Abstract
This study examined patterns of changes in post-cessation withdrawal symptoms and basal adrenocortical activity of smokers who were abstinent over a four-week period (n=18) and those who relapsed within the first week (n=35). Participants interested in smoking cessation attended a pre-quit assessment in which they completed multiple forms associated with smoking withdrawal and provided a saliva sample for cortisol and cotinine measures. Then, the participants were asked to set a quit day and were required to abstain from smoking for at least 24 hours. After that, the participants attended 4 weekly follow-up support sessions during which self-report measures on withdrawal symptoms and tobacco use and saliva samples were collected. Smoking status was confirmed biochemically. We found that, overall, the abstinent smokers reported lower withdrawal symptoms, craving, and negative affect than relapsed smokers. Further exploratory analysis indicated that pre-quit withdrawal severity was lower in those who were abstinent relative to those who eventually relapsed. Craving and physical symptoms in abstainers decreased while distress in relapsers increased during the follow-up period. Smoking urges diminished in both groups. Reported numbers of cigarettes per day after a failure of quit attempt were lower than their pre-cessation baseline. Cortisol did not differ by group or by time. Despite using a cross-sectional method, these results may suggest individual differences in negative symptoms while smoking regularly, and abstinence may be associated with reduction of craving and physical symptoms. The findings also suggested that relapsers may not immediately bring back their regular smoking habit after having relapsed.
Keywords: smoking, relapse, negative affect, withdrawal, cortisol
Introduction
Research has evidenced alterations in affective and various physiological activity in chronic smokers (Kirschbaum, Strasburger, & Langkrar, 1993; Steptoe & Ussher, 2006; al'Absi, Hatsukami, Davis, & Wittmers, 2004; Gilbert et al., 1999). However, time course of these associated with prolonged abstinence is still unclear (Kassel, Stroud, & Paronis, 2003; Shiffman, West, & Gilbert, 2004). Several studies suggest that withdrawal-related negative affect decreases within 2 to 4 weeks (Cohen & Lichtenstein, 1990; Dawkins, Powell, Pickering, Powell, & West, 2009; Hughes, 1992; Jorenby et al., 1996; Ward, Swan, & Jack, 2001; West & Hajek, 1997), while others report that the symptoms linger more than a month (West & Schneider, 1988; Gilbert et al., 2002). Inconsistency in findings may be associated with different research methodology such as including only smokers who maintained abstinence in data analysis, a lack of examining both pre-quit (or baseline) and post-quit symptoms, a poor control for selective dropouts, and different criteria used to define habitual smoking (Gilbert & McClernon, 2000; Kalman, 2002; Shiffman et al., 2004).
Another limitation is the absence of assessment of withdrawal-related changes in biological activity after smoking cessation (e.g., al'Absi et al., 2004; Gilbert et al., 1999). The hypothalamic-pituitary-adrenal (HPA) axis, which leads to production of cortisol in humans, is associated with tobacco use (al'Absi, 2006; Rohleder & Kirschbaum, 2006). Cortisol levels during the day tend to be higher among smokers relative to nonsmokers (Steptoe & Ussher, 2006), and greater levels during the morning hours are related to greater withdrawal symptoms (al'Absi et al, 2004). In addition, diminished cortisol response to stress predicted smoking relapse (al'Absi, Hatsukami, & Davis, 2005). These research findings suggest the important role of HPA activity in withdrawal experience after smoking cessation. However, very limited data are available concerning the time course of adrenocortical activity during prolonged smoking abstinence (Gilbert et al., 1999).
This study was designed to address limitations of prior work in examining trajectory of withdrawal symptoms and hormonal activity after a quit attempt. First, we included both smokers who were able to maintain abstinent for 4 weeks and those who relapsed by the first week. We included those who relapsed within 7 days because research has shown that the majority of smoking relapse occurs during this period (Hughes, Keely, & Naud, 2004). In addition, to our best knowledge, no study has directly assessed patterns of changes in smoking behavior and withdrawal symptoms among those who returned to smoking. Second, withdrawal symptoms and basal hormonal levels prior to as well as after smoking cessation were assessed. Third, individuals who had major medical or psychiatric disorders were not included to minimize potential confounds with smoking relapse. We hypothesized that abstinent smokers would report lower withdrawal symptoms and negative affect after smoking cessation than relapsed smokers. Also, the abstainers would exhibit lower basal cortisol activity after quitting smoking relative to the relapsers.
