Summary
Long-term potentiation (LTP) of synaptic transmission is thought to be a key cellular mechanism underlying memory formation. A widely accepted model posits that LTP requires the cytoplasmic tail of the AMPA receptor subunit GluA1. To find the minimum necessary requirement of the GluA1 C-tail for LTP in CA1 hippocampal pyramidal neurons, we used a single-cell molecular replacement strategy to replace all endogenous AMPA receptors with transfected subunits. In striking contrast to the prevailing model, we found no requirement of the GluA1 C-tail for LTP. In fact, replacement with the GluA2 subunit showed normal LTP, as did an artificially expressed kainate receptor not normally found at these synapses. The only conditions under which LTP was impaired were those with dramatically decreased AMPA receptor surface expression, indicating a requirement for a reserve pool of receptors. These results demonstrate the synapse’s remarkable flexibility to potentiate with a variety of glutamate receptor subtypes, requiring a fundamental change in our thinking with regard to the core molecular events underlying synaptic plasticity.
Introduction
Information storage in the brain is widely accepted to involve the rapid increase in synaptic strength between two neurons that can persist over long periods of time. This phenomenon, known as long-term potentiation (LTP), has been well described at glutamatergic synapses in the hippocampus, a region of the brain that is required for formation of new memories. At these CA1 synapses, LTP is expressed by the immediate increase in post-synaptic AMPA-type glutamate receptors (AMPARs) following coincident activation of pre- and post-synaptic neurons. However, the exact mechanism of rapid AMPAR insertion during LTP is not fully understood.
AMPARs mediate the majority of fast, excitatory synaptic transmission in the brain. A functional AMPAR is a tetramer of individual subunit proteins, of which there are four unique isoforms, GluA1 – 41,2. In CA1 pyramidal neurons, which serve as a model for understanding LTP, most receptors exist as GluA1/A2 heteromers, with a minor contribution from GluA2/A3 receptors3,4. Over the past decade, a large body of research has focused on how individual AMPAR subunits are trafficked. A widely held model posits that GluA1/A2 receptors are excluded from synapses unless an LTP-stimulus is provided, whereas GluA2/A3 receptors traffic to the synapse constitutively. This difference in trafficking behavior is mediated by the cytoplasmic tails (C-tails) of the individual subunit proteins5–7. Supporting this model is the finding that LTP is impaired in GluA1 knock-out mice8, but normal in GluA2/A3 double knock-outs9. Based on these findings, a broad consensus has emerged that LTP is mediated by synaptic insertion of GluA1-containing receptors via its C-tail interactions10–17.
Despite the consensus that GluA1 is required for LTP, no single phosphorylation site or protein-protein interaction in the GluA1 C-tail has been shown to be absolutely necessary. Our goal was to find the minimum requirement of the GluA1 C-tail for LTP, and, if found, use that region to identify crucial protein interactions that mediate synaptic AMPAR potentiation. To accomplish this, we used a single-cell molecular replacement strategy to replace all endogenous AMPARs with transfected subunits18,19. Using this approach, we systematically mutated the GluA1 C-tail and examined the effects on three stages of AMPAR trafficking: surface expression, synaptic transmission, and LTP. Surprisingly, we failed to identify any region in the GluA1 C-tail that was essential either for basal synaptic incorporation or for LTP. In fact, homomeric GluA2 receptors exhibited normal LTP. Most surprisingly, hippocampal synapses in which AMPARs had been replaced with kainate-type glutamate receptors (KARs) also expressed normal LTP. Only manipulations that severely compromised the extrasynaptic surface pool of receptors showed defects in potentiation.
The role of the GluA1 C-tail in surface expression
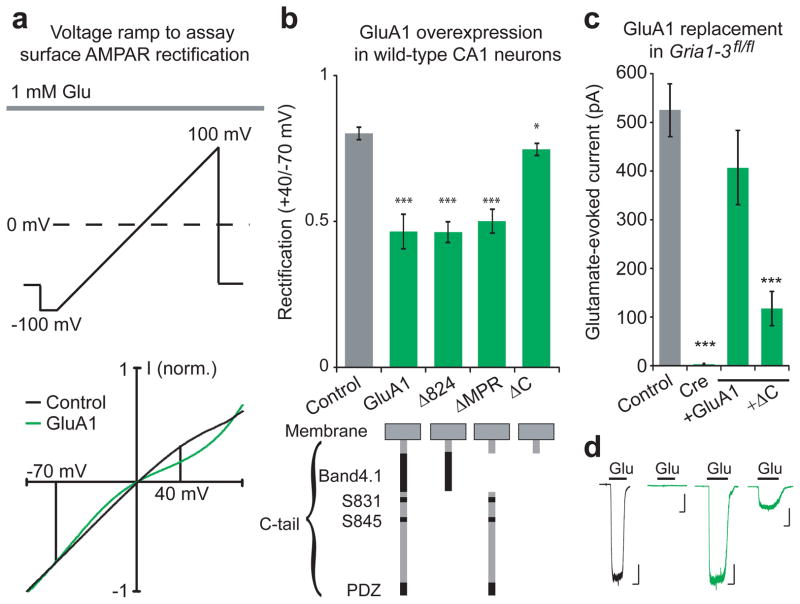
AMPAR trafficking can be broken down into three distinct steps: surface expression, basal synaptic targeting, and activity-dependent synaptic insertion. Since GluA1 is normally abundantly expressed on the neuronal surface3,20, we first screened for surface expression of various GluA1 C-tail truncations in wild-type neurons using somatic outside-out patches in organotypic slice culture. Because overexpressed receptors form inwardly rectifying homomers7, whereas endogenous heteromeric receptors show linear current-voltage (I–V) relationships21, we can detect surface expression of the expressed subunits as an increase in surface rectification (Fig 1a). Overexpression of full-length GluA1 by biolistic transfection into CA1 pyramidal neurons increased the rectification by approximately 40% compared to wild-type controls (Fig. 1b), indicating the presence of surface homomers. In contrast, overexpressing a GluA1 subunit with a full C-tail truncation (ΔC) showed rectification similar to wild-type neurons (Fig. 1b), indicating an impairment in trafficking to the surface. However, a less severe truncation up to amino acid 824 (GluA1Δ824), which removes two serine phosphorylation sites and the PDZ-binding domain, increased rectification to a similar degree as full-length GluA1. Selective excision of the remaining membrane proximal region (ΔMPR), which contains a well-characterized binding site of the protein Band4.1N22,23, also significantly increased rectification (Fig. 1b). Combined, these two modified subunits represent complementary truncations of the entire C-tail, ruling out a necessary role for any single part of the C-tail for steady-state surface expression.
