the detection of total HER2 protein (t-HER2) and phosphorylated HER2 protein (p-HER2) were used at 1:3000 dilutions. After addition of the substrate and enhancer solutions from a Super Signal-enhanced chemiluminescence kit (Pierce, Rockford, IL) to the membrane, the images were captured using C-Digit Blot Scanner (LI-COR Biotechnology, Lincoln, NE). A representative Western blot image was used for the final presentation.
Proximity Ligation Assay (PLA)
A proximity ligation assay was performed using the Duolink® II assay kit (Olink Bioscience, Uppsala, Sweden).34 SKBR-3 cells were incubated with 5 µM peptide for 48 h in 8-well Labtek® chamber slides (Fisher Scientific, Pittsburgh, PA). Cold methanol was used to fix the cells and 5% BSA was used as blocking solution. Primary antibodies for HER2 (Enzo Lifesciences, Farmingdale, NY) and HER3 (nanotools, Baden-Württemberg, Germany) diluted at 1:400 in blocking solution were added to the wells and incubated overnight. The cells were incubated for 1 h with PLA probes provided with the kit at 1:5 dilution in 2% BSA. Then ligation and amplification stocks were diluted as per manufacturer’s protocol and added to the wells. After incubation, the wells were thoroughly washed to remove unbound reagents. The slide was counterstained for nucleus using 4',6-diamidino-2-phenylindole (DAPI) in the mounting medium, covered with a coverslip, and observed under the microscope. Red fluorescent dots indicate the HER2–HER3 dimers. Fluorescent images were obtained using a Nikon Eclipse Ti-S system.
Docking
The crystal structure of HER2 protein extracellular domain was obtained from the Protein Data Bank (PDB ID 3N85).35,36 The structures of compounds were generated using Insight II software (Accelrys, Inc., San Diego, CA). Each compound structure was minimized with 100 steps of steepest descent method and was subjected to a simulated annealing procedure by carrying out the dynamics for 5 ps from 300 to 800 K and then decreasing the temperature back to 300 K in steps of 100 K. The final structure was minimized using a conjugate gradient method until the rms derivative was 0.03 kcal/mol. The structure of compound 21 was converted into mol2 files. For docking, AUTODOCK software (Molecular Graphics Laboratory, La Jolla, CA) was used.37 A grid with a box size of 126 × 126 × 126 points in each direction was created with domain IV of HER2 at the center. Mol2 files of compound 21 structure were prepared with AUTODOCK tools with all the side chain rotatable bonds as flexible. Docking calculations were performed with 150 starting structures using a genetic algorithm approach. 10 million energy evaluations were performed. Calculations were performed on a Linux cluster using HPC at LSU Baton Rouge via Louisiana Optical Network Initiative (LONI). Analysis was performed using AUTODOCK tools, and 50 low docking energy structures were analyzed. Among these, structures with docking energy of <2 kcal/mol from the lowest energy docked structure were used as representative structure. Final structures were converted into PDB files and visualized using PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC (Cambridge, MA).
RESULTS
Peptide Synthesis and Purification
Peptides were synthesized using an automatic peptide synthesizer as well as microwave-assisted synthesis.30 Microwave-assisted synthesis was adopted since it provided better yield and shorter reaction times. In some cases, the microwave-assisted synthesis provided >95 % pure peptides before HPLC purification. Analytical HPLC and MALDI-TOF and electrospray mass spectra indicated whether the peptides had the correct molecular ion as well as the purity (Table 1).
Structure-activity Relationships
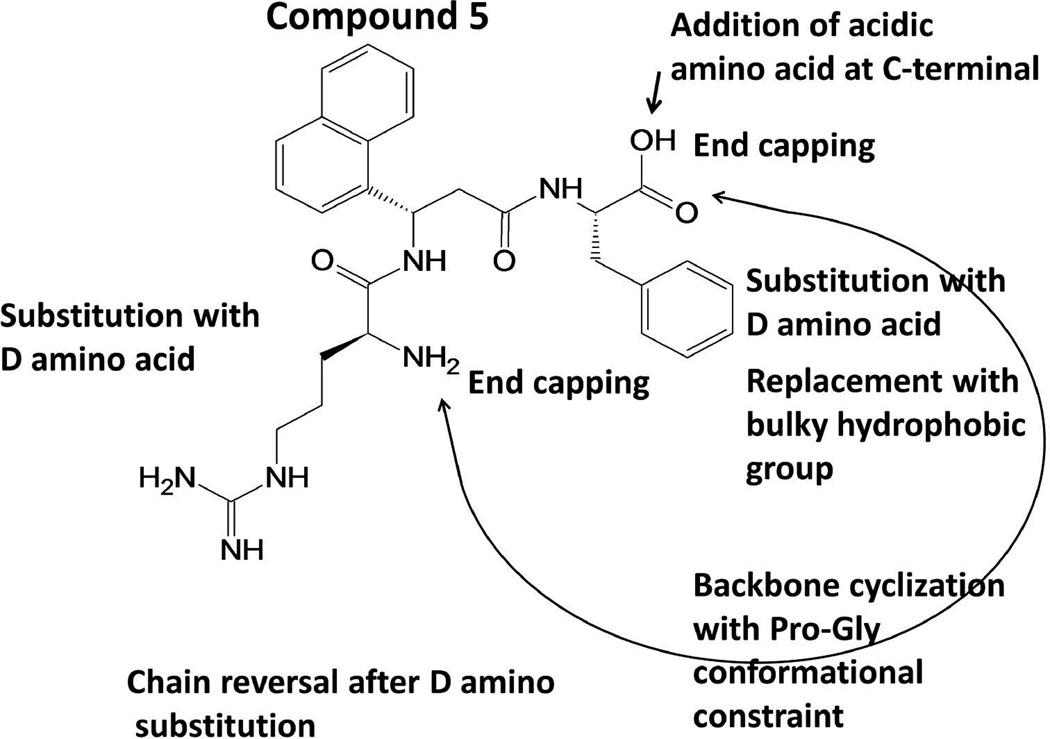
