Abstract
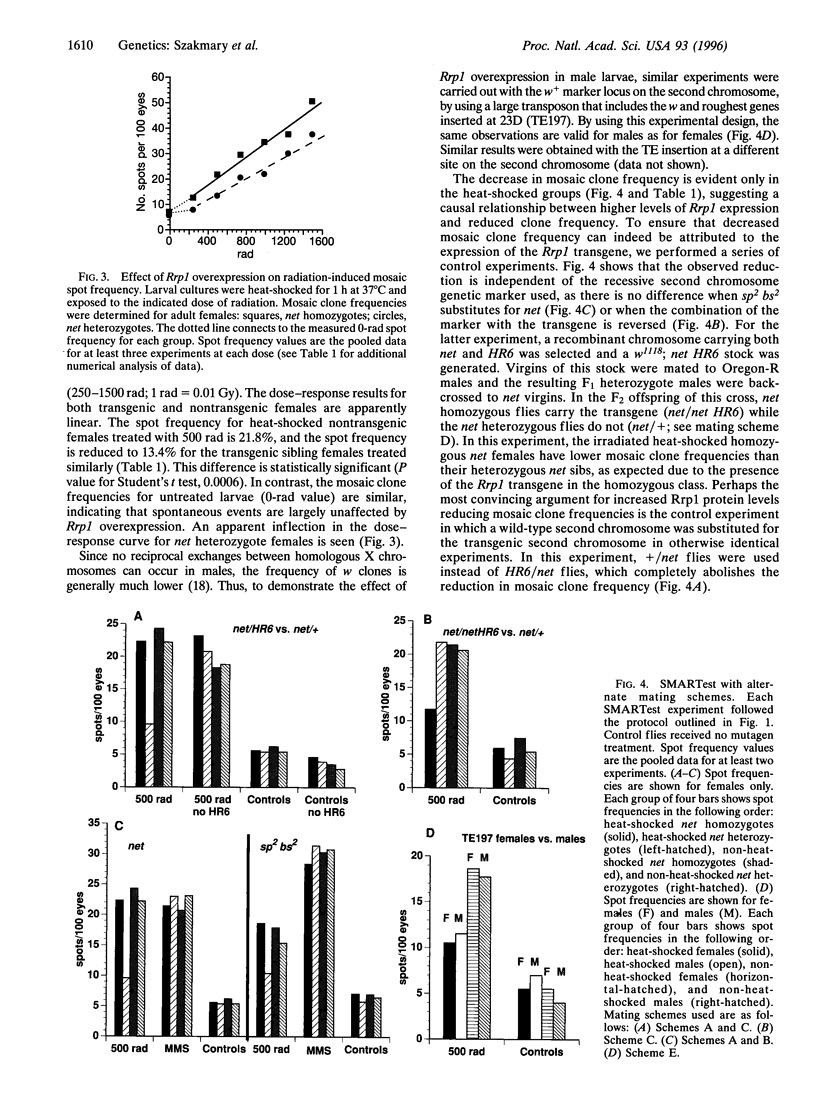
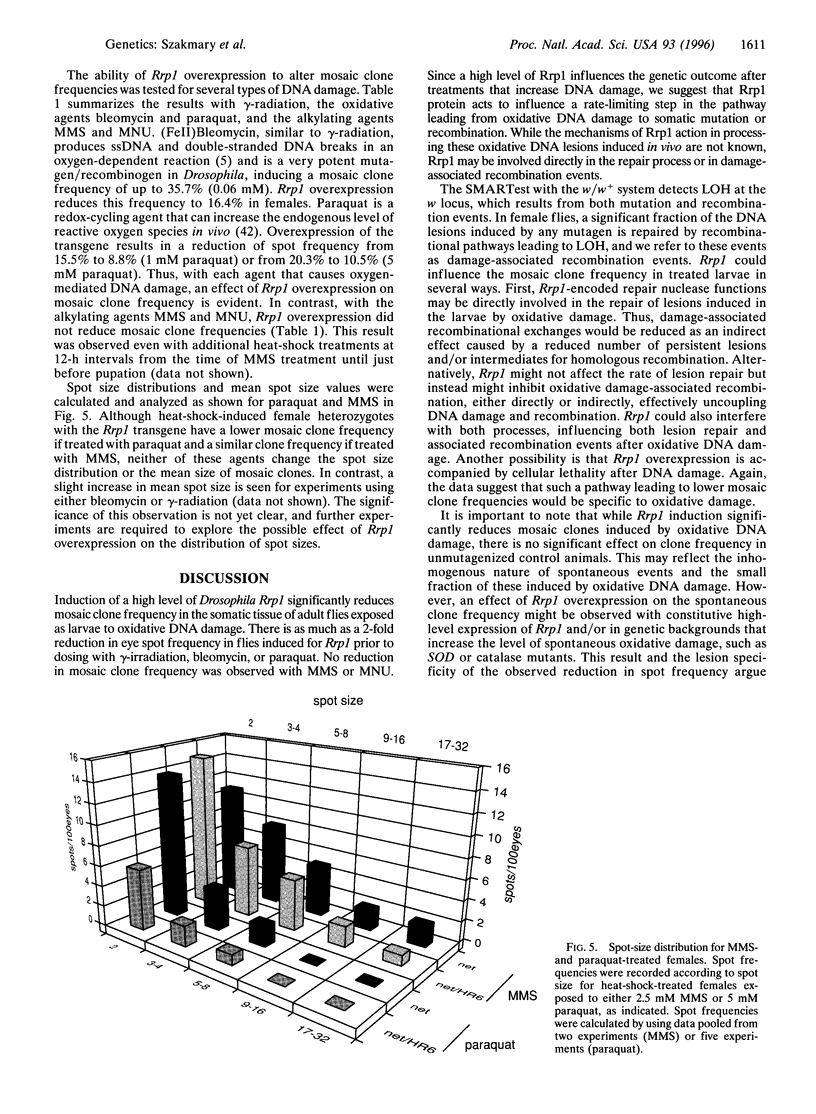
Recombination repair protein 1 (Rrp1) includes a C-terminal region homologous to several DNA repair proteins, including Escherichia coli exonuclease III and human APE, that repair oxidative and alkylation damage to DNA. The nuclease activities of Rrp1 include apurinic/apyrimidinic endonuclease, 3'-phosphodiesterase, 3'-phosphatase, and 3'-exonuclease. As shown previously, the C-terminal nuclease region of Rrp1 is sufficient to repair oxidative- and alkylation-induced DNA damage in repair-deficient E. coli mutants. DNA strand-transfer and single-stranded DNA renaturation activities are associated with the unique N-terminal region of Rrp1, which suggests possible additional functions that include recombinational repair or homologous recombination. By using the Drosophila w/w+ mosaic eye system, which detects loss of heterozygosity as changes in eye pigmentation, somatic mutation and recombination frequencies were determined in transgenic flies overexpressing wild-type Rrp1 protein from a heat-shock-inducible transgene. A large decrease in mosaic clone frequency is observed when Rrp1 overexpression precedes treatment with gamma-rays, bleomycin, or paraquat. In contrast, Rrp1 overexpression does not alter the spot frequency after treatment with the alkylating agents methyl methanesulfonate or methyl nitrosourea. A reduction in mosaic clone frequency depends on the expression of the Rrp1 transgene and on the nature of the induced DNA damage. These data suggest a lesion-specific involvement of Rrp1 in the repair of oxidative DNA damage.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER H. J. Uber Rontgenmosaikflecken und Defektmutationen am Auge von Drosophila und die Entwicklungsphysiologie des Auges. Z Indukt Abstamm Vererbungsl. 1957;88(3):333–373. [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Johnson A., Fung D. Exonuclease III and endonuclease IV remove 3' blocks from DNA synthesis primers in H2O2-damaged Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7731–7735. doi: 10.1073/pnas.83.20.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrús A., Garcia-Bellido A. Morphogenetic mutants detected in mitotic recombination clones. Nature. 1976 Apr 1;260(5550):425–426. doi: 10.1038/260425a0. [DOI] [PubMed] [Google Scholar]
- Frei H., Würgler F. E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat Res. 1995 Apr;334(2):247–258. doi: 10.1016/0165-1161(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Graf U., Würgler F. E., Katz A. J., Frei H., Juon H., Hall C. B., Kale P. G. Somatic mutation and recombination test in Drosophila melanogaster. Environ Mutagen. 1984;6(2):153–188. doi: 10.1002/em.2860060206. [DOI] [PubMed] [Google Scholar]
