Abstract
Introduction
Against the background of Ghana's ART program which scaled up rapidly since inception in 2003, the study assessed outcomes of an early cohort of patients initiating ART.
Methods
The study utilized the following methods: a cross-sectional study involving patient interviews using a structured questionnaire, a review of records and a retrospective cohort analysis of adults initiating ART between 2003 and 2008 from four selected clinics.
Results
The 683 study participants consisted of 464 females (67.9%) and the mean age was 41 years. Mean duration of treatment was 25 months (SD =13). More than 95% were on a regimen as per national guidelines. Ninety-five (14.1%) of the respondents had one or two drugs substituted. Seventy-three% of the substitutions were due to adverse drug reactions. On at least one occasion, over half (350) had defaulted on a clinic appointment. In the 3 months preceding the survey, 21.4% (146) had missed treatment doses. About 49% (334) had challenges meeting financial obligations related to care. The median weight increased by 5.9kg and 8.0kg at 6 and 12 months after initiating ART respectively over the median baseline weight of 54kg, (p-value = 0.001). The median CD4 count increased by 128, 170 and 256 cells/µl respectively at 6, 12 and 24 months from the median baseline of 125 cell/µl, (p-value = 0.035).
Conclusion
This study of Ghanaian PLHIV on ART from four facilities showed encouraging immunological and clinical outcomes. There were however issues of appointment default, sub-optimum adherence to treatment and cost of care barriers needing attention.
Keywords: Ghana, antiretroviral treatment, immunological, clinical, outcomes
Introduction
In consideration of the profound effects HIV has on the many people who are infected and affected particularly in sub-Saharan Africa, the World Health Organisation (WHO) in 2003 launched the “3 by 5” initiative which sought to make Anti-retroviral Therapy (ART) available to Persons Living with HIV (PLHIV) especially those in the developing world. [1] In tandem with this venture, Ghana embarked on its ART program in 2003. Counting from the 100 Ghanaian PLHIV who were first initiated on treatment in Ghana's ART pilot program in the first year, the number exceeded 10,000 by the end of 2007 and rose to over 47,000 by December 2010. [2, 3]
The introduction of highly active antiretroviral therapy (HAART) dramatically changed the course of HIV infection and has led to a significant reduction in HIV-related morbidity and mortality globally.[4] Several studies have documented long term outcomes among PLHIV on ART in sub-Saharan Africa demonstrating good immunologic, virologic, and clinical response to their treatment. [5–10] A number of these reports have highlighted various issues pertaining to life-long ART including the regimens used, adherence to treatment and adverse effects encountered providing valuable insight on the experience of PLHIV in the sub-region. In Ghana a limited number of studies have profiled various outcomes in PLHIV taking ART. [11–13] A study on short term outcomes among a sample of PLHIV initiated on treatment as Ghana scaled up ART was undertaken. We present these findings to fill in the gap in the picture of the history of ART provision in Ghana.
Methods
The study consisted of a cross sectional study and a chart review. The participants in this study were recruited from four purposively selected levels of health facilities namely teaching, regional, district and private hospitals offering ART services in Ghana. Two sites were public, one private and one faith based institution. The sites were Korle Bu Teaching Hospital (KBTH), Koforidua Regional Hospital (KRH), the St Dominic's Hospital (SDH) and Odorna clinic, a private provider. All four facilities were selected for being pioneers in their range to provide ART and were using the prevailing national guidelines issued by the National AIDS/STI Control Programme. The guidelines stipulated WHO clinical stages 3 or 4, CD4 < 250 and a least 2 sessions of adherence counseling as requirements before initiation of ART.
First line therapy first choice drugs consisted of Zidovudine (AZT), Lamivudine (3TC) and Nevirapine (NVP or. Efavirenz (EFV). Stavudine was an alternative to Zidovudine. Routine laboratory tests, weight and CD4 count were measured at initiation. Weight was repeated at each clinic visit, laboratory tests at 3 months and CD4 tests 6 monthly. Patient data was recorded in both manual and electronic formats at each visit. PLHIV accessing ART were required expected to contribute a monthly fee equivalent to $5.00 towards clinical services but were not denied treatment if they could not pay.
Inclusion Criteria
The study employed a retrospective cohort analysis of data from adult HIV positive patients18 years and above on ART. Eligible study participants consisted of those who had been on antiretroviral therapy for at least 6 months and were attending ART clinic for the first time in the four sites within the study period of June - August of 2008. During this time frame, consecutive patients attending the ART clinic, fitting into the inclusion criteria and who gave their consent were recruited into the study.