Method
Participants
Smokers were recruited from the community via newspaper advertisements and posters placed around the university. Potential participants completed an initial phone screening which included questions regarding medical or psychiatric disorders and medication intake. Those who reported major physical and psychiatric conditions (e.g., hypertension, diabetes, depression) or other substance use (e.g., marijuana) were not included in the current study. The participants must have smoked an average of 10 cigarettes or more per day for a minimum of 2 years, and reported a strong motivation to quit smoking. Those who met the criteria were invited to an on-site screening session. Participants were qualified if they had no history of a major illness or psychiatric disorder, weighed within ± 30% of Metropolitan Life Insurance norms, consumed two or less alcoholic drinks a day, and did not routinely use prescription medications (except contraceptives). Participants read and signed a consent form approved by the Institutional Review Board of the University of Minnesota. Seventy-two smokers (33 women and 39 men) completed the study.
Measures
A modified version of the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1998; Hughes & Hatsukami, 1986) was used to assess withdrawal symptoms. In this scale the wording of the item “craving” was changed to “desire to smoke.” This item was analyzed separately in light of evidence suggesting that patterns of changes in craving are different from other withdrawal symptoms (Hughes & Hatsukami, 1998). We also measured levels of distress (as assessed by items anxiety, irritability, impatience, and restlessness), positive affect (cheerfulness, content, calmness, controllability, and interest), and physical symptoms (headache, sweating, tremor, stomachache, drowsiness, fatigue, and coughing). Each item referenced a seven-point scale anchored by the end points, Not at All and Very Strong. Psychometric properties of these measures have been reported elsewhere (al'Absi et al., 2003). Levels of mood disturbance were measured by The Profile of Mood State questionnaire (POMS; McNair et al., 1992). The abbreviated version of the Questionnaire of Smoking Urges (QSU-B; Cox, Tiffany, & Christen, 2001; Tiffany & Drobes, 1991) was used to measure two components of craving (factor 1: appetitive urges; factor 2: aversive urges; Cox et al., 2001).
Measurement of cortisol and cotinine concentrations was accomplished by saliva samples. Participants provided 1-2 ml of saliva using cotton dental rolls held in the mouth until saturated. The saliva was collected into a plastic tube (Salivette® tubes, Sarstedt, Rommelsdorf, Germany) and the samples were stored in -70°C until assayed. Cortisol assays were performed in duplicate using a time-resolved fluorescence immunoassay with a cortisol-biotin conjugate as a tracer (Dressendorfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). This assay has a sensitivity of 0.4 nmol/l. The inter- and intra-assay coefficients of variation were less than 10 and 12%, respectively. Cotinine concentrations in saliva were measured by enzyme immunoassay (EIA; DRG Diagnostics, Marburg, Germany). Inter- and intra-assay variations were below 12%. Measurement of expired carbon monoxide (CO) was performed using MicroCO™ monitors (Micro Direct Inc., Auburn, Maine).
Demographic information, tobacco use (e.g., cigarettes per day, years of smoking), levels of nicotine dependence (Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) were also assessed.
Procedure
During the pre-cessation assessment, the participant completed forms of demographics, tobacco use, symptoms associated with smoking, and negative affect (described above). The participant also provided a breath sample for CO assessment and samples for cortisol and cotinine assays. Then, the participant was informed about potential benefits and risks regarding smoking cessation, and was asked to set a quit day. The participant was required to be abstinent at least for 24 hours and attended a laboratory session during the abstinent period (see al'Absi et al., 2005) for details). Smoking status was verified by self-report and CO measures; those who did not meet the criteria (e.g., CO levels higher than 8 ppm) were rescheduled. After that, the participant attended 4 weekly post-quit assessments. Each follow-up was scheduled between noon and 2:00 p.m. during which he or she completed the self-report questionnaires and provided a saliva sample. It also included a support session in which a psychologist encouraged the participant to reduce the rate of smoking (if he or she had relapsed) or maintain abstinence. Participants were monetarily compensated upon completion of the study regardless of their smoking status.