Figure 1. The role of the GluA1 C-tail in surface trafficking.
(a) Experimental protocol and example trace showing voltage ramps applied to outside-out patches of control (black) and GluA1-overexpressing (green) CA1 neurons. Rectification was measured as the normalized glutamate-evoked current at +40 mV over −70 mV. (b) Full-length GluA1, GluA1Δ824 and GluA1ΔMPR significantly increased rectification of surface currents compared to control. Overexpression GluA1ΔC slightly increased rectification (Control, n= 47; GluA1, n= 10, p < 0.001; GluA1Δ824; n = 13, p< 0.001; GluA1ΔMPR, n = 18, p < 0.001; GluA1ΔC, n = 8, p < 0.05). (c) Cre expression eliminates glutamate-evoked currents from in Gria1-3fl/fl CA1 neurons outside-out patches, which is rescued to control by co-expression with full-length GluA1, but not GluA1ΔC (Control, n = 28; Cre, n = 9, p < 0.001; GluA1, n = 11, p > 0.05; GluA1ΔC, n = 15, p < 0.001). (d) Example traces of glutamate-evoked current from Gria1-3fl/fl control neurons, Cre-expressing neurons, GluA1, and GluA1ΔC replacement neurons. Scale bars: 1 sec, 100 pA. Error bars represent mean ± s.e.m.
Because competition with endogenous receptors may have hindered GluA1ΔC trafficking, we wished to study surface expression in the absence of native AMPARs. To accomplish this, we used mice with the genes for GluA1, GluA2, and GluA3 flanked by loxP sites (Gria1-3fl/fl). A previous study has shown that expression of Cre into Gria1-3fl/fl neurons results in a complete absence of AMPARs within 12–15 days3, providing an effective AMPAR-null background onto which mutant GluA1 subunits can be expressed. We confirmed that Cre expression eliminated all glutamate-evoked current from somatic outside-out patches of Gria1-3fl/fl CA1 neurons, which can be rescued to control amplitudes by co-expression with full-length GluA1 (Fig. 1c,d), indicating full rescue of surface expression. Consistent with overexpression, molecular replacement with GluA1ΔC showed significantly decreased glutamate-evoked currents (Fig. 1c,d). This trafficking defect was not due to decreased association with TARPs, auxiliary subunits important for AMPAR trafficking24,25, as both full-length and GluA1ΔC subunits had KA/Glu ratios similar to control (Supplementary Fig 1a). Also, both GluA1 and GluA1ΔC replacement subunits showed strong inward rectification, confirming the absence of endogenous receptors (Supplementary Fig 1b). Since both GluA1ΔMPR and GluA1Δ824 showed normal surface trafficking, the GluA1ΔC subunit may be impaired owing to its severe truncation so close to the transmembrane region, which may inhibit proper protein folding.
Basal Synaptic Transmission does not require the GluA1 C-tail
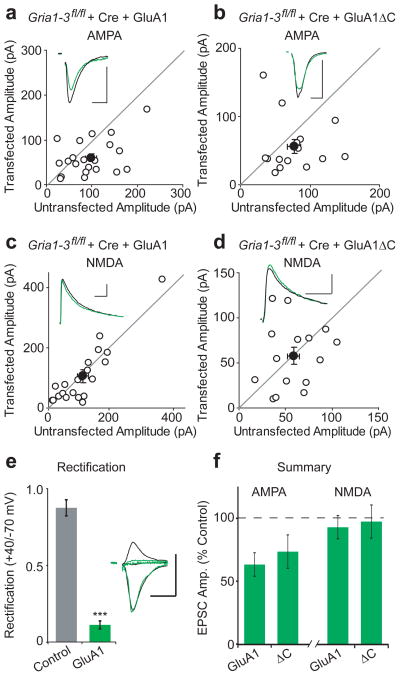
Given the decreased surface expression caused by complete truncation of the GluA1 C-tail, we next examined whether it would also impair basal synaptic targeting. Similar to surface currents, we assessed baseline synaptic transmission by transfecting Gria1-3fl/fl organotypic slice cultures with Cre and a replacement GluA1 subunit. After 17 days, we recorded evoked AMPAR excitatory post-synaptic currents (EPSCs) simultaneously from control and neighboring GluA1-replacement CA1 neurons. Similar to previously described results19, full-length GluA1 rescued AMPAR EPSC amplitudes to ~68% of control cells, while leaving NMDAR EPSCs unchanged (Fig. 2a,c). However, these results contrast with previous studies showing that GluA1 only traffics to synapses after an LTP stimulus5,7. For experimental exploration of this discrepancy, please refer to Supplementary Fig. 2. We also observed no change in paired-pulse ratio, indicating that GluA1 molecular replacement did not affect presynaptic release probability (Supplementary Fig. 3a). Synaptic EPSCs were strongly inwardly-rectifying compared to control, confirming the absence of endogenous receptors (Fig. 2e, Supplementary Fig. 3b). Replacement with GluA1ΔC rescued AMPAR EPSCs to the same degree as full-length GluA1, and also had no effect on the NMDA EPCS (Fig. 2b,d). Replacement with GluA1Δ824 produced similar results (Supplementary Fig. 3c–e). This demonstrates that despite having dramatically decreased somatic expression owing to its severe truncation, GluA1ΔC manages to effectively rescue basal synaptic transmission.