Data collection and analysis
Chart reviews included socio-demographic data, WHO staging, weight and CD4 counts, drug regimens, adherence records, treatment default and clinical progress specifically new opportunistic infections developed while on treatment. Face to face interviews were utilized to corroborate chart findings and also explore drug related side effects, affordability of treatment and factors related to accessing ART services. The data collected was entered into Microsoft Access database version and consistency was checked for quality control purposes. SPSS software/programme was then used to conduct the analysis. Descriptive statistics were used to outline the demographic, treatment and clinical characteristics. Outcomes of interest were changes in CD4 count and body weight over baseline at 6 months intervals. To evaluate the significance of changes in CD4 and weight over time using repeated measures, the Friedman test for comparing repeated median values was used.
Ethical clearance for this study was obtained from the Ethical and Protocol Review Committee of the University of Ghana Medical School. Written informed consent was obtained from all study participants.
Results
Socio-Demographic Characteristics Over the study period June to August 2008 a total of 683 respondents were recruited the majority of which were females (Table 1). Almost three quarters of the participants (496, 72.7%) fell within the age interval of 30 to 49 years. More than 80% (564) had at least primary education. Even though more than eight out of ten (568, 83.2%) of the respondents indicated they had children, most of those parents (70%, 398) did not know the HIV status of their children.
Table 1.
Demographic and treatment characteristics of PLHIV who had been taking ART at least 6 months by the time of the study (N=683)
| Number from study sites | n (%) |
|---|---|
| Korle Bu Teaching Hospital | 408 (59.8) |
| Koforidua Regional Hospital | 138 (20.2) |
| St Dominic's Hospital | 104 (15.2) |
| Odorna Clinic | 33 (4.8) |
| Age, years | |
| Mean (SD) | 41.4 (9.2) |
| Range | 18 – 72 |
| Women, n (%) | 464 (67.9) |
| Educational status, n (%) | |
| Nil | 119 (17.4) |
| Primary | 118 (17.3) |
| Junior secondary/middle school | 285 (41.7) |
| Senior secondary/high school | 116 (17.0) |
| Tertiary | 38 (5.6) |
| Other | 7 (1.0) |
| Religion, n (%) | |
| Christian | 612 (89.6) |
| Moslem | 60 (8.8) |
| Other | 11 (1.4) |
| Point of HIV diagnosis/ source referral to ART clinic, n (%) | |
| Medical diagnostic testing | 290 (42.5) |
| Pediatric diagnostic testing | 14 (2.0) |
| Surgical diagnostic testing | 8 (1.2) |
| TB clinic diagnostic testing | 24 (3.5) |
| PMTCT / ANC | 21 (3.1) |
| VCT | 60 (8.8) |
| Partner HIV positive | 48 (7.0) |
| Referral from other health Institutions | 206 (30.2) |
| Others | 12 (1.6) |
| Initial ART regimen, n (%) | |
| Zidovudine + Lamivudine + Nevirapine | 244 (35.7) |
| Zidovudine + Lamivudine + Efavirenz | 230 (33.7) |
| Stavudine + Lamivudine + Nevirapine | 103 (15.1) |
| Stavudine + Lamivudine + Efavirenz | 78 (11.4) |
| Other combinations | 28 (4.1) |
| Duration on ART, n (%) | |
| 6 – 12 months | 198 (28.0) |
| 13 – 24 months | 218 (32.0) |
| 25 – 36 months | 145 (21.2) |
| ≥37 months | 122 (18) |
| Means of paying ART services, n (%) | |
| Patient out of pocket | 456 (66.8) |
| Family | 87 (12.7) |
| Spouse | 46 (6.7) |
| Employee sponsored | 7 (1.0) |
| Medical insurance | 4 (0.6) |
| Other | 83 (12.2) |
| Times drug collected without making payment, n (%) | |
| Never | 179 (26.6) |
| 1-4 times | 293 (43.5) |
| 5-9 times | 136 (20) |
| >10 times | 64 (9.5) |
| No response | 11(1.6) |
Antiretroviral Therapy
Knowledge of HIV status and travel time to clinic Enquiring about how participants found out their HIV diagnosis and subsequently referred to the ART clinic revealed that 45.7% were through diagnostic HIV tests for a medical or surgical condition while 7% had had HIV testing after their partner tested positive for HIV. For 42.8% travelling time to the clinic was in the range of 30 minutes to an hour. A quarter spent 1 to 2 hours getting to the clinic whiles 13.7% usually travelled more than 2 hours to get to their treatment sites.