The present study did not include any pharmaceutical aids after smoking cessation as the primary purpose was to examine psychological and biological determinants associated with smoking relapse in unaided quitters.
Data Analysis
Relapse was defined as smoking at least one cigarette after the quit day (Hughes et al., 2003). We only included those who returned to smoking within a week of initial quit but completed all follow-up sessions in the relapsed group (n = 35; 18 men and 17 women) as they may represent the characteristics of those who are especially vulnerable to early smoking relapse (Hughes, Keely, & Naud, 2004). Those who remained abstinent throughout the 4 weekly follow-up periods were classified as abstainers (n = 18; 10 men and 8 women). Smokers who relapsed within a week but withdrew from the study by the fourth follow-up (n = 4) and those who relapsed two or three weeks after initial quit (n = 15) were not included in the analysis; however, preliminary analysis showed no difference in demographic and smoking variables from individuals who relapsed earlier or the abstainers.
Dependent variables were scores on withdrawal symptoms, craving, distress, positive affect, physical symptoms, total mood disturbance, smoking urges, cigarettes per day, and measures of CO, cotinine, and cortisol. Each variable was analyzed by a 2 groups (abstinent, relapsed) × 5 periods (pre-cessation baseline, post-cessation week 1, 2, 3, 4) repeated measures ANOVA. Greenhouse-Geisser correction was used for these tests and Bonferroni correction was applied to post-hoc analysis. Log transformation was performed to the cortisol data to meet the assumption of normality. Demographic and smoking history variables were analyzed using a one-way ANOVA. Due to missing data, variations exist between sample size and degrees of freedom for the reported variables.
Results
Abstinent and relapsed smokers were comparable in demographics and history of tobacco use except that the abstainers were older, and reported more caffeine consumption, longer hours of nightly sleep, and longer duration of smoking (Fs > 4.3, ps < .05) than the relapsers (see Table 1).
Table 1. Participant characteristics and smoking-related variables prior to quitting smoking.
| Abstinent (n=18) | Relapsed (n=35) | p | |
|---|---|---|---|
| Age (years) | 42.4 (3.2) | 33.5 (2.3) | .030 |
| BMI (kg/m2) | 26.2 (0.9) | 24.5 (0.7) | ns |
| Education (years) | 14.0 (0.7) | 14.6 (0.5) | ns |
| Caffeine (per day) | 5.9 (0.7) | 3.3 (0.5) | .003 |
| Sleep (hr per day) | 7.4 (0.3) | 6.8 (0.2) | .04 |
| Cigarettes (per day) | 17.8 (1.8) | 20.9 (1.3) | ns |
| Duration (years) | 15.7 (2.4) | 9.8 (1.6) | .047 |
| Previous quit attempts | 6.1 (3.4) | 7.1 (2.5) | ns |
| Longest quit (days) | 18.2 (5.4) | 6.6 (3.8) | ns |
| Motivation to quit | 5.9 (0.2) | 6.1 (0.1) | ns |
| FTND | 5.0 (0.5) | 5.9 (0.4) | ns |
| CO (ppm) | 23.5 (2.5) | 23.3 (1.8) | ns |
| Cotinine (ng/mL) | 181 (29.1) | 203 (21.5) | ns |
Entries show mean and standard error of the mean. Note. FTND = Fagerström Test of Nicotine Dependence. CO = carbon monoxide. MNWS = the Minnesota Nicotine Withdrawal Scale. QSU-B = the Questionnaire of Smoking Urges. POMS = the Profile of Mood State questionnaire.