- Gu L., Huang S. M., Sander M. Drosophila Rrp1 complements E. coli xth nfo mutants: protection against both oxidative and alkylation-induced DNA damage. Nucleic Acids Res. 1993 Oct 11;21(20):4788–4795. doi: 10.1093/nar/21.20.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Huang S. M., Sander M. Single amino acid changes alter the repair specificity of Drosophila Rrp1. Isolation of mutants deficient in repair of oxidative DNA damage. J Biol Chem. 1994 Dec 23;269(51):32685–32692. [PubMed] [Google Scholar]
- Jowett T., Wajidi M. F., Oxtoby E., Wolf C. R. Mammalian genes expressed in Drosophila: a transgenic model for the study of mechanisms of chemical mutagenesis and metabolism. EMBO J. 1991 May;10(5):1075–1081. doi: 10.1002/j.1460-2075.1991.tb08047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Henson E. S., Roche H., Ramotar D., Nunoshiba T., Demple B. Specificity of the mutator caused by deletion of the yeast structural gene (APN1) for the major apurinic endonuclease. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8165–8169. doi: 10.1073/pnas.91.17.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laval F. Expression of the E. coli fpg gene in mammalian cells reduces the mutagenicity of gamma-rays. Nucleic Acids Res. 1994 Nov 25;22(23):4943–4946. doi: 10.1093/nar/22.23.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Merriam J. R., Garcia-Bellido A. A model for somatic pairing derived from somatic crossing over with third chromosome rearrangements in Drosophila melanogaster. Mol Gen Genet. 1972;115(4):302–313. doi: 10.1007/BF00333169. [DOI] [PubMed] [Google Scholar]
- Minakami H., Fridovich I. Relationship between growth of Escherichia coli and susceptibility to the lethal effect of paraquat. FASEB J. 1990 Nov;4(14):3239–3244. doi: 10.1096/fasebj.4.14.2172063. [DOI] [PubMed] [Google Scholar]
- Morata G., Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975 Feb;42(2):211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Nugent M., Huang S. M., Sander M. Characterization of the apurinic endonuclease activity of Drosophila Rrp1. Biochemistry. 1993 Oct 26;32(42):11445–11452. doi: 10.1021/bi00093a023. [DOI] [PubMed] [Google Scholar]
- Ono Y., Furuta T., Ohmoto T., Akiyama K., Seki S. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat Res. 1994 Jul;315(1):55–63. doi: 10.1016/0921-8777(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Ripoll P. Behavior of somatic cells homozygous for zygotic lethals in Drosophila melanogaster. Genetics. 1977 Jun;86(2 Pt 1):357–376. [PMC free article] [PubMed] [Google Scholar]