ART regimens
All the respondents had been taking ARVs for 6 months or more, with the mean duration of treatment being 25.15 months (SD =13.04). The longest period a participant had been on treatment was 59 months. Before initiating treatment, most people (90.7%) had undergone at least 2 adherence counselling sessions. Almost all the respondents (95.1%) were on a recommended first line regimen. More than two-thirds of these (69.4%) were on a regimen consisting of Zidovudine and Lamivudine with either Nevirapine or Efavirenz. combination. During the course of ARV therapy, 95 (14.1%) of the respondents had one or two of the ARVs medication substituted. Substitutions were made for the following numbers (proportions) of person on the respective ARVs as follows: Stavudine 20 (11%), AZT 35 (7.3%), NVP 25 (7.2%) and Efavirenz 15 (4.9%). For seven out of ten of the substitutions (73%), the reason was due to adverse drug reaction. For 13% it was due to initiating TB treatment. Three percent of the substitutions were as a result of pregnancy.
Treatment and appointment default
About half, 350 (51.2%), of the respondents admitted to defaulting on their clinic appointments but a review of the medical charts showed that 77.7% of the respondents had defaulted on their appointment on at least one occasion. Reasons cited for defaulting varied from financial/ economical difficulties to travelling out of town and forgetfulness (Table 2). “Other reasons” included a myriad of responses such as delayed laboratory results, having to attend to a sick relative and mix up of clinic dates. Adherence to ARVs within the 3 months before the survey as per patient self report was assessed. Out of 146 respondents (21.4% of study population) who admitted defaulting on their treatment in the last 3 months, 118 (80.8%) had missed taking their drugs between 1 to 4 times and the rest missed their doses 5 or more times. Reasons given for the non-adherence to ARV varied from forgetfulness to running out of drugs (Table 2). Ten respondents (1.5% of those surveyed) said they stopped taking the ARVs entirely because of unpleasant effects including weakness and gastro-intestinal side effects.
Table 2.
Reasons given by PLHIV taking ART for defaulting on clinic appointment and missing doses
| Reasons given by PLHIV for defaulting on clinic appointment least once (N = 352) | Number (%) |
|---|---|
| Economic reasons | 110 (31.3) |
| Travelled | 68 (19.3) |
| Forgetfulness | 41 (11.6) |
| Still had some drugs | 33 (9.4) |
| Felt unwell | 13 (3.7) |
| Other | 88 (24.7) |
| Reasons given by PLHIV for missing their doses at least once in the preceding 3 months (N = 146) | Number (%) |
| Forgetfulness | 66 (45.2) |
| Travelled without them | 31 (21.2) |
| Ran out of drugs | 27 (18.4) |
| Unpleasant side effects | 8 (5.5) |
| Problems related to meals | 7 (4.8) |
| Other | 11 (7.5) |
Payment for Treatment
At the time of the study, as a national policy, PLHIV were charged Gh ¢ 5 (approximately US$5) monthly for as contribution towards cost of care for HIV management. This amount was usually collected at the pharmacy at the point of drug pick up. Enrolment in National Health Insurance Scheme (NHIS) did not exempt a person from paying this fee so even the 53% of respondents who reported being enrolled in the NHIS were still expected to pay the service charge. Patients who could not pay however because of inability to afford the fee or did not have money available on the clinic day were still entitled to pick up their drugs. About two-thirds of participants stated that they paid for their treatment out of pocket. More than 7 out of ten respondents however indicated that on at least one occasion they had collected their drugs without paying the required fee (Table 1). About half of the study participants (334) had an outstanding bill to pay at the time of being interviewed for the study.
Opportunistic infections and tuberculosis
Opportunistic infections (OI)/conditions that the study population had been managed for while on ART were recorded from the patient charts. At least one episode of OI was recorded for 460 out of 683 participants (67%). The number treated for consecutive second, third, fourth and fifth episodes of OI whiles on ARVs were 276 (40%), 169 (25%), 103 (15%) and 64 (9%) respectively. The three commonly reported conditions managed for each episode were respiratory disorders, malaria and skin lesions. These three accounted for over 70% of the opportunistic infections/ conditions reported in each episode. A history of being investigated for TB was elicited by checking for assessment of sputum smears and or chest x-rays from medical chart reviews and collaboration from respondent interviews. It was found that 42% (286) of the respondents had been investigated for TB. Of the 267 who had undergone sputum smear testing, 22.8% (61) had a smear positive sputum for AFB while 28.5% (71) of the 249 who had had chest X-ray taken had findings suggestive of TB.