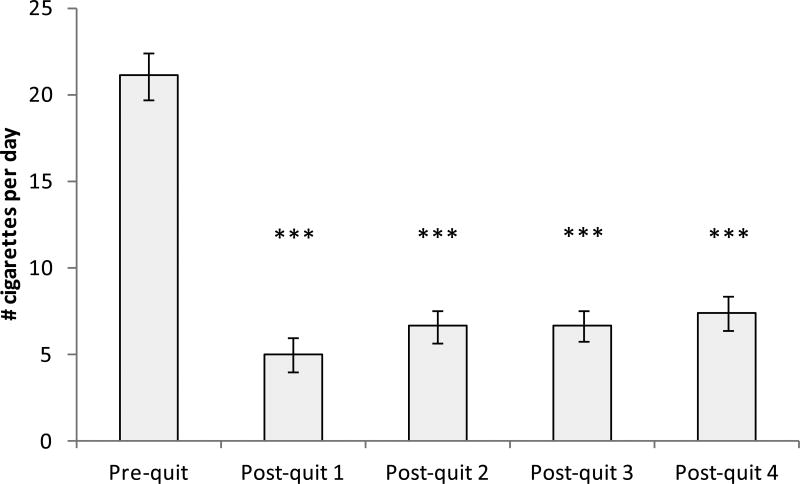
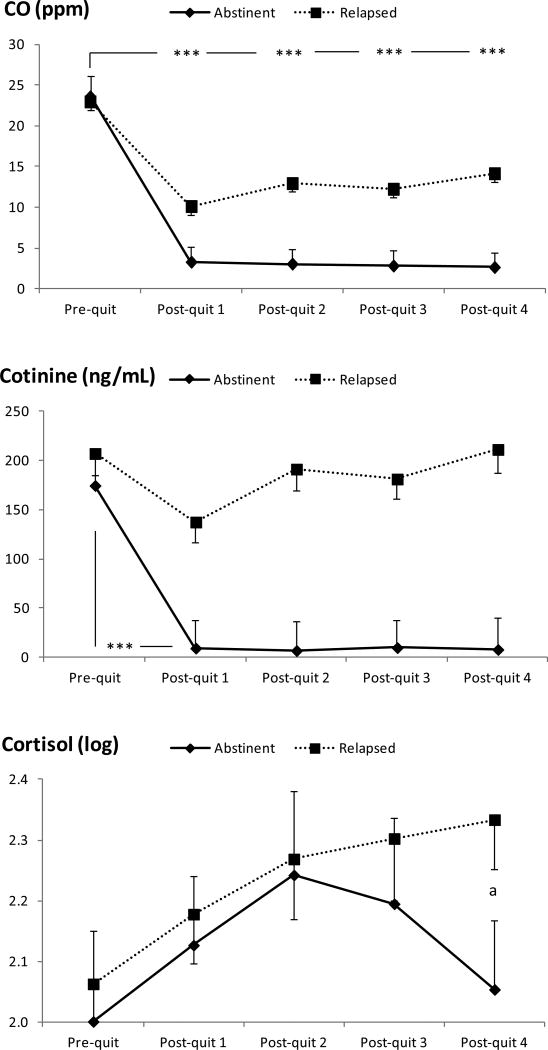
Reported cigarette smoking decreased over time (F (2, 111) = 181, p < .001). A repeated analysis conducted only among relapsers also found a significant time effect (F (2, 74) = 73.2, p < .001), indicating that the number of cigarettes at each follow-up were lower than the pre-cessation baseline (ps < .001; see Figure 1). There was a significant group × time interaction in CO (F (2, 115) = 8.04, p < .001) reflecting greater decrease from the baseline to the first post-quit week among abstainers than in relapsers (see Figure 2). However, CO levels observed at each follow-up period among relapsers were also lower than their baseline (time effect: F (3, 100) = 26.2, p < .001; post-hoc tests: ps < .001). A significant group × time effect found in cotinine (F (3, 142) = 8.43, p < .001) suggested a greater decrease from pre-cessation to the first follow-up period among abstainers than in relapsers. Low levels of CO and cotinine observed among abstainers suggest the compliance with the smoking abstinence restriction (cotinine: ≤ 10 ng/mL; CO: < 9 ppm). Cortisol levels did not change by group or across time (ps > .16; see Figure 2). To further explore the data, group difference in cortisol was examined at each period. It was found that abstainers exhibited lower levels than relapsers at the fourth follow-up (F (1, 49) = 4.09, p < .05).