- Ripoll P., García-Bellido A. Viability of Homozygous Deficiencies in Somatic Cells of DROSOPHILA MELANOGASTER. Genetics. 1979 Mar;91(3):443–453. doi: 10.1093/genetics/91.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Ryo H., Yoo M. A., Fujikawa K., Kondo S. Comparison of somatic reversions between the ivory allele and transposon-caused mutant alleles at the white locus of Drosophila melanogaster after larval treatment with X rays and ethyl methanesulfonate. Genetics. 1985 Jul;110(3):441–451. doi: 10.1093/genetics/110.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sander M., Carter M., Huang S. M. Expression of Drosophila Rrp1 protein in Escherichia coli. Enzymatic and physical characterization of the intact protein and a carboxyl-terminally deleted exonuclease-deficient mutant. J Biol Chem. 1993 Jan 25;268(3):2075–2082. [PubMed] [Google Scholar]
- Sander M., Huang S. M. Characterization of the nuclease activity of Drosophila Rrp1 on phosphoglycolate- and phosphate-modified DNA 3'-termini. Biochemistry. 1995 Jan 31;34(4):1267–1274. doi: 10.1021/bi00004a021. [DOI] [PubMed] [Google Scholar]
- Sander M., Lowenhaupt K., Lane W. S., Rich A. Cloning and characterization of Rrp1, the gene encoding Drosophila strand transferase: carboxy-terminal homology to DNA repair endo/exonucleases. Nucleic Acids Res. 1991 Aug 25;19(16):4523–4529. doi: 10.1093/nar/19.16.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M., Lowenhaupt K., Rich A. Drosophila Rrp1 protein: an apurinic endonuclease with homologous recombination activities. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6780–6784. doi: 10.1073/pnas.88.15.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Szabad J., Würgler F. E. A genetic assay to detect chromosome gain and/or loss in somatic cells of Drosophila melanogaster. Mutat Res. 1987 Oct;180(2):201–206. doi: 10.1016/0027-5107(87)90215-6. [DOI] [PubMed] [Google Scholar]
- Vogel E. W. Evaluation of potential mammalian genotoxins using Drosophila: the need for a change in test strategy. Mutagenesis. 1987 May;2(3):161–171. doi: 10.1093/mutage/2.3.161. [DOI] [PubMed] [Google Scholar]
- Vogel E. W., Nivard M. J. Performance of 181 chemicals in a Drosophila assay predominantly monitoring interchromosomal mitotic recombination. Mutagenesis. 1993 Jan;8(1):57–81. doi: 10.1093/mutage/8.1.57. [DOI] [PubMed] [Google Scholar]
- Vogel E. W. Tests for recombinagens in somatic cells of Drosophila. Mutat Res. 1992 Dec 1;284(1):159–175. doi: 10.1016/0027-5107(92)90030-6. [DOI] [PubMed] [Google Scholar]
- Vogel E. W., Zijlstra J. A. Mechanistic and methodological aspects of chemically-induced somatic mutation and recombination in Drosophila melanogaster. Mutat Res. 1987 Oct;182(5):243–264. doi: 10.1016/0165-1161(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Walker L. J., Craig R. B., Harris A. L., Hickson I. D. A role for the human DNA repair enzyme HAP1 in cellular protection against DNA damaging agents and hypoxic stress. Nucleic Acids Res. 1994 Nov 25;22(23):4884–4889. doi: 10.1093/nar/22.23.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]