SUMMARY
Background
Immunomodulator medications (IMM) play a vital role in the care of patients with inflammatory bowel disease (IBD). IBD practice guidelines recommend myelosuppression monitoring after initiation of IMM.
Aim
To identify adherence rates and predictors of myelosuppression monitoring after IMM initiation in a large practice setting.
Methods
We identified a national cohort of VA users with IBD for the fiscal years 2003–2009 using the Veterans Affairs administrative datasets. Subjects with filled prescriptions for IMM were included. The primary endpoint was the proportion of subjects who had a white blood cell (WBC) test completed within 90 days of the IMM index date. Determinants of myelosuppression monitoring were identified by univariate and multivariate analyses.
Results
A total of 6045 unique IBD patients were identified with filled IMM prescriptions. Overall, only 57% of subjects completed a WBC test within 90 days of IMM index date. Monitoring rates increased over time, from 48% in 2003 to 75% in 2009. There was variability of monitoring rates by facility, ranging from 0 to 83%. In multivariate analyses, older age at IMM index date was associated with a lower rate of monitoring. Frequency of VA encounters and IMM index date were associated with increased rates of myelosuppression monitoring.
Conclusions
Monitoring for myelosuppression among veterans with inflammatory bowel disease after immunomodulator medications initiation is low with wide variability based on facility. This may reflect a low quality of care among veterans with IBD. Provider- and system-wide interventions are needed to improve adherence and reduce variability of immunomodulator medications monitoring across facilities.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are collectively referred to as inflammatory bowel diseases (IBD) and result in a chronic inflammation of the digestive system. Immunosuppressive medications are the mainstay of therapy of patients with moderate-to-severe IBD. Corticosteroids are fast acting and efficacious at achieving rapid response and remission in IBD, but are associated with complications, such as hyperglycemia, osteoporosis, osteonecrosis, infection and increased risk of mortality.1, 2 Immunomodulator medications (IMM) such as azathioprine (AZA), mercaptopurine (MP) and methotrexate (MTX) play a vital role in the care of patients with IBD as steroid-sparing agents.2, 3 However, immunomodulators are associated with myelosuppression, which has been reported in up to 27% of patients, with an incidence of severe leukopenia in 0.16 cases per 100 person months.4–6 Although IMM-associated myelosuppression may occur at any time, the risk appears greatest in the first several weeks after initiation.6, 7
Risk of IMM-related myelosuppression may be predicted in a subset of patients who demonstrate low activity or genetic mutation in the enzyme thiopurine methyltransferase (TPMT).7 However, myelosuppression may still occur in patients with normal TPMT activity and genotype.7, 8 Therefore, monitoring for myelosuppression in patients receiving IMM is recommended by the package insert, the Food and Drug Administration and multiple professional societies in the form of practice guidelines (Table 1).2, 3, 9–11 Although there are differences among the guidelines in the specific frequency of monitoring, there is consensus that monitoring for myelosuppression after IMM initiation should occur.2, 3, 9–11
Table 1.
Practice guidelines of myelosuppression monitoring after immunomodulator initiation
| American College of Gastroenterology10 | ‘Routine monitoring of complete blood count, initially every 1–2 weeks, then, at least every 3 months is recommended to avoid the risk of acute or delayed bone marrow suppression.’ |
| American Gastroenterology Association11 | ‘When initiating therapy with either 6-MP or ZA, measurement of complete blood count with differential is advocated at least every other week as long as doses of medications are being adjusted. Thereafter, the measurement of complete blood count with differential should be performed as clinically appropriate at least once every 3 months. Periodic measurement of liver-associated chemistries is also advocated. (Grade C)’. |
| British Society of Gastroenterology9 | ‘Manufacturers recommend weekly full blood counts (FBCs) for the first 8 weeks of therapy followed by blood tests atleast every 3 months. There is no evidence that this is effective. One fairly common practice is to perform a full blood count every 2–4 weeks for 2 months and then every 4–8 weeks.’ |
| World Gastroenterology Organization2 | ‘Before starting AZA or 6MP measuring thiopurine methyl transferase levels (TPMT) phenotype (enzyme levels) or genotype will help direct dosing and if enzyme levels very low then risk may be too high to use these drugs. Where this testing is not available CBC needs to be obtained at 2 weeks, 4 weeks, and every 4 weeks thereafter. Even where this testing is available monthly CBCs still indicated.’ |
Despite consensus that monitoring for myelosuppression should occur, actual practice patterns of monitoring after IMM initiation have not been reported. Variation in practice patterns, suggesting either over- or underutilisation of resources, may serve as a quality of care indicator.12 Identification of practice pattern variations and predictors of adherence may provide important information to improve the quality of care of IBD patients. The aim of this study was to evaluate the prevalence and determinants of myelosuppression monitoring after IMM initiation among a national cohort of veterans with IBD receiving care in the VA.