Peptidomimetics were designed to target HER2-overexpressed breast cancer cell lines. Analogs of compound 5 were designed based on the previous results from our laboratory.18,21 The three main categories in the analogs were a) modifications of N- and C-terminals of amino acids, b) peptidomimetics based on the protein-protein interaction site of EGFR domain IV, and c) D-amino acid substitution (Figure 1). Structure-activity relationships of compounds 5 and 9 were described in previous reports.21 Compound 16, an analog of 5 with N-terminal Arg replaced with Trp, did not show antiproliferative activity (Table 2). Similarly, when Phe in the C-terminal was replaced with a bulky Trp residue, the activity was lost. Compounds 19 and 20 were cyclic peptidomimetics with amino acid residues from the PPI interaction site of the protein; they were cyclized by Pro and Gly β-turn strategy.38 The antiproliferative activity was >100 µM, suggesting the inability of these peptidomimetics to target HER2-overexpressed cancer cell lines. Compounds 21 to 28 were analogs of 5 and 9 with D-amino acid substitution in the peptidomimetic sequence. Compound 21, an analog of 5 having D-amino acid substitution for Phe and Arg is the retro-sequence of 5 with N- and C-terminal capping. It showed improved activity compared to compounds 5 and 9. Furthermore, compound 21 exhibited higher selectivity for HER2-overexpressing cancer cell lines BT-474 (IC50 0.595 ± 0.270 µM) and SKBR-3 (0.373 ±0.150 µM) compared to MCF-7 cell lines that do not overexpress HER2 (IC50 >50 µM) (Table 2). Compounds 22 and 23, which are analogs of 9, lost their antiproliferative activity. Compounds 24 and 25, analogs of 5 with D-amino acids without chain reversal, retained the activity in HER2-overexpressing cancer cell lines. However, selectivity for HER2-overexpressing cancer cell lines was lost because they exhibited antiproliferative activity in MCF-7 cell lines. Compounds 26 to 28 with D-amino acids and with C-terminal modification or without capping did not show any improved activity or selectivity (Table 2). Overall, structure-activity studies suggested that compound 21, an analog of compound 5, exhibited antiproliferative activity comparable to that of compound 5, and better selectivity for HER2-overexpressing cancer cell lines. When D-amino acid was substituted for N- and C-terminal residues (Arg and Phe), the direction of the peptide chain had to be reversed. The N- and C-termini could be capped with acetyl and amide groups without the loss of activity and selectivity. Substitution of the sequence with D-amino acids without the chain reversal resulted in loss of activity as shown by compounds 23 and 26 (Tables 1 and 2).
FIGURE 1.
Structure of compound 5 and conformational constraints imposed on it to generate different analogs of 5.
Table 2.
Antiproliferative activity of compounds in HER2-overexpressing cancer cell lines BT-474, SKVO3, and Calu-3. For comparison, antiproliferative activity of compounds in MCF-7 cells lines that do not overexpress HER2 is also shown. Activity is represented as IC50 values in µM.
| Code | Peptide Sequence | BT-474 | MCF-7 | SKOV3 | Calu-3 |
|---|---|---|---|---|---|
| 5 | H2N-R(Anapa)F-OH | 0.895 ± 0.029 | 16.9 ± 1.0 | 0.658±0.041 | 0.601±0.020 |
| 9 | H2N-R(Anapa)FD-OH | 0.785 ± 0.011 | 45 | 0.417±0.055 | 0.847±0.071 |
| 16 | H2N-W(Anapa)F-OH | >100 | >100 | ||
| 17 | H2N-R(Anapa)W-OH | >100 | >100 | ||
| 19 | p1P2G3W4L5T6 | >100 | >100 | ||
| 20 | G1P2R3(Anapa)4F5D6E7F8W9R10 | >100 | >100 | >50 | >100 |
| 21 | Ac-f(Anapa)r-NH2 | 0.595 ± 0.272 | >50 | 0.373±0.150 | 1.09±0.31 |
| 22 | Ac-df(Anapa)r-NH2 | >100 | >100 | ||
| 23 | Ac-r(Anapa)fd-NH2 | >50 | >50 | >100 | >100 |
| 24 | Ac-r(Anapa)f-NH2 | 3.65 ± 0.82 | 5.11 ± 0.09 | ||
| 25 | H2N-r(Anapa)f-OH | 0.521 ± 0.080 | 4.368 ± 1.6 | ||
| 26 | H2N-r(Anapa)fd-OH | >100 | >100 | ||
| 27 | H2N-r(Anapa)f-NH2 | 1.17 ± 0.24 | 0.266± 0.080 | ||
| 28 | H2N-r(Anapa)fd-NH2 | >100 | >100 | ||
| Control CP | H2N-K[3-amino 3-(biphenyl)-propionic acid]-Phe-OH | >100 | >100 | >100 | >100 |
| lapatinib | 0.025 ± 0.004 |
Anapa = [3-amino-3(napthyl)]-propionic acid. Capital letters represent L-amino acid in the sequence and small letters refer to D-amino acids in the sequence. Lapatinib is a dual kinase inhibitor56
For cyclic peptides 19 and 20, cyclization is indicated by underline suggesting the link between first and last amino acid in the sequence.