Treatment outcomes
After the initiation of ART, changes in CD4 counts over the baseline levels were analyzed at 6 month interval using Freidman's Test for repeated measures to assess significance in the changes. Due to the different starting points of ART for the study participants and unavailability of data in some cases, the number of those who had CD4 count available for analysis decreased over time from 579 at baseline to 200 after 12 months and 183 after 24 months on treatment. The median CD4 count increased by 128, 170 and 256 cells per microliter (µl) respectively at 6, 12 and 24 months over the median baseline of 125 cell/µl (p = 0.035). Figure 1 shows the median CD4 counts at the various intervals for the specified numbers of participants whose CD4 counts were available for analysis. Similarly an assessment of changes in the median weight increase over the baseline was tracked again using Freidman's Test for repeated measures to assess whether the differences were significant. The number of participants for whom weight was available for analysis at baseline, 6 months and 12 months was 528, 293 and 156. Increases of 5.9 kg and 8.0 kg over the median baseline weight of 54 kg (p = 0.001) at 6 and 12 months after initiating ART respectively were noted. Thereafter, there was stabilization of the median weight among the relatively fewer number of patients for whom weight data was available.
Figure 1.
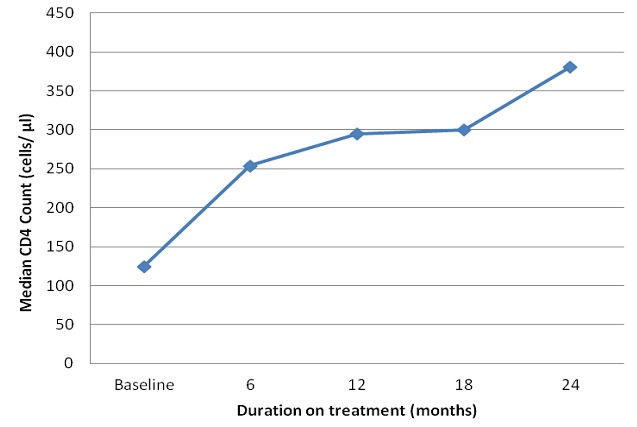
Changes in median CD4 count of patients on ART from baseline at six monthly intervals
Discussion
This study on the experience of an early cohort of PLHIV initiated on ART during the scale up of Ghana's ART program points to significant improvement in weight and CD4 counts over the course of their treatment. Almost all the participants had been initiated on the first line drug regimen recommended in the national guidelines. More than a fifth however admitted to missing their drugs on at least one occasion in the three months preceding the survey. About half had been unable to honour their contribution to the cost of care. The ability of the study to bring out these findings as well as other characteristic related to initiating and taking ART including issues pertaining to entry to care, drug substitutions clinic attendance and the common opportunistic conditions encountered while on ART highlights its strength. As such, the study contributes to the available literature by providing the missing piece of data on the early years of ART in Ghana. This is in spite of the following study limitations: dependence on routine clinic records and patient reports with the associated problems of missing data and recall bias respectively and the exclusion of those on treatment who had been transferred out or were lost to follow up at the time of the study.
The study participants comprised of more women than men, similar to the situation in most sub-Saharan countries [5, 6, 15] and also reflective of the national proportion of males and females receiving ART [16]. The age ranges and the educational level were found to be comparable with that from a study of PLHIV on ART in Kumasi, another city in Ghana. [13]. It was surprising to note that among those who had children, the HIV status of two-thirds of the children was not known by the study participants. Considering the possibility of vertical or horizontal transmission, every opportunity should be taken to recommend HIV testing and counselling for family members, especially young children, to facilitate treatment and care for those who might need them. [17]
As antiretroviral therapy is scaled-up in Ghana, there should be a push for early identification of persons with HIV infection so they can benefit from the services provided. The study showed that diagnostic testing was the major source of patient identification. This is not too surprising since all our study participants were on ART indicating they were at a late stage of HIV infection to warrant treatment. It is possible that they underwent diagnostic testing because they presented at a health facility with a Stage 3 or 4 disease. In their report on the outcomes of PLHIV initiated on ART in the early part of Botswana's National ART Program, Bussman and colleagues also highlighted the point that a large proportion of their patients showed evidence of advanced stages of HIV infection. [6] To reduce late initiation of ART for better outcomes however it is prudent for PLHIV to be diagnosed early in the infection. [5, 18] Thus efforts at improving access to counselling and testing (CT) such as offering outreach CT services at the community level and provider initiated routine offer of CT should be actively pursued and scale up [17].