Figure 2. GluA1ΔC has normal synaptic targeting.
Paired whole-cell recordings from control and Cre/GluA1 or Cre/GluA1ΔC-expressing CA1 neurons in Gria1-3fl/fl organotypic slice cultures. (a,c) Full-length GluA1 rescued synaptic AMPAR EPSCs to 68% of control cells (n = 13 p > 0.05), while NMDA EPSCs remained unchanged between control and transfected cells (p > 0.05). (b,d) Replacement with GluA1ΔC results in 73% rescue of AMPA EPSCs without a change in the NMDA EPSC (n = 15, both p > 0.05). (e) Replacement with GluA1 showed inwardly rectifying EPSCs (n = 8, p < 0.01). (f) Summary graph of AMPA and NMDA EPSC rescue between GluA1 and GluA1ΔC. Example traces show average EPSCs for paired control (black) and replacement (green) neurons. Scale bars: 20 msec (AMPA), 100 msec (NMDA), 50 pA. Error bars represent mean ± s.e.m.
No GluA1 C-tail domains are required for LTP
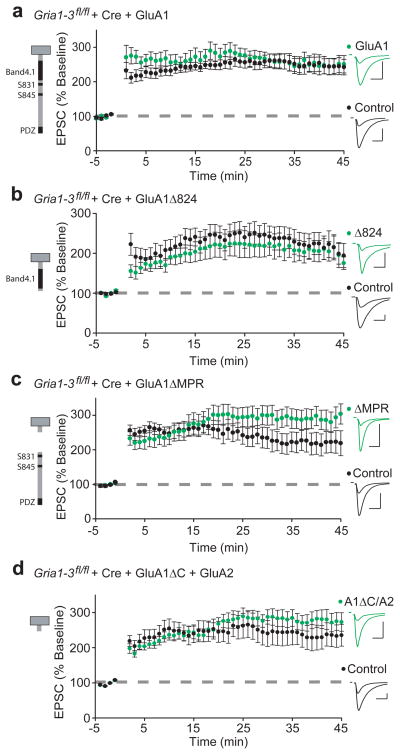
To assess how GluA1 C-tail truncations affect LTP, we transfected Cre and GluA1 into the hippocampus of ~E15.5 Gria1-3fl/fl mouse embryos by electroporation. Like biolistic transfection, this results in sparse expression of transfected cells. Electroporation of Cre alone results in complete absence of an AMPAR EPSC by p10 with no effect on NMDAR EPSCs, and no AMPAR EPSCs appearing following an LTP stimulus (Supplementary Fig. 4a–c). In p17–20 acute hippocampal slices, we induced LTP after recording stable (3–5 minute) baseline AMPAR EPSCs simultaneously from control and GluA1-replacement neurons. We found that replacement with full-length GluA1 exhibited normal LTP (Fig. 3a), confirming that the GluA1 subunit is sufficient. To avoid the confounding effect of decreased surface expression seen by GluA1ΔC, we next assessed the competence of GluA1Δ824 and GluA1ΔMPR subunits, which represent overlapping truncations of the entire C-tail. Both expressed LTP comparable to control (Fig. 3b,c), as did neurons replaced with a truncated GluA1Δ824 subunit with S816A and S818A (GluA1Δ824-AA) mutations to specifically prevent Band4.1N binding26 (Supplementary Fig. 5a,b). We also found that expressing GluA1ΔC with GluA2 to produce more natural GluA1/A2 heteromers was able to rescue the surface trafficking defect of GluA1ΔC (Supplementary Fig. 6a–d), and show synaptic responses similar to controls (Supplementary Fig. 6e–h). Finally, LTP was fully rescued by replacement with GluA1ΔC/GluA2 (Fig. 3d). Combined, these data show that the GluA1 C-tail is not required for LTP.
Figure 3. LTP requires no single portion of the GluA1 C-tail.
Paired whole cell recordings from control CA1 neurons and neighboring Cre/GluA1-expressing neurons in p17–20 Gria1-3fl/fl acute slices. LTP is similar to control in GluA1 (a), GluAΔ824 (b), GluA1ΔMPR (c), and GluA1ΔC/GluA2 (d) replacement neurons (GluA1, n = 11; GluA1Δ824, n = 11;. GluA1ΔMPR, n = 20; GluA1ΔC/GluA2, n = 11; all p > 0.05). Example traces show EPSC before and 45 minutes after LTP induction in paired control (black) and GluA1-replacement neurons (green). Scale bars: 20 ms, 100 pA. Error bars represent mean ± s.e.m.