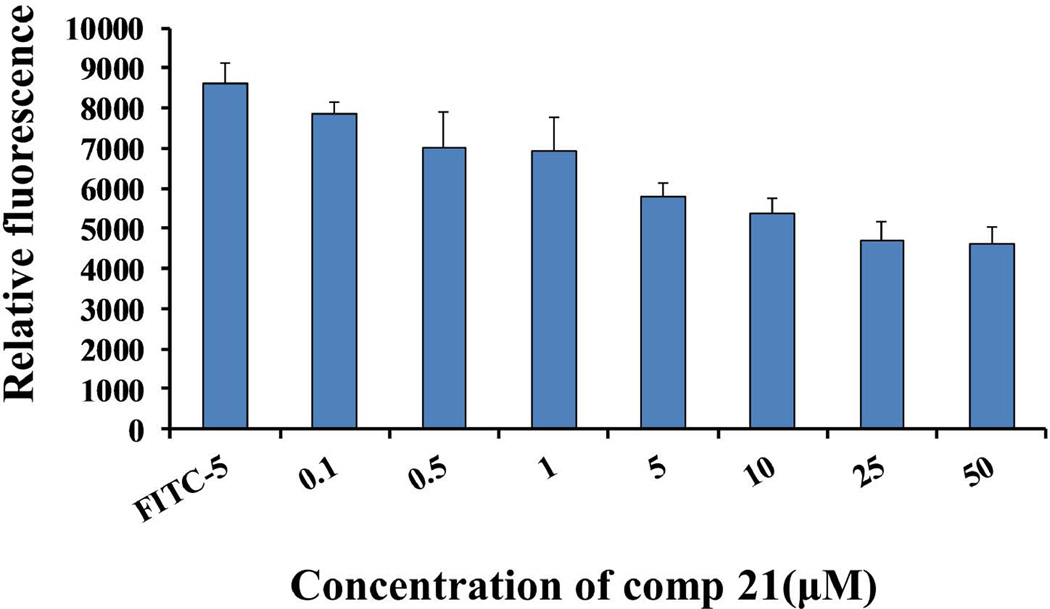
HER2 is also known to be overexpressed in human ovarian and lung cancer cells.39,40 SKOV3 and Calu-3 cells, which overexpress HER2, are derived from ovarian and lung cancers, respectively. The compounds that showed antiproliferative activity in BT-474 cells were chosen to be evaluated for activity in SKOV3 and Calu-3 cell lines (Table 2). All three compounds—5, 9, and 21—exhibit antiproliferative activity in the lower micromolar range in ovarian and lung cancer cell lines, suggesting that these peptidomimetics are specific for HER2-overexpressed cell lines. Since compound 21 is the lead compound from the structure-activity relationship studies, we chose this compound for competitive binding studies to evaluate the binding ability to HER2-overexpressing BT-474 cell lines. Figure 2 shows the competitive binding of compound 21 to HER2-overexpressing cells determined using FITC-labeled compound 5 (which was known from previous studies to bind to domain IV of HER219). A dose-dependent inhibition of FITC-5 binding to BT-474 cells with increasing concentration of compound 21 was observed, suggesting that compound 21 binds to domain IV of HER2. A control compound (CP) did not show any competitive binding with FITC-5 (Supporting Information).
FIGURE 2.
Competitive binding assay for compound 21. FITC-labeled compound 5 was incubated with different concentrations of compound 21 with BT-474 cells. Cells were washed and fluorescence was detected using a plate reader. Ex λ 485 nm, Em λ 535 nm. A decrease in the fluorescence indicates binding of compound 21 to BT-474 cells expressing HER2 protein. As a control binding of FITC-5 is also shown.
PPI Inhibition and Phosphorylation
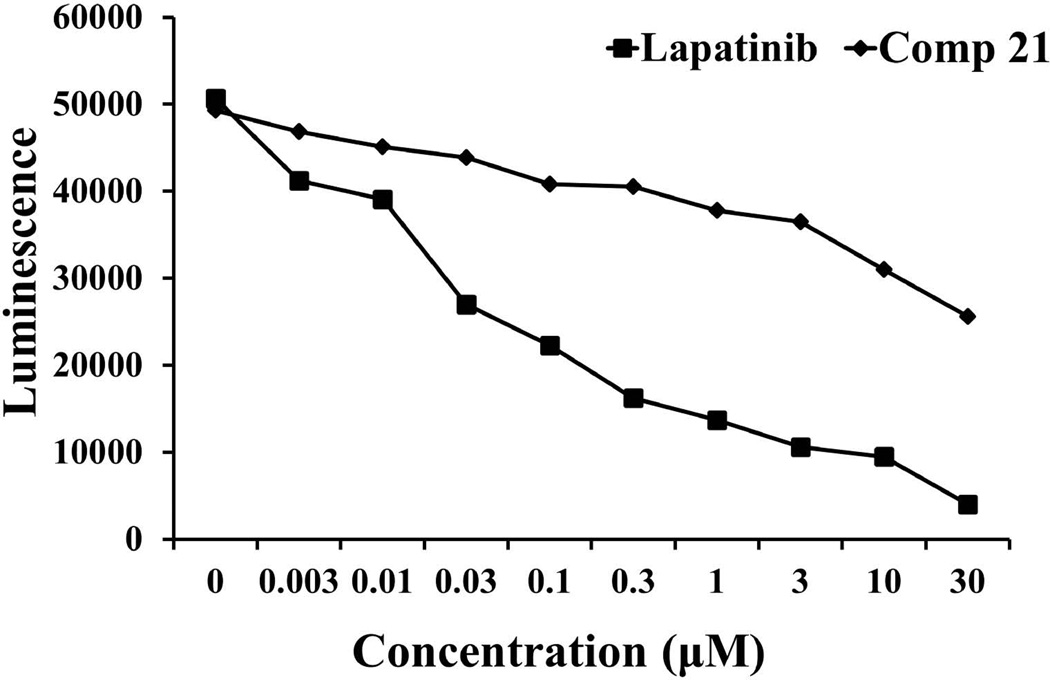
Using PathHunter® assay, Western blot, and PLA, compound 21 was evaluated for the ability to inhibit protein-protein interactions and the phosphorylation of the kinase domain of HER2. In the PathHunter® assay, the PPI interaction is detected using a β-galactosidase enzyme based assay.32 Upon PPI interaction of HER2:HER3, the fully functional galactosidase enzyme formed is measured using a chemiluminescent substrate, a part of the detection kit. Compound 21 was incubated with U2OS cell lines that are transfected and genetically modified to express only HER2 and HER3 proteins. Heregulin, a ligand for HER3, induces the dimerization of HER2 and HER3. The dose-response of compound 21 on the heterodimerization of HER2–HER3 was measured by β-galactosidase activity in the form of luminescence. Figure 3 suggests that compound 21 inhibits the heterodimerization of HER2–HER3 in a concentration-dependent manner compared to the control compound (CP) and lapatinib, a well-known HER2 kinase inhibitor.
FIGURE 3.
PathHunter assay for inhibition of heterodimerization of HER2–HER3 in HER2–HER3-transfected U2OS cells by compound 21 at different concentrations. Dose-response curve for inhibition of heterodimerization by compound 21 (filled diamonds) and lapatinib (filled squares), a well-known kinase inhibitor, in the presence of 0.3 µM heregulin.