Figure 1.
Reported number of cigarettes consumed per day among relapsed smokers. p < .001.
Figure 2.
Changes in biochemical and hormonal measures during the study peirod. Asterisks indicate significant differences found in omnibus models, p < .001. a Significant group differences were found during post-quit week 4.
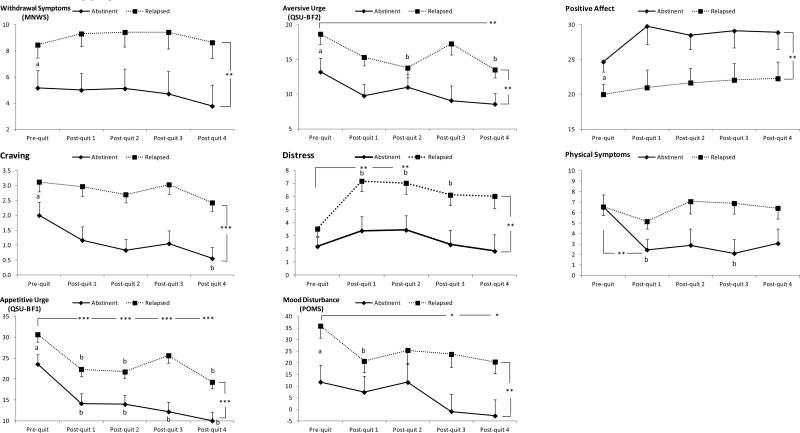
Results of omnibus repeated measures models indicated that relapsed smokers reported greater levels of withdrawal symptoms (F (1, 49) = 8.90, p < .005) and craving (F (1, 49) = 21.4, p < .001) than abstainers as indicated by significant main effects of group (see Figure 3). QSU-B factor 1 was greater in relapsers than in abstainers (F (1, 48) = 18.7, p < .001) but the levels during each follow-up assessment were lower than the pre-cessation baseline (time effect: F (3, 162) = 20.2, p < .001; post-hoc tests: ps < .001). Relapsers also had higher scores on Factor 2 than the abstainers (F (1, 48) = 8.56, p < .01); the levels during the fourth follow-up were lower than the baseline (time effect: F (3, 168) = 5.16, p < .001; post-hoc tests: ps = .005). The relapsed group reported greater levels of mood disturbance than their counterparts (F (1, 47) = 6.83, p = .01). The levels during the third and fourth follow-up were lower than the baseline (time effect: F (3, 162) = 4.66, p < .005; post-hoc tests: ps ≤ .02). The relapsers reported greater distress than abstainers (F (1, 49) = 10.2, p < .005), and the levels during the first and second follow-ups were greater than the baseline (time effect: F (4, 179) = 4.66, p < .005; post-hoc tests: ps ≤ .01). Positive affect was lower in relapsers than in abstainers (F (1, 49) = 8.59, p < .01). A group × time interaction in physical symptoms (F (3, 167) = 3.05, p < .05) reflected a significant decline from pre-cessation baseline to the first follow-up among the abstainers (time effect: F (2, 38) = 7.33, p < .005; post-hoc tests: p =.01) but this was not the case with the relapsers (p = .33).
Figure 3.
Differences patterns of changes, withdrawal symptoms, craving, and mood state measures between abstinent and relapsed smokers. Note: Asterisks indicate significant differences found from omnibus models *p < .05, ** p < .01, *** p < .001. a Significant group differences were found during pre-quit baseline. b Significant time differences were found within each smoking group.