GluA2 is sufficient for LTP
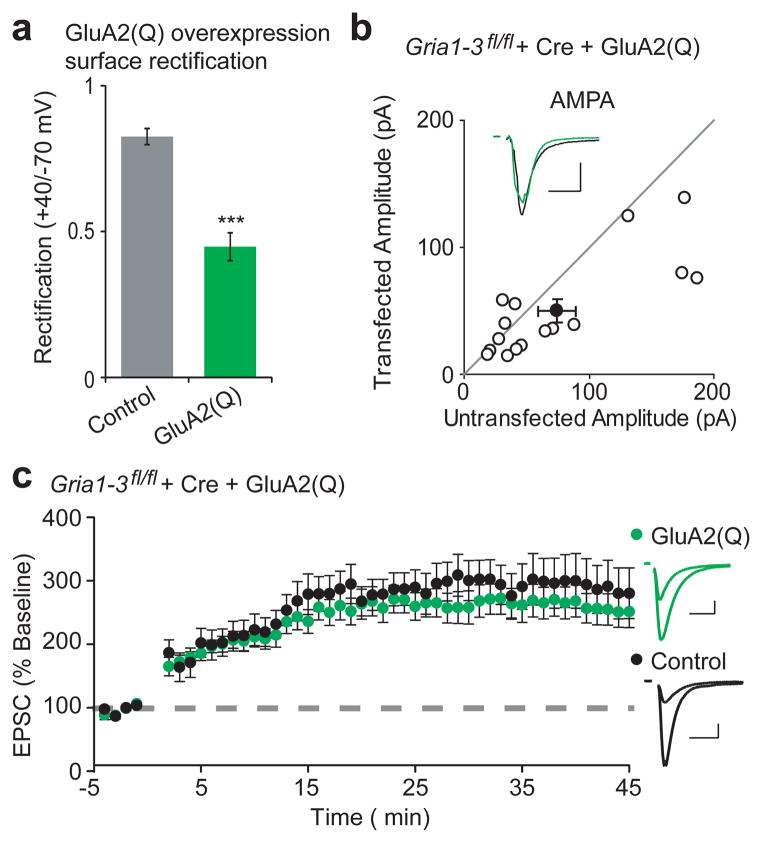
Given that no individual portion of the GluA1 C-tail was necessary for LTP, we hypothesized that expression of an alternative AMPAR subunit might also rescue LTP. GluA2 is another such subunit with limited C-tail homology to GluA115 that is normally highly expressed in CA1 neurons, but is ineffective at forming homomers and trafficking to the cell surface3,20. This is attributable to Q/R RNA editing in the pore of the receptor, which severely limits channel permeability and may make formation of homomers energetically unfavorable27. Expression of un-edited GluA2(Q) resulted in abundant appearance of homomers on the neuronal surface, as observed by increased rectification (Fig. 4a). Like GluA1ΔC, GluA2(Q)ΔC also showed impaired surface expression (Supplementary Fig. 7d). Similarly, both full-length GluA2(Q) (Fig. 4b) and GluA2(Q)ΔC (Supplementary Fig. 7b) trafficked to the synapse, arguing against any necessary role for the GluA2 C-tail in synaptic targeting, in agreement with prior experiments21. NMDA EPSCs and paired-pulse ratio remain unchanged in these replacement neurons, and complete replacement of endogenous receptors was confirmed by synaptic rectification (Supplementary Fig. 7a,b). Moreover, LTP in Gria1-3fl/fl neurons that expressed only GluA2(Q) was indistinguishable from control cells (Fig. 4c), despite lacking any of the intracellular phosphorylation sites and protein-protein binding sites of GluA1. Similarly intact synaptic targeting and LTP was seen in a GluA2(Q) truncation that lacks the majority of its C-tail and known protein-interaction sites (Supplementary Fig. 7c–e).
Figure 4. GluA2(Q) is sufficient to express LTP.
(a) Overexpression of GluA2(Q) caused significantly increased surface rectification compared to control, (Control, n = 8; GluA2(Q), n = 14, p < 0.001). (b) Paired whole-cell recordings between control and Cre/GluA2(Q)-expressing Gria1-3fl/fl CA 1 neurons show rescue of AMPA EPSCs (GluA2(Q), n = 16, p < 0.05). Average AMPA EPSC example traces are shown for paired control (black) and GluA2-replacement neurons (green). (c) Expression of Cre/GluA2(Q) shows LTP similar to control (n = 14, p > 0.05, minute 45). Example traces show average AMPA EPSCs before and 45 minutes after LTP induction. Scale bars: 20 ms and 50 pA. Error bars represent mean ± s.e.m.