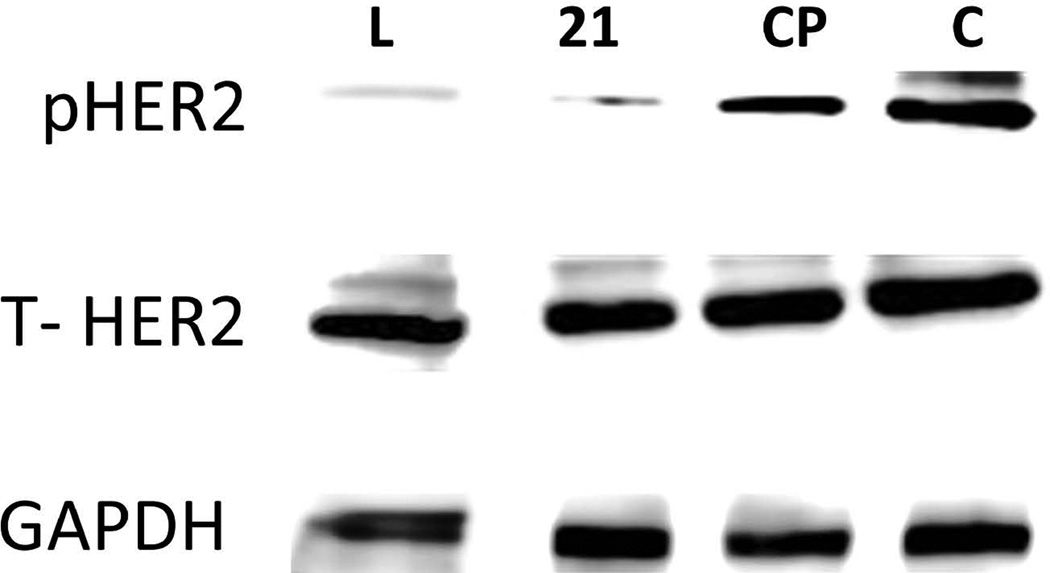
Heterodimerization of extracellular domains leads to transphosphorylation of the kinase domain. Western blot analysis of HER2 protein in BT474 cells treated with compound 21 suggests that it inhibits the phosphorylation of the kinase domain of HER2 (Figure 4). As a control, total HER2 protein and the effect of the kinase inhibitor lapatinib are also shown in the Western blot (Figure 4). Also, to assess the specific inhibition of HER2–HER3 dimerization by compound 21, PLA was performed. It is evident from the images that the red fluorescent dots, which indicate dimerization of HER2 and HER3, have decreased with the treatment of compound 21 as compared to that of control (Figure 5). These results clearly suggest that compound 21 targets HER2-overexpressed cells and inhibits the signaling for cell growth by inhibiting protein-protein interaction between HER2 and HER3.
FIGURE 4.
Western blot analysis of phosphorylated HER2. BT-474 cells that overexpress HER2 protein upon treatment with compound 21 (1 µM) and lapatinib (0.07 µM). The visualization of GAPDH was used to ensure equal sample loading in each lane. Phosphorylation was detected using p-HER2 antibody. CP is for control compound and C is for control without any treatment. Total HER2 protein is also shown.
FIGURE 5.
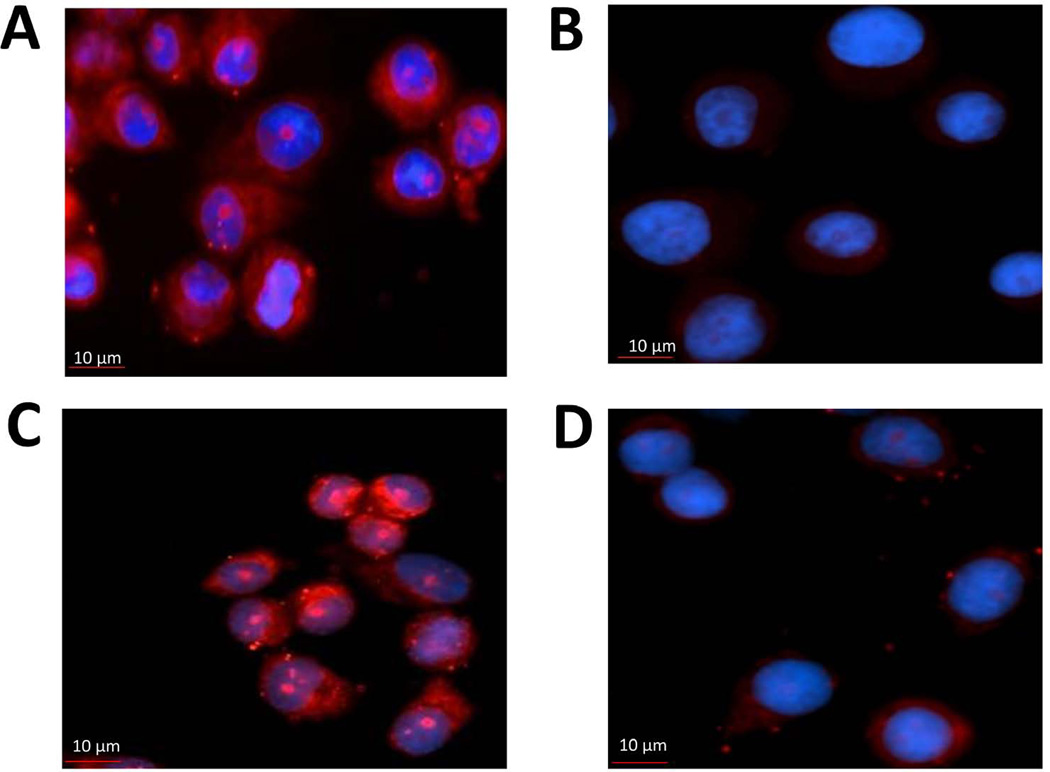
PLA assay to show HER2:HER3 heterodimerization and its inhibition by compound 21 in SKBR-3 cells. A) HER2:HER3 heterodimerization detected by PLA probe shown as red dots. B) SKBR-3 cells without any antibody to HER2 and HER3 and with PLA probes. There are no red dots, indicating the specificity of the assay. C) In the presence of control compound (CP), red dots are observed, suggesting that the control compound does not inhibit heterodimerization. D) in the presence of compound 21, the number of red dots decreased significantly compared to the control, suggesting the inhibition of HER2:HER3 heterodimerization.