Despite a lack of group x time interactions in most self-report measures from omnibus models, additional analyses were conducted to further explore the current results. A series of one-way ANOVAs examining pre-quit group differences found that individuals who eventually relapsed reported greater levels of withdrawal symptoms (F (1, 51) = 4.29, p < .05), craving (F (1, 51) = 4.50, p < .05), QSU-B factor 1 (F (1, 51) = 6.48, p < .05) and factor 2 (F (1, 51) = 4.73, p < .05), and mood disturbance (F (1, 49) = 7.48, p < .01), and lower levels of positive affect (F (1, 51) = 6.95, p < .05) than those who maintained abstinent after quitting smoking (Figure 3). In addition, a series of repeated measures ANOVAs were conducted in each smoking group. The results indicated that some symptoms varied across time only in abstainers. There was a significant time effect in reported levels of craving (F (2, 39) = 5.32, p < .01) and physical symptoms (F (2, 37) = 7.33, p < .01) in this group. Follow-up analysis with appropriate adjustments found that craving at fourth week post-quit was lower than pre-cessation baseline (p < .05) and physical symptoms at the first and third weeks were lower than the baseline (ps < .05). These findings were not observed in relapsers (ps > .33). QSU-B factor 1 decreased in both abstainers (time effect: F (1, 28) = 13.4, p < .001); follow-up tests: levels at each follow-up week were lower than the baseline; ps ≤ .05) and in relapsers (time effect: F (3, 111) = 10.0, p < .001; follow-up tests: levels at first, second, and fourth weeks were lower than the baseline, ps < .001). Some symptoms changed among relapsers only. There was a time effect in distress (F (3, 114) = 6.07, p < .001), QSU-B factor 2 (F (3, 103) = 4.83, p < .01), and mood disturbance (F (3, 101) = 3.45, p < .05) in this groupi. Distress levels at first, second, and third week follow-up were greater than pre-quit baseline (ps < .05) while factor 2 at second and fourth week (ps < .05) and mood disturbance at the first follow-up week (p< .05) were lower than pre-quit baseline. These findings were not observed in abstainers (ps > .06). MNWS and positive affect did not change across time in both groups (ps > .19).
Discussion
The current study evidenced differences in patterns of changes in withdrawal symptoms between smokers who remained abstinent for 4 weeks and those who relapsed within 7 days. The results from omnibus models indicated lower withdrawal symptoms, craving, smoking urges, distress, negative affect, and physical symptoms and greater positive affect in abstainers than in relapsers. Further exploratory analyses indicated group differences in withdrawal severity prior to quitting smoking. Individuals who eventually relapsed reported greater levels of withdrawal symptoms, craving, smoking urges, and mood disturbance, and lower levels of positive affect than those who abstained from smoking cigarettes. The analyses also found group-specific trajectory of symptoms after smoking cessation. In abstainers craving and physical symptoms during post-cessation assessments were lower than those during pre-quit baseline, while in relapsers distress increased but aversive urges (factor 2 of QSU-B) and negative affect decreased during the follow-up period. Appetitive urges (QSU-B factor 1) diminished after quitting in both groups.
Findings in abstainers are consistent with research showing that absence of smoking is associated with reduction of withdrawal-related negative affect (Cohen & Lichtenstein, 1990; Dawkins et al., 2009; Hughes, 1992; Jorenby et al., 1996; Ward et al., 2001; West & Hajek, 1997). Findings in relapsers are also in agreement with prior work reporting that urges tend to fluctuate with ongoing smoking status, and patterns of changes in urges might be different from other withdrawal symptoms (Hughes & Hatsukami, 1998). The fact that smokers reported reduced smoking after relapse may support this model. The finding that reported distress levels increased but mood disturbance decreased during post-quit assessments in relapsers may appear contradicting; however, we note that these measures assessed levels in different time frames (i.e., distress: in the past 30 minutes; negative mood: in the past week). A failure of quit attempt may have different influences on changes in state vs. trait mood. These findings, however, should be treated with caution due to the cross-sectional nature of the study. That is, whether smoking abstinence (or relapse) causes changes in withdrawal symptoms and negative affect or whether pre-existing individual differences (e.g., trait mood, sensitivity to pharmacological effects of nicotine) associated with withdrawal symptoms causes one's ability to quit smoking cannot be determined from the current results. Third factors such as gender and socioeconomic status may account for the findings a well. Nevertheless, our results suggest that there may be individual differences in severity of withdrawal symptoms before smoking cessation, and that these differences may be related to an ability to maintain smoking abstinence and time course of post-quit symptoms.