It is reassuring to note that practically everyone had the minimum number of 2 adherence counselling sessions as recommended in the ART guidelines. [14] Also gratifying is the fact that the recommended combinations of first line ART for treatment were being prescribed to over 95% of the patients in sync with what was found for attendees in another major ART clinic in the Ghana program.[13]
The proportion of respondents on respective ARVs who had their drugs substituted is comparable to what others have found. [12, 19] Most of the substitutions in our study participants were because of adverse events. Other researchers also cite these reasons among the significant reasons for substitution. [12, 19–21] Gastro-intestinal symptoms were among the unpleasant effects reported by our study respondents who stopped taking their ARV entirely. This concurs with the study by O'Brien and colleagues who also report that gastrointestinal adverse events of ARVs were the most frequently cited reason for discontinuation of treatment.[22] It is therefore imperative for health staff caring for PLHIV on ART to regularly ask clients about adverse drug reactions and side effects to facilitate appropriate management according to national guidelines.
From the study about half of the respondents admitted they had defaulted on their treatment appointment but the review of the medical chart revealed a much greater proportion had defaulted. Other studies have reported a relatively lower appointment default rate probably due to the differences in the definitions used which in our case was missing at least one appointment. [23, 24] It is important to note that successful treatment with highly active antiretroviral therapy (HAART) requires the patient to maintain consistent adherence to the prescribed regimen and keeping of clinic appointments enable patients come for follow up and refill prescriptions. [25] So defaulting on appointments poses a risk to treatment adherence and steps have to be taken to address barriers. The reasons our study participants gave for defaulting have been corroborated in multiple studies. [25] Similarly the proportion of those who missed taking their drugs and the reasons they gave for failing to do so are comparable to other Ghanaian PLHIV on ART. [13] To facilitate adherence it is key to adopt effective adherence support interventions which include treatment supporters, patient centred counselling and addressing barriers such as cost.[17, 26] This perhaps buttresses the need for having an adherence monitor as stated in the ART guidelines since the monitors are expected to help patients adhere to treatment through reminders and other means. It is also important to look at the role played by economic constraints on appointment defaults and therefore running out of drugs. Two-thirds of the study participants said they paid for their care out of pocket and about half had not kept up with the payment of the monthly fee and owed various amounts at the clinic. Much as in principle clients are not denied treatment because of inability to pay, it is possible that embarrassment at owing money may deter some people from showing up at the clinic. [13] There is a need to reconsider the issue of payment for HIV care as it may have implication for treatment adherence.
The opportunistic infections/conditions reported in our study are among those commonly reported in PLHIV. [27–29] Over a fifth of our participants investigated for TB were found to have a positive results reiterating the point that PLHIV are still very much at risk for contracting TB even though they may have initiated ART. [27] Our study findings show that the study participants responded well to treatment over time. Immune recovery evident by increasing CD4 and weight gain was progressive in the course of the year of starting treatment. These findings are in keeping with what is expected when immunity builds up and HIV infection is controlled in a PLHIV on treatment. [5, 6, 30] These desired outcomes are made possible when clients are managed according to national guidelines, barriers to taking treatment are addressed and treatment is taken as prescribed.[17] As Ghana continues to scale up and aims for universal access to HIV prevention, treatment and care services, it is encouraging to note that those enrolled in the program are making good progress. It is however imperative that the follow up of those on treatment is maintained to address issues related to taking their treatment including adverse drug reactions. This will contribute to PLHIV who are effectively managed on ART living productive lives and minimize the emergence of HIV drug resistance.[17] Building on this study, further analysis of long term outcomes is warranted to assess whether the clinical and immunological outcomes are maintained. Future studies should also assess viral suppression in response to treatment.