Docking
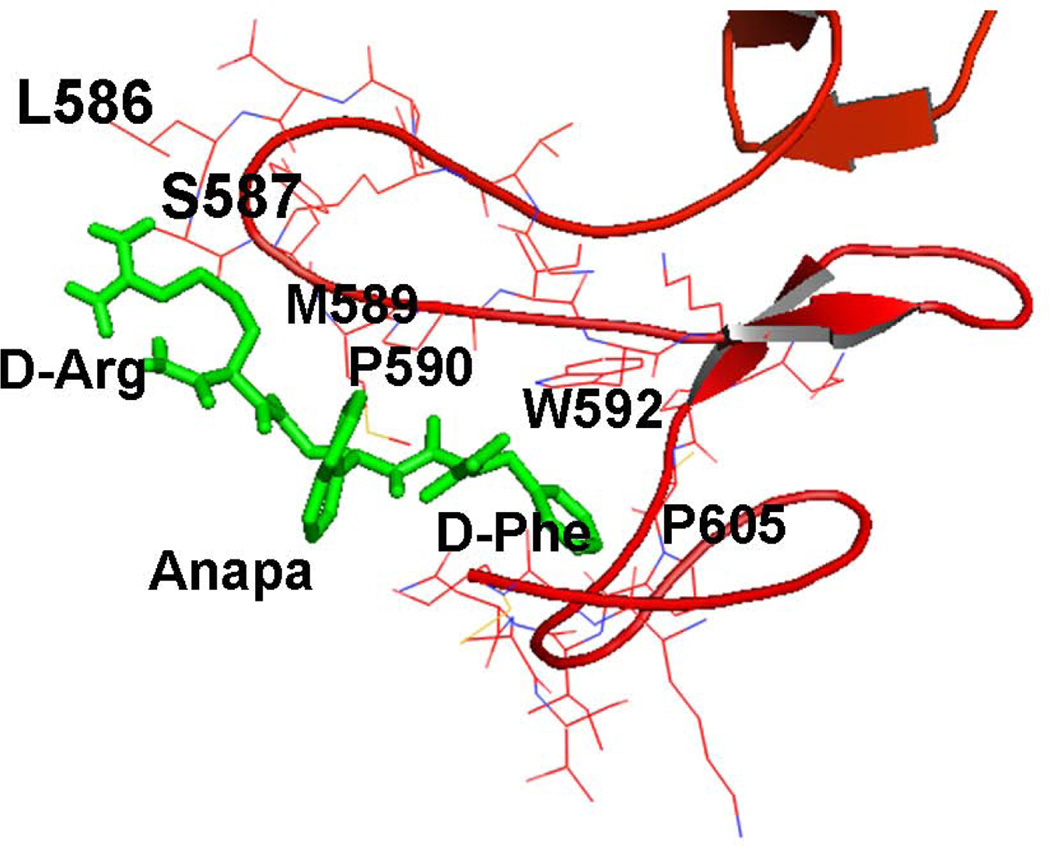
To model the possible binding mode of compound 21 on HER2 domain IV, docking was performed using AUTODOCK.37 Using the crystal structure of HER2 with 620 amino acid residues of the extracellular domain that represents domain IV of HER2,36 compound 21 was docked to the region around domain IV. The grid box was big enough to cover the entire domain IV with nearly 100 amino acids. The low energy docked structures that had energy values within 2 kcal of the lowest energy docked structures were analyzed as possible binding modes. The lowest energy docked structure had docking energy of −6.2 kcal/mol. Ten structures within 2 kcal/mol were chosen as possible docking modes of compound 21. All the structures were docked at the C-terminal part of domain IV of HER2 (Supporting Information). There was a major cluster of docked structures of compound 21 around residues Leu586, Trp592, and Pro605 of the HER2 domain IV. Only two structures were in another cluster near amino acids Arg577, Trp592, and Pro595. The lowest energy structure with docked conformation is shown in Figure 6. This structure produced a hydrophobic interaction with HER2 domain IV. The Phe and β-naphthyl groups of compound 21 participate in hydrophobic interactions with Trp592 and Pro605 of HER2 domain IV. No hydrogen bonding was observed in the lowest energy docked structure. The docked structure was near the protein-protein interaction site of HER2 with other EGFR.15 Docking studies were also performed on all the analogs studied. However, all the structures docked to the C-terminal domain IV of HER2 with docking energy of −6 ± 2.0 kcal/mol. There was no indication of specificity of the peptides in the docking results. Hence docking studies can only serve to propose a model for compound 21 and HER2 protein domain IV interactions based on the results of binding studies and PPI inhibition.
FIGURE 6.
Docking of compound 21 to HER2 domain IV. Lowest energy docked structure showing the hydrophobic interaction with domain IV of HER2. β-napthyl group of Anapa and Phe side chain of compound 21 interact with P590 and W592 of HER2. For the sake of clarity, amino acids of the protein are labeled with a single letter code and those of compound 21 are labeled with a three letter code.
DISCUSSION
The inhibition of protein-protein interactions using small molecules and peptides is challenging. Antibodies and fusion proteins have been used successfully to inhibit PPI.23,41,42 However, development of useful small molecules or peptides is still in progress. In an attempt to study PPI and their inhibition, we chose to investigate EGFR. The homo- and heterodimerization of EGFR is well known in the pathogenesis of cancer.10,11,43 It is known that in HER2-positive breast cancer HER2–HER3 interaction is more dominant than EGFR-HER2 interaction.22 Current therapies for different types of cancer are focused on antibodies or kinase domain inhibitors.23,44,45 Our interest was in designing peptidomimetics that inhibit PPI of extracellular domains of EGFR. Peptides and peptidomimetics are obvious choices for inhibition of PPI since the interface of PPI is a peptide epitope. We designed a peptidomimetic based on the binding site of the trastuzumab binding region of HER2.24 In our earlier studies we have shown that compounds 5 and 9 exhibit antiproliferative activity in HER2-overexpressed cancer cell lines and inhibit PPI of EGFR-HER2 and HER2–HER3 using a variety of techniques.19,20,46 However, compounds 5 and 9 are peptidomimetics with N- and C-termini viable for enzymatic degradation. The in vivo stability of these compounds may be limited due to their possible enzymatic degradation. We used several strategies to modify the peptidomimetics for stability and have evaluated their antiproliferative activity and PPI inhibition ability.