In relapsers, the number of cigarettes per day after having relapsed did not exceed 10 throughout 4 weekly follow-up assessments, which was less than 50% of what they used to smoke prior to quitting (see Figure 1). CO levels during the follow-up periods also remained low. These results may suggest that a failure of quit attempt may not immediately take the individual back to his or her pre-quit smoking habit. Although the reason for this is unclear, one possibility is that an individual may be stressed or feel guilty when they take up cigarettes again. Cutting down cigarettes therefore may reflect compensatory behavior for negative emotional states due to smoking relapse. Trajectory of emotional states and biological activity after smoking relapse should be elucidated in future research.
The current findings that abstainers reported lower withdrawal-related distress than relapsers after smoking cessation appear to be inconsistent with previous work that found increased negative symptoms during absence of tobacco use in this group (Gilbert et al., 2002). Differences in methodology may account for this discrepancy however. For instance, the current study did not have control over participants' smoking status after the quit day. In other words, whether to return to smoking was thoroughly dependent on each individual. Thus, reported withdrawal symptoms in this study were compared between unaided quitters who were successful in cessation (for 4 weeks) and those who failed to remain abstinent. In Gilbert and colleague's study, financial incentives were used to minimize attrition of abstainers, and time course of withdrawal symptomatology was compared between those who were requested to maintain abstinence and continue smoking during the study period. The associations between research methodology and trajectory of abstinence-related symptoms are not well understood. More research is warranted to identify experimental designs in which abstinence-related changes in psychophysiological activity represent those in the natural environment.
It was unexpected to find stability of basal cortisol levels across abstinent and relapsed groups. This suggests that abstinence-related adrenocortical activity may not be resolved in a month (Gilbert et al., 2004; Gilbert et al., 1999). We note, however, that our additional analysis suggested a trend of reduction in cortisol levels at the fourth follow-up week in abstainers relative to relapsers. The current findings, however, should be treated with caution because we only collected saliva samples once during each period. Longer-term assessment with repeated sample collections during each follow-up visit may improve reliability of data to examine the trajectory of basal hormonal levels after smoking cessation. For example, it would be interesting to examine whether basal cortisol levels may continue to decrease after the fourth week among abstainers (Figure 2). A trend of increased hormonal activity observed in relapsers is consistent with the literature showing the link between enhanced activity in HPA axis and tobacco use (al'Absi, 2006; Rohleder & Kirschbaum, 2006; Steptoe & Ussher, 2006). Future study should also examine the extent to which abstinence of smoking is related to normalization of emotional and neuroendocrine response to acute stress.
The present results may have clinical implications. For example, knowing psychological benefits of quitting smoking, such as reduction of desire to smoke and physical discomfort, may encourage self-aided quitters to maintain their motivation to stay away from cigarettes. The current results may also be useful in cessation intervention because a failure of quit attempt may not be desperate; our data suggest that those who took up cigarettes again may not immediately be motivated to retrieve their regular smoking habit after having relapsed. Lower urges to smoking may also indicate that the system was not “ready” for normal rate of smoking. Providing relapsers with emotional support and additional opportunities to quit smoking may reduce the risk of full relapse if these were delivered promptly after a failure of quit attempt. This model however has not been directly examined.
The results of the current study should be considered preliminary for a number of reasons. First, the findings were correlational in nature. Thus, no statements can be made regarding causal direction. Also, the findings from additional analyses (e.g., ANOVA conducted to examine smoking group differences in pre-cessation self-report measures, repeated measures AVOVA conducted in each group) should be interpreted with caution since group x time effects were not found in omnibus models. We note, however, that the analyses were informative in terms of an attempt to fully appreciate data with a small sample size. While a larger sample with equal number of men and women should improve the results, this may not be ethical because each smoker's smoking status (to maintain abstinence or relapse) cannot be manipulated or randomized. Second, individuals with current major physical or psychiatric diseases were not included to minimize confounding associated with comorbility. However, the role of clinical conditions such as depression in post-quit symptomatology should be elucidated. Third, psychosocial influences should be taken into account. Nonetheless, this study included subjective as well as physiological measures and assessed these indices prior to and after smoking cessation to examine differences in affective and adrenocortical activity between smokers who were abstinent and those who relapsed.