LTP requires a reserve pool of AMPARs
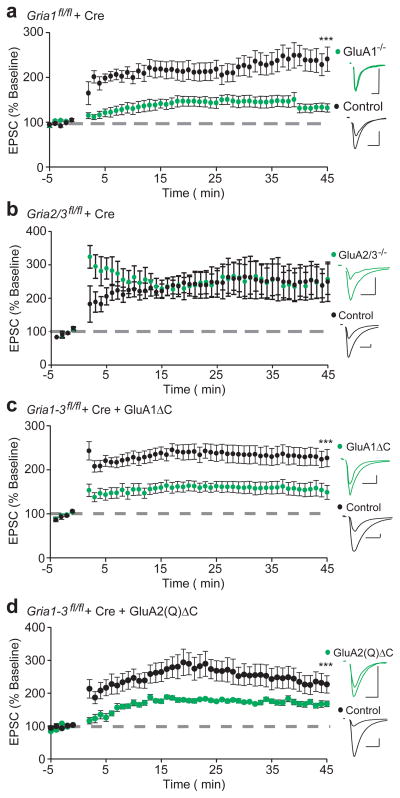
Previous studies have shown that LTP is impaired in mice with constitutive deletion of GluA18, but not GluA2 or GluA39, demonstrating that GluA1 is both necessary and sufficient for LTP. These findings appear to be at odds with our data showing that GluA2(Q) homomers readily express LTP. We therefore reexamined the requirement for GluA1 in single-cell conditional knockouts and found that conditional deletion of GluA1 alone did indeed impair LTP (Fig. 5a). Furthermore, deletion of GluA2 or 3 separately (Supplementary Fig. 8a,b) or in combination (Fig. 5b) had no effect. How can this data be reconciled with our previous experiments? One profound difference between deleting GluA1 and deleting GluA2/A3 is that in the former condition there is an absence of extrasynaptic receptors3,8,20, whereas in the latter condition this pool remains entirely intact3. Also, unlike endogenous GluA2, our replacement GluA2(Q) showed abundant surface expression. We reasoned then that perhaps it is the depletion of this pool that accounts for the loss of LTP in the GluA1 KO. To test this possibility, we returned to the extreme C-tail truncations of both GluA1 and GluA2(Q), in which surface expression is impaired, but synaptic targeting is maintained (Fig. 1b,c; Supplementary Fig. 7d). Indeed, LTP was substantially impaired in both GluA1ΔC and GluA2(Q)ΔC replacement neurons (Fig. 5c,d). These findings suggest that the minimum requirement for LTP is a reserve pool of extrasynaptic AMPARs, regardless of the subunit type.
Figure 5. Lack of surface expression corresponds with loss of LTP in GluA1 conditional knock-outs, and GluA1ΔC and GluA2(Q)ΔC replacement neurons.
(a) Conditional GluA1 knock-out cells (Gria1fl/fl + Cre) demonstrate impaired LTP compared to control (n = 13, p < 0.001, 45 min). (b) GluA2/3 knock-out cells (Gria2/3fl/fl + Cre) demonstrate comparable LTP to control (n = 6, p > 0.05, 45 min). (c,d) Molecular replacement with either GluA1ΔC or GluA2(Q)ΔC results in reduced expression of LTP (GluA1ΔC, n = 16, p < 0.05; GluA2(Q)ΔC, n = 10, p < 0.05, both at 45 min). Example traces show averaged AMPA EPSCs before and 45 minutes following induction of LTP in paired experimental neurons (green) and control cells (black). Scale bars: 20 msec, 50 pA. Error bars represent mean ± s.e.m.
GluK1 is sufficient for mediating LTP
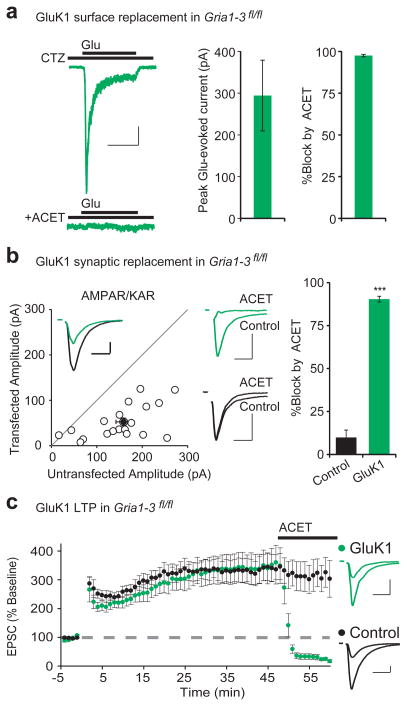
Having failed to identify any specific domains in the C-tails that are important for LTP, we wondered whether other domains in the AMPAR are required. In search of a null condition to conduct domain-swapping experiments, we turned to kainate receptors (KARs), a separate class of fast, ionotropic glutamate receptor which differs in fundamental ways from AMPARs. They bind to different auxiliary subunits and have no sequence homology in their C-tails28. We therefore set out to replace all endogenous AMPARs with KARs at CA1 synapses. CA1 pyramidal neurons do not express synaptic KARs, as shown by the absence of synaptic currents in the presence of the AMPAR-selective antagonist GYKI (Supplementary Fig. 9a). However, co-expression of the KAR subunit GluK1 with the auxiliary subunit Neto229,30 in wild type CA1 neurons generated a GYKI-resistant current that was blocked by NBQX, an antagonist that blocks both KARs and AMPARs (Supplementary Fig. 9a), This indicates that overexpressed KARs are capable of being targeted to the synapse and contribute to EPSCs. To examine KAR currents in isolation, we co-expressed Cre with GluK1 and Neto2 in Gria1-3fl/fl CA1 neurons. In this case, we recorded a population of pure KARs on the surface that desensitize to glutamate even in the presence of cyclothiazide and are completely blocked by ACET, a highly specific GluK1 antagonist31 (Fig. 6a). Furthermore, in this AMPAR-null background, these neurons exhibit EPSCs that are entirely blocked by ACET, while the EPSCs in neighboring controls neurons are unaffected (Fig. 6b), further demonstrating that exogenously expressed KARs are capable of targeting to synapses. As with AMPAR replacement, NMDA EPSCs were unaffected (Supplementary Fig. 9b). Finally, we tested whether neurons expressing only KARs could express LTP. To our surprise, we found that the KAR EPSC showed potentiation indistinguishable from that recorded simultaneously from neighboring control neurons (Fig. 6c). To ensure that the EPSC in the KAR expressing neuron was, in fact, mediated entirely by KARs, we applied ACET at the end of the experiments and found that it abolished the EPSC, but had no effect on neighboring control neurons (Fig. 6c). We also wished to confirm that LTP mediated by KARs, which are also Ca2+-permeable, was not induced by a fundamentally different mechanism than wild-type LTP. We therefore tried inducing LTP in the presence of NMDAR-antagonist APV, and saw no significant potentiation (Supplementary Fig. 9d,e). These experiments demonstrate that even neurons completely lacking AMPARs can undergo LTP, so long as they are provided with an alternative fast, ionotropic glutamate receptor.