The structures of compound 5 and the designed analogs are shown in Figure 1 and Table 1. It is well known that the PPI hot spot site is dominated by hydrophobic amino acid residues such as Trp, Tyr, and Phe, as well as positively charged amino acids such as Arg.47 The amino acid Trp has a bulky hydrophobic group with hydrogen bond donors in the side chain. To evaluate the effect of Trp in the peptidomimetic compound 5, Trp was introduced in the N- and C-termini, resulting in compounds 16 and 17. The crystal structure of a homodimer of EGFR has been reported.15 It is known that domains IV of EGFR interact with one another in the C-terminal region. In the EGFR homodimer, in domain IV, residues Leu582, Trp583, Tyr602, and Thr614 stabilized EGFR domain IV interaction. Based on this protein-protein interaction region, we postulated that peptides with Trp and Leu could fit into the hydrophobic pocket of the EGFR domain IV PPI site. These hydrophobic amino acids can be placed at proper distance in a molecule using a β-turn conformational constraint. It is well known that β-turn can be stabilized by inserting L-Pro and D-Pro sequence in the peptide structure.48 L-Pro and D-Pro residues were introduced to stabilize the compound with β-turn structure. Gly and Thr residues were introduced in the structure to provide flexibility and solubility of the compound. For cyclization of a peptide, a minimum of five amino acids is required.49,50 However, four amino acid residues containing cyclic peptides can have cis or trans peptide bonds.50 Thus, compound 19 was designed with 6 amino acids with PPI interaction site and conformational constraint residues, to make it suitable as a cyclic hexapeptide. However, compound 19 did not exhibit antiproliferative activity in any of the cancer cell lines studied in this report. Compound 5 with Arg, Anapa, and Phe showed specificity for HER2-overexpressing cancer cell lines. When Asp was introduced into the C-terminal (compound 9), the activity of the compound was retained. We wanted to cyclize compound 9 with the introduction of a Pro-Gly β-turn. Compound 20 was designed based on compounds 5, 9, and 17 with cyclization and conformation constraints. As seen in compound 19, D-Pro-L-Pro turn resulted in loss of antiproliferative activity. Hence, we introduced a Pro-Gly flexible β-turn in the peptide sequence, resulting in compound 20. The antiproliferative activities of compounds 16 to 20 were evaluated in HER2-overexpressing cancer cell line BT-474. None of the compounds in this series showed antiproliferative activity (Table 2).
Our next strategy was to keep the amino acid residues in the compounds the same and change the chirality of the amino acids/analogs in compounds 5 and 9. It is well known that introduction of D-amino acids provides stability to the peptides/peptidomimetics against proteolytic degradation.28,51 Furthermore, peptides/peptidomimetics can be made stable via N- and C-termini capping by acetyl and amide functional groups.52,53 Compounds 21 to 28 were analogs of compounds 5 and 9 with N-terminal or C-terminal capping and D-amino acid substitution. For all of these compounds, the chirality of the central β-amino acid Anapa was in S configuration. The structure-activity relationships of 21–28 suggested that replacement of D-amino acids in the N- and C-termini resulted in a slight decrease in the activity compared to that of compounds 5 and 9. When the sequence of the amino acids was reversed after the chirality was changed, the compounds exhibited better antiproliferative activity. Among the compounds studied, compound 21 showed the best activity against HER2-overexpressing breast cancer cell lines (Table 2) and, hence, it was chosen as the lead compound for further studies. These structure-activity studies suggested a trend in the antiproliferative activity. Compound 5 and its analogs, 21, 24, 25, and 27, all exhibited antiproliferative activity in the lower micromolar range. Changing the chirality of the amino acids or reversing the sequence and N-and C-terminal capping did not affect the activity of the compounds drastically. On the other hand, analogs of compound 9—22, 23, 26, and 28—exhibited a significant decrease in activity after changing the chirality or reversing the sequence and changing the chirality (retro-inverse). Compared to 21, compound 22 has an additional amino acid, Asp. Compound 9 with Asp at the C-terminal has good antiproliferative activity in the lower micromolar range. When the chirality of the amino acid was changed and the sequence was reversed (compound 22), the activity was lost. Compound 23, which is similar to 9, lost its antiproliferative activity when the chirality was changed and the N-and C-termini were capped. The same results could be seen with compounds 26 and 28. We believe that the positions of hydrophobic and charged residues in the molecule along with chirality are important for activity. Introduction of negatively charged Asp at the N-terminal leads to a decrease in activity.
HER2 is also known to be overexpressed in ovarian and lung cancers. Compared to that of breast cancer and ovarian cancer, the mortality rate for lung cancer is higher.54–56 Thus, to broaden the scope beyond breast cancer without shifting our focus, we also tested the effect of compound 21 along with the previously described compounds 5 and 9 against HER2-overexpressing ovarian (SKOV3) and lung (Calu-3) cancer cell lines. All three lead compounds (5, 9, and 21) exhibited antiproliferative activity in the lower micromolar concentration in HER2-overexpressed cancer cell lines. Furthermore, these compounds showed high selectivity for HER2-overexpressing cancer cell lines compared to MCF-7 cell lines (Table 2).