Conclusion
In summary, this study of an early cohort of PLHIV from the four treatment facilities initiated on ART during the scale up of ART showed encouraging outcomes immunologically and clinically. HIV care management was as per national guidelines. There were however issues of defaulting on appointment, sub-optimum adherence to treatment and cost of care barriers needing attention. To maximise the benefits of ART, it is imperative to maintain regular follow up of PLHIV on treatment and ensure appropriate clinical and immunological monitoring. Information on drug effects should be regularly elicited from PLHIV to facilitate prompt and optimum management. This will enable those not achieving the desired outcomes to be identified for effective management measures to be taken. It is recommended that clinicians and policy makers take note of the findings from this study to inform management of PLHIV and planning as even more people are enrolled on treatment to ensure quality of care of the ART program.
Acknowledgments
The authors thank and wish to acknowledge with gratitude Ms Theresa Womegah, Ms Janet Antwi, Ms Vera Botchway and Ms Rhoda Joy Lartey who collected the data and the patients and staff of the ART clinic in Korle Bu Teaching Hospital Koforidua Central Hospital KBTH St Dominic's Hospital and Odorna Clinic for the diverse assistance and cooperation in this study. The study was supported by World Health Organization through the World Bank funded Treatment Acceleration Program.
Competing interests
The authors declare that they have no conflicts of interests.
Authors’ contributions
Nii Akwei Addo, Sally-Ann Ohene, Morkor Newman, Margaret Lartey, Francisca Zigah and TN designed the study. Sally-Ann Ohene, Francisca Zigah, Sampson Ofori, MAR and Tania Sheriff implemented the study and supervised the data collection in the sites. Tom Ndanu did the analyses with input from Nii Akwei Addo, Sally-Ann Ohene, Morkor Newman, Margaret Lartey and Francisca Zigah. The paper was drafted by Sally-Ann Ohene and Francisca Zigah with contribution from Margaret Lartey, Morkor Newman, and Nii Akwei Addo. All authors agreed on the final manuscript.
References
- 1.World Health Organization. Geneva: World Health Organization; 2003. Treat 3 Million by 2005 Initiative. Treating 3 million by 2005: making it happen: the WHO strategy: the WHO and UNAIDS global initiative to provide antiretroviral therapy to 3 million people with HIV/AIDS in developing countries by the end of 2005. [Google Scholar]
- 2.Ministry of Health. Accra: Ministry of Health/Ghana Health Service; 2006. Towards universal access to antiretroviral therapy: Ghana national ART scale up plan: 2006-2010. [Google Scholar]
- 3.National AIDS/STI Control Programme. Accra, Ghana: National AIDS/STI Control Programme; 2011. 2010 Annual Report. [Google Scholar]
- 4.World Health Organization. Geneva: World Health Organization; 2011. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. [Google Scholar]
- 5.Lowrance David, Ndamage Francois, Kayirangwa Eugenie, Ndagije Felix, et al. Adult clinical and immunologic outcomes of the national antiretroviral treatment program in Rwanda during 2004-2005. J Acquir Immune Defic Syndr. 2009;52(1):49–55. doi: 10.1097/QAI.0b013e3181b03316. [DOI] [PubMed] [Google Scholar]
- 6.Bussmann Hermann, Wester William, Ndwapi Ndwapi, Grundmann Nicolas, et al. Five-year outcomes of initial patients treated in Botswana's National Antiretroviral Treatment Program. AIDS. 2008;22(17):2303–11. doi: 10.1097/QAD.0b013e3283129db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahuerta Maria, Lima Josue, Elul Batya, Okamura Mie, et al. Patients enrolled in HIV care in Mozambique: baseline characteristics and follow-up outcomes. J Acquir Immune Defic Syndr. 2011;58(3):e75–e86. doi: 10.1097/QAI.0b013e31822ac0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nglazi Mweete, Kranzer Katharina, Holele Pearl, Kaplan Richard, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infectious Diseases. 2012;12:21. doi: 10.1186/1471-2334-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etard JF, Ndiaye I, Thierry-Mieg M, Guèye NF, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20(8):1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 10.Stringer JS, Zulu I, Levy J, Stringer EM, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 11.Howley IW, Lartey M, Machan JT, Talbot EA, et al. Highly active antiretroviral therapy and employment status in Accra, Ghana. Ghana Med J. 2010;44(4):144–149. doi: 10.4314/gmj.v44i4.68907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collini Paul, Schwab Uli, Sarfo Stephen, Obeng-Baah Joseph, et al. Sustained immunological responses to highly active antiretroviral therapy at 36 months in a Ghanaian HIV cohort. Clin Infect Dis. 2009;48(7):988–91. doi: 10.1086/597353. [DOI] [PubMed] [Google Scholar]