Figure 6. GluK1 expresses on the neuronal surface, targets to synapses, and mediates LTP.
(a) Co-expression of Cre, GluK1, and Neto2 in Gria1-3fl/fl neurons results in robust glutamate-evoked currents from somatic outside-out patches (n = 10). The current desensitizes in the presence of 100 μM cyclothiazide (CTZ), and is completely blocked by 1 μM ACET. (b) Paired recordings from Cre/GluK1/Neto2-expressing and neighboring control CA1 neurons resulted in a 33% rescue of synaptic EPSCs (n = 20, p < 0.001). Example trace (inset) shows paired control (black) and GluK1-replacement (green) EPSCs. 1 μM ACET completely blocks the GluK1 replacement EPSCs (green example traces, upper middle), with no block of control cell EPSCs (black example traces, lower middle) (n = 14, p < 0.001). (c) Paired whole-cell recording from control and Cre/GluK1/Neto2-expressing Gria1-3fl/fl CA1 neurons show similar levels of LTP (n =12, p > 0.05, minute 45). 1 μM ACET completely blocks the GluK1-replacement EPSC, but not control (n = 11, p < 0.001, minute 60). Example traces show average EPSCs before and 45 minutes following LTP induction in control (black) and GluK1-replacement neurons (green). Scale bars: 1 sec (a), 20 msec (b,c) and 50 pA in (a–c). Error bars represent mean ± s.e.m.
Discussion
Using a single-cell molecular replacement approach that gave us complete control over the complement of expressed AMPA receptors, we found no requirement for the GluA1 C-tail for basal synaptic transmission or for LTP. In fact, we found no requirement for the GluA1 subunit generally, as both GluA2(Q), another AMPAR subunit, and GluK1, an entirely separate class of glutamate receptor, exhibited normal levels of LTP. Previous studies that have implicated the GluA1 C-tail in LTP demonstrated phenotypes with a largely normal initial stage of potentiation, followed by a gradual decrease in EPSC amplitude towards baseline5,6,28,32. The most compelling of these studies demonstrate impaired LTP in mice with posphonull knock-in mutations of two key phosphorylation sites, and complete absence of long-term depression (LTD)32. Another study with phosphomimetic knock-in mutations also demonstrated a decreased threshold for LTP induction33. Given these findings, we cannot rule out the possibility that the C-tails play some modulatory effect on synaptic plasticity. In the present experiments, however, we saw immediately impaired potentiation in GluA1 conditional KO cells and with GluA1ΔC and GluA2ΔC replacement, which more closely mimics the absence of LTP seen with pharmacological blockade of NMDA receptors. With all three of these manipulations, there was a profound decrease in the pool of extrasynaptic receptors, indicating that the main requirement for LTP is an adequate reserve pool of glutamate receptors. Another equally plausible model is that AMPAR-containing recycling endosomes are required for LTP and conditions that deplete the surface receptor pool also deplete this pool34 (see Supplementary Discussion).
Fundamentally, our results suggest that synapses can cluster a broad variety of receptors following LTP, shifting the focus of LTP expression from the receptor subunits to the synapse itself and specifically the post-synaptic density (PSD). Our data suggests a model in which AMPARs freely diffuse on the neuronal surface, and are trapped at the PSD for use in synaptic transmission35. LTP, then, can be understood as an immediate increase in the ability of the PSD to trap receptors that relies on a reserve pool of freely diffusing surface receptors. This model is consistent with evidence from 2-photon glutamate uncaging experiments, which show an immediate increase in the volume of post-synaptic spines following LTP induction36–38, suggesting significant alterations to the synapse and PSD. Despite this shift of focus, research on AMPARs and their auxiliary subunits, such as TARPs, remain important for identifying LTP-related PSD proteins. In the absence of a role for the GluA1 C-tail, the question remains exactly which specific interactions cluster AMPARs at the synapse both basally and during plasticity. Identification of these interactions may be crucial to understanding the synaptic modifications that underlie learning in the brain.
Methods
Mouse Genetics
Animals were housed according to the IACUC guidelines at the University of California, San Francisco. Mice with the Gria1fl/fl, Gria2fl/fl, and Gria3fl/fl (Gria1-3fl/fl) were generated and genotyped as previously described3.