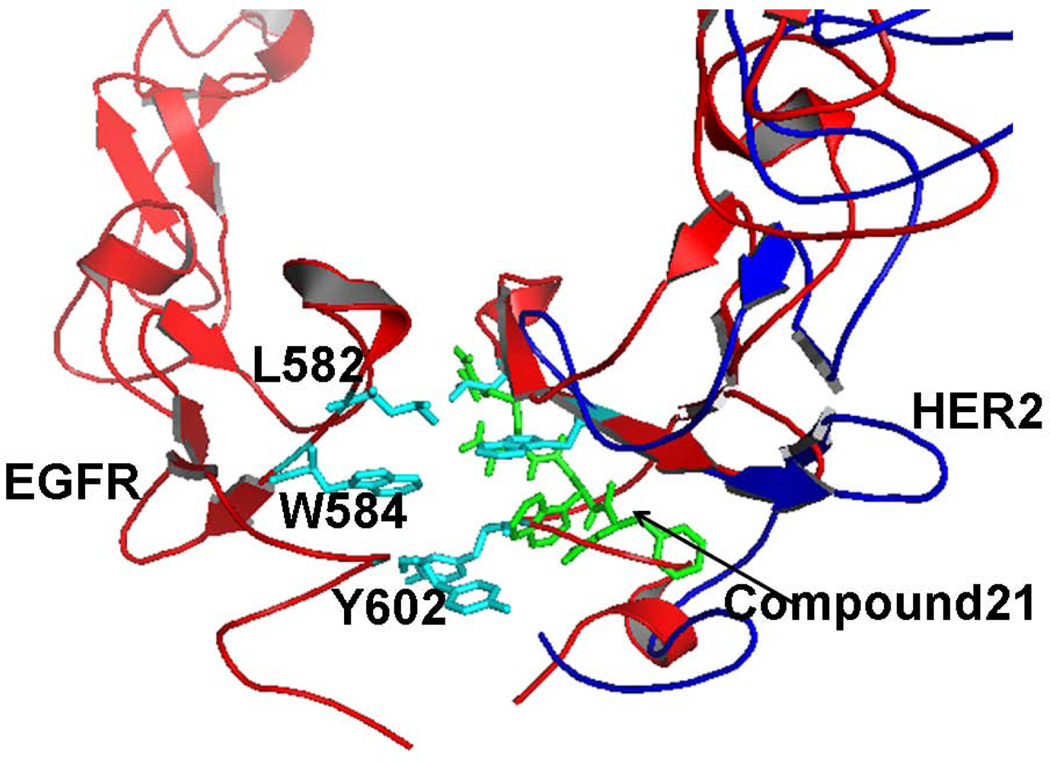
To understand the molecular mechanism of action of compound 21, it was evaluated for PPI inhibition activity using PathHunter® assay, Western blot, and PLA. The results clearly suggest that compound 21 inhibits the protein-protein interaction of HER2–HER3 and the phosphorylation of the kinase domain of HER2 (Figures 3–5). Antiproliferative activity and phosphorylation were comparable to those of a well-known kinase inhibitor lapatinib.57 Thus, inhibition of extracellular domain PPI results in inhibition of intracellular signaling due to the kinase domain. Our idea was to design compounds that bind to HER2 specifically at domain IV and inhibit PPI of the extracellular domain of HER2 interaction with other EGFR. Compound 21 exhibited competitive binding with FITC-labeled compound 5, suggesting that compound 21 and compound 5 bind to the same region on HER2 protein. Our previous surface plasmon resonance studies have suggested that compound 5 binds to HER2 domain IV. Based on this result and competitive binding results presented here (Figure 2), we can conclude that compound 21 binds to domain IV of HER2 protein and inhibits PPI of HER2 with other EGFRs. To understand the binding of compound 21 to HER2 protein, docking calculations were performed. Although the grid was created for the entire domain IV of HER2 covering more than 100 amino acids, compound 21 docked to HER2 domain IV in the C-terminal region near amino acid residues Leu586, Trp592, and Pro605 of HER2. When we compared the binding region of compound 21 on HER2 protein with the EGFR homodimer structure, it was found that compound 21 binds to the PPI interaction site of EGFR homodimer (Figure 7). At present, the crystal structure of HER2 interaction with other EGFR (heterodimer structures) is not available. If we assume that the PPI interaction site of HER2 is similar to that of EGFR, we can propose that compound 21 binds to the pocket of HER2 PPI site in domain IV and thus inhibits protein-protein interactions of HER3-HER2 as well as EGFR-HER2, hence inhibiting the signaling for cell growth. The peptides we designed have Arg, hydrophobic group β-naphthyl and Phe in the sequence. As mentioned earlier, the PPI interface is dominated by residues such as Trp, Arg, Tyr, and Phe. The β-naphthyl group is an analog of Trp. With Arg, β-naphthyl, and Phe, our compound mimics the PPI interface and, hence, inhibits PPI of EGFR. Such peptidomimetics are not only useful as therapeutic agents but will also be helpful in understanding the underlying mechanism of PPI.
FIGURE 7.
Proposed model for inhibition of protein-protein interaction of EGFR by compound 21. Low energy docked structure of compound 21 on HER2 domain IV is shown as sticks (green). Ribbon (blue) is representation of HER2 protein domain IV. HER2 protein was overlapped with EGFR homodimer structure to propose the PPI inhibition. Amino acid residues that are involved in PPI of EGFR homodimer are shown as sticks (cyan). Notice that compound 21 docks near the interface of PPI of EGFR.
CONCLUSIONS
The peptidomimetics designed on the basis of HER2-trastuzumab crystal structure showed good antiproliferative activity. Compound 21 was the most potent and selective analog of compound 5, and also showed competitive binding to HER2 in the presence of compound 5. The antiproliferative activity of the compound was similar to that of compound 5; however, there was an increase in selectivity in 21 to HER2-overexpressed cell lines compared to MCF-7 cells that do not overexpress HER2 protein. Although the mode of action of the compound is not completely clarified as yet, we have concluded from PathHunter® assay, Western blot analysis, and PLA that the peptidomimetic is capable of inhibiting the dimerization and phosphorylation of HER2. The peptidomimetic mimics the PPI interface with amino acids Arg, Trp, and Phe and thus is a suitable tool to understand the PPI. In the future, studies such as PLA, flow cytometry, and TUNEL cytoxicity assay would help in investigating the mechanistic details of the peptide.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 8P20GM103424. The authors would like to thank Nancy Harmony for help with editing the manuscript.