- 13.Ohene S, Forson E. Care of patients on anti-retroviral therapy in Kumasi Metropolis. Ghana Med J. 2009;43(4):144–149. [PMC free article] [PubMed] [Google Scholar]
- 14.National AIDS/STI Control Programme. Accra, Ghana: National HIV/ AIDS/ STI Control Programme, Ministry of Health/ Ghana Health Service; 2005. Guidelines for Anti Retro-viral Therapy in Ghana. [Google Scholar]
- 15.Rosen S, Ketlhapile M, Sanne I, Desilva MB. Characteristics of patients accessing care and treatment for HIV/AIS at public and nongovernmental sites in South Africa. J Int Assoc Physicians AIDS Care. 2008;7(4):200–7. doi: 10.1177/1545109708320684. [DOI] [PubMed] [Google Scholar]
- 16.Dako-Gyeke Phyllis, Snow Rachel, Yawson Alfred. Who is utilizing anti-retroviral therapy in Ghana: an analysis of ART service utilization. Int J Equity Health. 2012;11:62. doi: 10.1186/1475-9276-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Geneva: Version 2.0 World Health Organization; 2010. Priority intervention: HIV/AIDS prevention, treatment and care in the health sector. [Google Scholar]
- 18.Geneva: World Health Organization; 2011. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. [Google Scholar]
- 19.Boulle A, Orrel C, Kaplan R, Van Cutsem G, et al. International Epidemiological Databases to Evaluate AIDS in Southern Africa Collaboration. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12(5):753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 20.Forna F, Liechty CA, Solberg P, Asiimwe F, et al. Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2007;44(4):456–62. doi: 10.1097/QAI.0b013e318033ffa1. [DOI] [PubMed] [Google Scholar]
- 21.Sivadasan A, Abraham OC, Rupali P, Pulimood SA, et al. High rates of regimen change due to drug toxicity among a cohort of South Indian adults with HIV infection initiated on generic, first-line antiretroviral treatment. J Assoc Physicians India. 2009;57:384–8. [PubMed] [Google Scholar]
- 22.O'Brien ME, Clark RA, Besch CL, Myers L, Kissinger P. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003;34(4):407–14. doi: 10.1097/00126334-200312010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm AK, Paci G, Lartey M, Kwara A. Treatment Default among Patients Initiating HAART at the Korle-Bu Teaching Hospital in Accra, Ghana. West Afr J Med. 2012 Sep-Oct;31(4):264–9. [PubMed] [Google Scholar]
- 24.Boateng Daniel, Kwapong Golda, Agyei-Baffour Peter. Knowledge, perception about antiretroviral therapy (ART) and prevention of mother-to-child transmission (PMTCT) and adherence to ART among HIV positive women in the Ashanti Region, Ghana: a cross-sectional study. BMC Women's Health. 2013;13:2. doi: 10.1186/1472-6874-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills Edward, Nachega Jean, Bangsberg David, Singh Sonal, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zachariah R, Van Engelgem I, Massaquoi M, Kocholla L, et al. Payment for antiretroviral drugs is associated with a higher rate of patients lost to follow-up than those offered free-of-charge therapy in Nairobi, Kenya. Trans R Soc Trop Med Hyg. 2008 Mar;102(3):288–93. doi: 10.1016/j.trstmh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Huang Laurence, Crothers Kristina. HIV-associated opportunistic pneumonias. Respirology. 2009;14(4):474–485. doi: 10.1111/j.1440-1843.2009.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Focà Emanuele, Odolini Silvia, Brianese Nigritella, Carosi Giampiero. Malaria and HIV in adults: when the parasite runs into the virus. Mediterr J Hematol Infect Dis. 2012;4(1):e2012032. doi: 10.4084/MJHID.2012.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harish MR, Shanmukhappa, Kumar S, Kumar M, Gowda MS. A study of cutaneous manifestations in HIV infected persons. J Indian Med Assoc. 2012;110(10):726–7. [PubMed] [Google Scholar]
- 30.Koethe JR, Lukusa A, Giganti MJ, Chi BH, et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009;53(4):507–13. doi: 10.1097/QAI.0b013e3181b32baf. [DOI] [PMC free article] [PubMed] [Google Scholar]