MATERIALS AND METHODS
Data sources
We used the Veterans Health Administration National Patient Care Database for the fiscal years 2003–2009 (October 2002 to September 2009), specifically the Inpatient and Outpatient Medical SAS datasets. The Inpatient and Outpatient Medical SAS datasets contain individual-level data from inpatient and outpatient encounters, respectively, with information on demographics (age, gender, ethnicity), encounters or visits (date, frequency), diagnosis codes [International Classification of Diseases, 9th Revision, (ICD-9)], and medical and surgical procedure codes (Current Procedural Terminology).
The Veterans Health Administration Decision Support System compiles clinical and fiscal data, including pharmacy and select laboratory studies performed in the VA system, into National Data Extracts. Both laboratory and pharmacy utilisation data were collected in National Data Extracts starting in fiscal year 2002.
IBD IMM user case definition
VA users with IBD were identified by ICD-9 diagnosis codes for CD (555.x) or UC (556.x) that were recorded on at least two VA encounters from the fiscal year 1998 to 2009. We required at least one code to occur from an outpatient encounter as previously described and validated in VA datasets with a positive predictive value of 86% for UC.13 ICD-9 codes for CD using VA data have also been validated to have a positive predictive value of 88%.14 VA users with ICD-9 codes for both CD and UC were classified as indeterminate colitis (IC). Among VA users with IBD, IMM users were defined as having at least one filled prescription of AZA, MP, thioguanine (TG), or MTX, in VA pharmacy data from the fiscal year 2003 to 2010. VA pharmacy data were available starting in fiscal year 2002. The date of the first IMM filled prescription was defined as the IMM index date. We excluded patients who were on IMM maintenance therapy at the time of IMM index date, defined as a filled prescription for IMM prior to fiscal year 2003. Additionally, subjects who died within 90 days of IMM index date, had no laboratory results, or had less than a 30 day supply of IMM in Veterans Health Administration Decision Support System were excluded from the analysis.
Myelosuppression monitoring definition
The primary endpoint for analyses was the completion of at least one white blood cell (WBC) test within 90 days of index IMM date based on the least stringent interpretation of practice guidelines.2, 3, 9–11 WBC testing was identified through Veterans Health Administration Decision Support System within 90 days after IMM index date. Frequency of WBC monitoring within 30 days after IMM index date was evaluated as a secondary outcome.
Statistical analyses
The myelosuppression monitoring rate was calculated as the number of IBD IMM users who had a WBC completed within 90 days of the IMM index date divided by the total number of IBD IMM users. Determinants of myelosuppression monitoring (outcome variable) were identified by univariate and multivariate analyses, including race, gender, geographic region, concomitant IBD medications, frequency of VA encounters, facility load of IBD users on IMM, fiscal year of IMM index, IBD type, co-morbidities (Deyo score) and VA priority level. Concomitant IBD medication use was defined as a filled prescription in VA pharmacy data for anti-tumor necrosis factor medication, steroid, aminosalicylate, or antibiotic (quinolone, metronidazole) within 90 days of IMM index date. Variables with a P-value of less than 0.1 were included in multivariate logistic regression models. Pearson correlation coefficient was used to evaluate for correlation between myelosuppression monitoring and facility IBD IMM user patient volume.
Sensitivity analyses were performed for rates and determinants of myelosuppression monitoring based on VA priority level, a surrogate for socioeconomic status, to indirectly evaluate for health resource utilisation outside the VA, and for thiopurine users (AZA, MP and TG) alone.
ETHICAL CONSIDERATIONS
This study was approved by the Institutional Review Boards of Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas.
RESULTS
A total of 6045 VA users were identified as patients with IBD who used IMM in fiscal years 2003–2009 (Table 1). This cohort consisted of 2882 cases of CD (48%), 2276 cases of UC (39%), and 787 cases of IC (13%). IMM users were 92% male, 73% Caucasian, 11% African American, 3% Hispanic, 2% other, and 11% in whom race could not be identified (Table 2). Mean age at time of IMM index date was 55.5 ± 15.5. The overall myelosuppression monitoring rate within 90 days was 57%, and increased steadily over time from 48% in 2003 to 75% in 2010. Two per cent of subjects who received WBC testing within 90 days were admitted to an inpatient facility at the time of testing.