REFERENCES
- 1.Chene P. ChemMedChem. 2006;1:400–411. doi: 10.1002/cmdc.200600004. [DOI] [PubMed] [Google Scholar]
- 2.Wells JA, McClendon CL. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 3.Sperandio O, Reynes CH, Camproux AC, Villoutreix BO. Drug discovery today. 2010;15:220–229. doi: 10.1016/j.drudis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Fuller JC, Burgoyne NJ, Jackson RM. Drug discovery today. 2009;14:155–161. doi: 10.1016/j.drudis.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson KM. Annual review of biophysics. 2008;37:353–373. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. Molecular cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 7.Landgraf R. Breast cancer research : BCR. 2007;9:202. doi: 10.1186/bcr1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahta R, Esteva FJ. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:5078–5084. [PubMed] [Google Scholar]
- 9.Chang JC. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1–3. doi: 10.1158/1078-0432.CCR-06-2405. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Swain SM. Nature reviews Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran H, Wiley HS, Resat H. Biophysical journal. 2006;90:3993–4009. doi: 10.1529/biophysj.105.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 13.Mi LZ, Grey MJ, Nishida N, Walz T, Lu C, Springer TA. Biochemistry. 2008;47:10314–10323. doi: 10.1021/bi801006s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxon ML, Lee DC. The Journal of biological chemistry. 1999;274:28356–28362. doi: 10.1074/jbc.274.40.28356. [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Mi LZ, Grey MJ, Zhu J, Graef E, Yokoyama S, Springer TA. Molecular and cellular biology. 2010;30:5432–5443. doi: 10.1128/MCB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulyok GA, Gibson C, Goodman SL, Holzemann G, Wiesner M, Kessler H. Journal of medicinal chemistry. 2001;44:1938–1950. doi: 10.1021/jm0004953. [DOI] [PubMed] [Google Scholar]
- 17.Benyamini H, Friedler A. Future medicinal chemistry. 2010;2:989–1003. doi: 10.4155/fmc.10.196. [DOI] [PubMed] [Google Scholar]
- 18.Satyanarayanajois S, Villalba S, Jianchao L, Lin GM. Chemical biology & drug design. 2009;74:246–257. doi: 10.1111/j.1747-0285.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banappagari S, Corti M, Pincus S, Satyanarayanajois S. Journal of biomolecular structure & dynamics. 2012;30:594–606. doi: 10.1080/07391102.2012.687525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banappagari S, Ronald S, Satyanarayanajois SD. Journal of biomolecular structure & dynamics. 2010;28:289–308. doi: 10.1080/07391102.2010.10507360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banappagari S, Ronald S, Satyanarayanajois SD. MedChemComm. 2011;2:752–759. doi: 10.1039/C1MD00126D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. Cancer research. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 23.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Nature reviews Clinical oncology. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 24.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Yang PL, Gray NS. Nature reviews Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamman JH, Enslin GM, Kotze AF. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2005;19:165–177. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- 27.McGregor DP. Current opinion in pharmacology. 2008;8:616–619. doi: 10.1016/j.coph.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Tugyi R, Uray K, Ivan D, Fellinger E, Perkins A, Hudecz F. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:413–418. doi: 10.1073/pnas.0407677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitsche C, Behnam MA, Steuer C, Klein CD. Antiviral research. 2012;94:72–79. doi: 10.1016/j.antiviral.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Gorske BC, Jewell SA, Guerard EJ, Blackwell HE. Organic letters. 2005;7:1521–1524. doi: 10.1021/ol0502984. [DOI] [PubMed] [Google Scholar]
- 31.Tolliday N. Current protocols in chemical biology. 2010;2:153–161. doi: 10.1002/9780470559277.ch100045. [DOI] [PubMed] [Google Scholar]
- 32.Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. The Journal of biological chemistry. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ, Finn RS. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1509–1519. doi: 10.1158/1078-0432.CCR-09-1112. [DOI] [PubMed] [Google Scholar]
- 34.Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. Nature biotechnology. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 35.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. Nucleic acids research. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher RD, Ultsch M, Lingel A, Schaefer G, Shao L, Birtalan S, Sidhu SS, Eigenbrot C. Journal of molecular biology. 2010;402:217–229. doi: 10.1016/j.jmb.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. Journal of computational chemistry. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonti R, Gopi HN, Muddegowda U, Ragothama S, Balaram P. Chemistry. 2013;19:5955–5965. doi: 10.1002/chem.201204327. [DOI] [PubMed] [Google Scholar]
- 39.Ou CC, Chen YW, Hsu SC, Sytwu HK, Loh SH, Li JW, Liu JY. Evidence-based complementary and alternative medicine : eCAM. 2012;2012:350239. doi: 10.1155/2012/350239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosell R. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1171–1173. doi: 10.1200/JCO.2004.01.904. [DOI] [PubMed] [Google Scholar]
- 41.Drucker AM, Wu S, Dang CT, Lacouture ME. Breast cancer research and treatment. 2012;135:347–354. doi: 10.1007/s10549-012-2157-7. [DOI] [PubMed] [Google Scholar]
- 42.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Cancer cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 43.Tao RH, Maruyama IN. Journal of cell science. 2008;121:3207–3217. doi: 10.1242/jcs.033399. [DOI] [PubMed] [Google Scholar]
- 44.Posy SL, Hermsmeier MA, Vaccaro W, Ott KH, Todderud G, Lippy JS, Trainor GL, Loughney DA, Johnson SR. Journal of medicinal chemistry. 2011;54:54–66. doi: 10.1021/jm101195a. [DOI] [PubMed] [Google Scholar]
- 45.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. Nature biotechnology. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 46.Banappagari S, McCall A, Fontenot K, Vicente MG, Gujar A, Satyanarayanajois S. European journal of medicinal chemistry. 2013;65C:60–69. doi: 10.1016/j.ejmech.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreira IS, Fernandes PA, Ramos MJ. Proteins. 2007;68:803–812. doi: 10.1002/prot.21396. [DOI] [PubMed] [Google Scholar]
- 48.Saha I, Chatterjee B, Shamala N, Balaram P. Biopolymers. 2008;90:537–543. doi: 10.1002/bip.20982. [DOI] [PubMed] [Google Scholar]
- 49.Buttner F, Norgren AS, Zhang S, Prabpai S, Kongsaeree P, Arvidsson PI. Chemistry. 2005;11:6145–6158. doi: 10.1002/chem.200500249. [DOI] [PubMed] [Google Scholar]
- 50.Oakley MT, Oheix E, Peacock AF, Johnston RL. The journal of physical chemistry B. 2013;117:8122–8134. doi: 10.1021/jp4043039. [DOI] [PubMed] [Google Scholar]
- 51.Hruby VJ, Cai M, Nyberg J, Muthu D. Expert opinion on drug discovery. 2011;6:543–557. doi: 10.1517/17460441.2011.565743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hruby VJ. Nature reviews Drug discovery. 2002;1:847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 53.Rezansoff AJ, Hunter HN, Jing W, Park IY, Kim SC, Vogel HJ. The journal of peptide research : official journal of the American Peptide Society. 2005;65:491–501. doi: 10.1111/j.1399-3011.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 54.Greenlee RT, Murray T, Bolden S, Wingo PA. CA: a cancer journal for clinicians. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 55.Naqvi SH, Bandukda MY, Naqvi SM. Infectious agents and cancer. 2013;8:17. doi: 10.1186/1750-9378-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. European journal of cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 57.Hegde PS, Rusnak D, Bertiaux M, Alligood K, Strum J, Gagnon R, Gilmer TM. Molecular cancer therapeutics. 2007;6:1629–1640. doi: 10.1158/1535-7163.MCT-05-0399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.