Experimental Constructs
Flip-isoform GluA1, GluA2(Q), and Cre:mCherry were cloned into the pFUGW expression plasmid by PCR and In-Fusion® HD Cloning System (Invitrogen). pFUGW-GluA1 and GluA2(Q) co-expressed with GFP behind an internal ribosomal entry site (IRES). GluA1 and GluA2(Q) truncations were generated by overlapping extension PCR. GluA1ΔC ended in amino acid 812, with the last 4 amino-acids being EFCY. GluA1Δ824 ended in amino acid 824, with the sequence MKGF. GluAΔ824-AA contained the C-tail sequence EFCYKSRAEAKRMKGF. GluA1ΔMPR had the following amino acids excised from the C-tail: KSRSESKRMKGFC, with the rest of the C-tail intact. GluA2(Q)ΔC also truncated to amino acids EFCY, and GluA2(Q)Δ847 ended in amino acids MKGF. GluK1 and Neto2 were cloned into the pCAGGs expression plasmid with GFP and mCherry, respectively, co-expressed behind an IRES.
Neuronal Transfection
Sparse biolistic transfections of organotypic slice cultures were performed as previously described3. Briefly, 80 ug total of mixed plasmid DNA was coated on 1 uM-diameter gold particles in 0.5 mM spermidine, precipitated with 0.1 mM CaCl2, and washed 4x in pure ethanol. The gold particles were coated onto PVC tubing, dried using ultra-pure N2 gas, and stored at 4 degrees in desiccant. DNA-coated bullets were shot with a Helios Gene Gun (BioRad). Cre expression was confirmed by mCherry epifluorescence, and replacement AMPA/KAR subunits confirmed by GFP epifluorescence.
For in-utero electroporations, ~E15.5 pregnantGria1-3fl/fl mice were anesthetized with 2.5% isoflurane in 02 and injected with buprenorphine for analgesic. Embryos within the uterus were temporarily removed from the abdomen and injected with 2 μl of mixed plasmid DNA into the left ventricle via a beveled micropipette. pFUGW-Cre:mCherry was typically diluted to approximately 0.5 μg/μl in 2–3 μg/μl of the replacement pFUGW AMPAR or pCAGGS GluK1 plasmid. Each embryo was electroporated with 5x50 msecond, 35 volt pulses. The positive electrode was placed in the lower right hemisphere and the negative electrode placed in the upper left hemisphere. Following electroporation, the embryos were sutured into the abdomen, and sacrificed on p17–20 for LTP recording. For further detail on electroporation, please see Navaroo-Quirogo et al42.
Electrophysiology
Voltage-clamp recordings were taken from CA1 pyramidal neurons in either acute hippocampal slices or organotypic slice cultures. For acute slices, 300 μM transverse slices were cut using a Microslicer™ DTK-Zero1 (Ted Pella, Inc.) in chilled high sucrose cutting solution containing (in mM): 2.5 KCl, 7 MgSO4, 1.25 NaH2PO4, 25 NaHCO3, 7 glucose, 210 sucrose, 1.3 ascorbic acid, 3 sodium pyruvate. The slices were then incubated for 30 minutes at 34 degrees in artificial cerebral spinal fluid (aCSF) containing (in mM): 119 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose. For acute slices, 2.5 mM CaCl2 and 1.3 mM MgSO4 were added to the aCSF, and for organotypic slice cultures 4 mM CaCl2 and MgSO4 were added. The aCSF was bubbled with 95% O2 and 5% CO2 to maintain pH, and the acute slices allowed to recover at room temperature for 45 minutes to 1 hour. Cultured slices were prepared as previously described39, and recorded between 7–24 DIV depending on the experiment. During recording, slices were transferred to a perfusion stage on an Olympus BX51WI upright microscope and perfused at 2.5 ml/min. with aCSF containing 0.1 mM pictrotoxin for acute slices experiments, and 0.01 mM gabazine, and 2–5 μM 2-Cl-adenosine for organotypic slice cultures. Synaptic responses were evoked by stimulating with a monopolar glass electrode filled with aCSF in stratum radiatum of CA1. To ensure stable recording, membrane holding current, input resistance, and pipette series resistance were monitored throughout recording. Data was gathered through a MultiClamp 700B amplifier (Axon Instruments), filtered at 2 kHz, digitized at 10 kHz.
Whole-cell synaptic recordings and LTP
Simultaneous dual whole-cell recordings were made between GFP and/or mCherry positive experimental cells as identified by epifluorescence, and neighboring non-transfected control cells. Internal recording solution contained (in mM): 135 CsMeSO4, 8 NaCl, 10 HEPES, 0.3 EGTA, 5 QX-314, 4 Mg-ATP, 0.3 Na-GTP, and 0.1 spermine. Osmolarity was adjusted to 290–295 mOsm, and pH buffered at 7.3–7.4. AMPAR- and KAR- mediated responses were isolated by clamping the cell −70 mV, while NMDA responses were recorded at +40 mV, with amplitudes taken 100 msec following stimulation to avoid contamination by AMPA receptor current. Paired-pulse ratios of AMPAR EPSCs were taken by stimulating twice at a 40 ms interval. To examine AMPA receptor rectification, 0.1 mM D-AP5 was washed in to block NMDA receptors. LTP was induced by stimulating at 2 Hz for 90 sec while clamping the cell at 0 mV, after recording a stable 3–5 minute baseline, but not more than 6 minutes after breaking into the cell. To minimize run-up of baseline responses during LTP, slices were stimulated for ~10 minutes prior to breaking in, and both cells held cell-attached for 2–5 minutes before breaking into the whole cell. Prior to breaking in, stimulation intensity was calibrated just below the threshold required to elicit an action potential from the wild-type control neuron. Rectification was calculated as the ratio of the slopes of the lines connecting AMPA EPSC amplitude from 0 to +40 mV and from −70 mV to 0 mV. This calculation can be taken as follows: R.I. = 7(I40 – I0)/4(I0–I70) where Ix represent EPSC amplitude at x mV.