In summary, this study showed that smokers who were able to reach a 4-week abstinence reported lower craving and physical symptoms post-cessation than the pre-quit levels, while relapsers reported increased distress but reduced smoking urges after relapse as compared with the pre-quit baseline. Individuals who eventually relapsed reported greater levels of withdrawal symptoms and negative affect prior to the quit day than those who maintained abstinence. Among relapsers, reported numbers of cigarettes per day and CO levels after relapse were lower than their pre-cessation period. The results suggested individual differences in negative symptoms before quitting smoking, and certain symptoms may be diminished during smoking abstinence. Also, patterns of tobacco use may not return to those prior to quitting at least for 4 weeks after relapse. Evaluating pre-cessation withdrawal intensity and its relationships with patterns of changes in post-quit symptoms and tobacco use may be useful in identifying mechanisms linked to prolonged abstinence.
Acknowledgments
This work was supported in part by the National Cancer Institute (CA 88272) and the National Institute on Drug Abuse (DA 013435 and DA 016351).
Footnotes
The authors do not have any conflict of interests with preparing the manuscript.
References
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology. 2006;59(3):218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181(1):107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73(3):267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior. 2003;74(2):401–410. doi: 10.1016/S0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychology. 1990;9(4):466–478. doi: 10.1037/0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104(5):850–858. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43(7):683–692. doi: 10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ. A smoke cloud of confusion. American Psychologist. 2000;55(10):1158–1159. doi: 10.1037/0003-066X.55.10.1158. [DOI] [PubMed] [Google Scholar]
- Gilbert D, McClernon J, Rabinovich N, Sugai C, Plath L, Asgaard G, et al. Effects of quitting smoking on EEG activation and attention last for more than 31 days and are more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine & Tobacco Research. 2004;6(2):249–267. doi: 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S, et al. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: relations to depressive traits, nicotine exposure, and dependence. Experimental and Clinical Psychopharmacology. 1999;7(4):427–443. doi: 10.1037/1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, et al. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. Journal of Consulting and Clinical Psychology. 2002;70(1):142–152. doi: 10.1037/0022-006X.70.1.142. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7(1):92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. Journal of Consulting and Clinical Psychology. 1992;60(5):689–697. doi: 10.1037/0022-006X.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5(1):13–25. doi: 10.1093/ntr/5.1.13. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen S, Jensen J, et al. Characterization of tobacco withdrawal symptoms: transdermal nicotine reduces hunger and weight gain. Psychopharmacology (Berl) 1996;128(2):130–138. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- Kalman D. The subjective effects of nicotine: methodological issues, a review of experimental studies, and recommendations for future research. Nicotine & Tobacco Research. 2002;4(1):25–70. doi: 10.1080/14622200110098437. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacology Biochemistry and Behavior. 1993;44(3):527–531. doi: 10.1016/0091-3057(93)90162-M. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. POMS Manuel-Profile of Mood Questionnaire. San Diego: Edits; 1992. [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. International Journal of Psychophysiology. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West R, Gilbert D. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research. 2004;6(4):599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. International Journal of Psychophysiology. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addictive Behaviors. 2001;26(3):311–327. doi: 10.1016/S0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. What happens to anxiety levels on giving up smoking? The American Journal of Psychiatry. 1997;154(11):1589–1592. doi: 10.1176/ajp.154.11.1589. [DOI] [PubMed] [Google Scholar]
- West R, Schneider N. Drop in heart rate following smoking cessation may be permanent. Psychopharmacology (Berl) 1988;94(4):566–568. doi: 10.1007/BF00212857. [DOI] [PubMed] [Google Scholar]