Outside-out patches
Outside-out patches were taken from CA1 cells by obtaining whole-cell access to CA1 pyramidal neurons at −70 mV with a 4–5 MΩ patch pipette, then slowly pulling the pipette away from the soma until a high-resistance seal reformed. HEPES-aCSF containing (in mM): 150 NaCl, 2.5 KCl, 10 HEPES, 10 glucose, 1 MgCl2, 2 CaCl2, 0.1 D-AP5, 0.1 picrotoxin, 0.1 cyclothiazide, and 0.5 uM TTX was then perfused over the tip of the pipette. Glutamate and kainate currents were evoked by perfusion of HEPES-ACSF containing 1 mM L-glutamic acid and 1 mM kainic acid, respectively. A ValveLink 8 (AutoMate Scientific Inc.) was used for fast perfusion of control, glutamate, and kainate containing HEPES-aCSF. During outside-out patch experiments, experimental cells were interleaved with non-transfected control cells. Rectification was calculated as in synaptic experiments.
Statistics
For all experiments involving un-paired data, including all outside-out patch data, a Mann-Whitney U-test with Bonferonni correction for multiple comparisons was used. For all experiments using paired whole-cell data, including all synaptic replacement and synaptic overexpression, a two-tailed Wilcoxon signed-rank test was used. LTP data was gathered as pairs of control and experimental neurons, but occasionally during experiments one of the cells would be lost. Comparisons were therefore made using the Mann-Whitney U-test, and the reported n-values represent the number of cells at the end of each experiment. Data analysis was carried out in Igor Pro (Wavemetrics), Excel (Microsoft), and R (The R Project for Statistical Computing, http://www.r-project.org/).
Supplementary Material
Acknowledgments
We thank A. Jackson, J. Levy, S. Fischbach, K. Lovero, N. Sheng, S. Shipman, and M. Younger for critical discussions and reading of the manuscript; K. Bjorgen for technical help with organotypic slice cultures; L. Subramanian from the Kriegstein lab for technical help with in utero electroporations. We thank Drs. P. Seeburg and R. Sprengel for the Gria1-3fl/fl mice. A.J.G. was supported by the National Science Foundation Graduate Research Fellowship. R.A.N. is supported by the National Institute of Health.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature
Author Contributions M.C. carried out electroporations and maintained Gria1-3fl/fl mice. Y.S. collected GluK1 over-expression data. W.L. was involved in study design and cloned several constructs. A.J.G. designed the study, collected and analyzed data, and wrote the paper. R.A.N. conceived of the study, contributed to the design of experiments and wrote the paper. All authors discussed the results and commented on the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Correspondence and requests for materials should be addressed to R.A.N. (nicoll@cmp.uscf.edu).
References
- 1.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 2.Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993;3:291–8. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- 3.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–68. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–9. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–43. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 6.Boehm J, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–25. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi Y, et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 8.Zamanillo D, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–11. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 9.Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–76. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- 10.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;3:461–9. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–62. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 13.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 15.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 16.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–79. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 18.Granger AJ, Gray JA, Lu W, Nicoll RA. Genetic analysis of neuronal ionotropic glutamate receptor subunits. J Physiol. 589:4095–101. doi: 10.1113/jphysiol.2011.213033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu W, Isozaki K, Roche KW, Nicoll RA. Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc Natl Acad Sci U S A. 107:22266–71. doi: 10.1073/pnas.1016289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrasfalvy BK, Smith MA, Borchardt T, Sprengel R, Magee JC. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J Physiol. 2003;552:35–45. doi: 10.1113/jphysiol.2003.045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panicker S, Brown K, Nicoll RA. Synaptic AMPA receptor subunit trafficking is independent of the C terminus in the GluR2-lacking mouse. Proc Natl Acad Sci U S A. 2008;105:1032–7. doi: 10.1073/pnas.0711313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–40. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinanen K. Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J Neurosci. 2003;23:798–806. doi: 10.1523/JNEUROSCI.23-03-00798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–99. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomita S, et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–8. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 26.Lin DT, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–87. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007;30:407–16. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–63. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–96. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J Neurosci. 2011;31:7334–40. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dargan SL, et al. ACET is a highly potent and specific kainate receptor antagonist: characterisation and effects on hippocampal mossy fibre function. Neuropharmacology. 2009;56:121–30. doi: 10.1016/j.neuropharm.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HK, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–43. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 33.Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. PNAS. 2011;108:8450–5. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opazo P, Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci. 2011;46:1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakoshi H, Yasuda R. Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci. 2012;35:135–43. doi: 10.1016/j.tins.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson M, Yasuda R. Signalling pathways underlying structural plasticity of dendritic spines. Br J Pharmacol. 2011;163:1626–38. doi: 10.1111/j.1476-5381.2011.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 40.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. PNAS. 2002;21:13902–7. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. PNAS. 2008;105:20953–8. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro-Quiroga I, Chittajallu R, Gallo V, Haydar TF. Long-term selective gene expression in developing adult hippocampal pyramidal neurons using focal in utero electroporation. J Neurosci. 2007;27:5007–11. doi: 10.1523/JNEUROSCI.0867-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.