Table 2.
Demographic characteristics of inflammatory bowel disease immunomodulator users
| Number (%) |
WBC within 90 days (%) |
P-value | |
|---|---|---|---|
| Total | 6045 | 56.9 | |
| IBD type | <0.01 | ||
| Crohn’s disease | 2882 (48) | 55.9 | |
| Ulcerative colitis | 2376 (39) | 54.0 | |
| Indeterminate colitis | 787 (13) | 69.3 | |
| Race | <0.01 | ||
| Caucasian | 4438 (73) | 57.7 | |
| African American | 636 (11) | 65.6 | |
| Hispanic | 185 (3) | 60.5 | |
| Other | 112 (2) | 63.4 | |
| Unknown | 674 (11) | 41.3 | |
| Gender | <0.01 | ||
| Male | 5573 (92) | 56.2 | |
| Female | 472 (8) | 64.6 | |
| Age at IMM index | <0.01 | ||
| <35 | 816 (14) | 62.0 | |
| 35–49 | 1164 (19) | 60.7 | |
| 50–64 | 2372 (39) | 61.3 | |
| 65+ | 1693 (28) | 45.5 |
Patient-level factors
In univariate analyses, African Americans were significantly more likely than patients of other races to have received myelosuppression monitoring (Table 3); however, the significance of this effect did not persist in the multivariate analyses. Similarly, females were more likely to have received myelosuppression monitoring (65% vs. 56%, P < 0.01) compared with males, but this effect did not persist on multivariate analyses. Subjects who were 65 years or older at the time of IMM index were less likely to receive myelosuppression monitoring compared with those less than 35 years of age at IMM index (46% vs. 62%; P < 0.01). This association remained significant after multivariate analyses [odds ratio (OR) = 0.73; 95% CI: 0.59–0.90]. Subjects who were on concomitant IBD medications were significantly more likely to have received myelosuppression monitoring (Table 2). Subjects with low VA priority level (i.e. high income) were less likely to have received a WBC in the VA (42%) compared with subjects with high VA priority level (61%). In univariate analyses, low VA priority level was associated with lower monitoring rates (OR 0.46, 95% CI: 0.41–0.52); however, this effect was not significant on multivariate analyses. There were no differences in monitoring rates between CD and UC patients or co-morbidity (Deyo score).
Table 3.
Univariate and multivariate logistic regression of determinants for myelosuppression monitoring after immunomodulator initiation
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds Ratio |
95% Wald Confidence limits |
Odds Ratio |
95% Wald Confidence limits |
|
| Patient-level variables | ||||
| Race | ||||
| Caucasian | Ref | Ref | ||
| African American | 1.40 | (1.17–1.66) | 1.03 | (0.84–1.25) |
| Hispanic | 1.13 | (0.83–1.52) | 0.74 | (0.59–1.05) |
| Other | 1.27 | (0.86–1.87) | 1.04 | (0.67–1.60) |
| Unknown | 0.52 | (0.44–0.61) | 0.93 | (0.76–1.13) |
| Age at IMM index (years) | ||||
| <35 | Ref | Ref | ||
| 35–49 | 0.95 | (0.79–1.14) | 0.94 | (0.76–1.16) |
| 50–64 | 0.97 | (0.82–1.14) | 0.83 | (0.69–1.01) |
| ≥65 | 0.51 | (0.43–0.61) | 0.73 | (0.59–0.90) |
| IBD type | ||||
| Crohn’s disease | Ref | Ref | ||
| Ulcerative colitis | 0.93 | (0.83–1.03) | 1.04 | (0.91–1.18) |
| Indeterminate colitis | 1.78 | (1.50–2.10) | 1.48 | (1.22–1.79) |
| Concomitant medications | ||||
| Anti-tumor necrosis factor | 2.98 | (2.37–3.75) | 1.59 | (1.24–2.03) |
| Steroid | 2.83 | (2.54–3.14) | 1.76 | (1.55–1.98) |
| Aminosalicylate | 1.31 | (1.18–1.46) | 1.16 | (1.02–1.32) |
| Antibiotic | 2.64 | (2.28–3.04) | 1.53 | (1.30–1.80) |
| Co-morbidity (Deyo score ≥3 vs. <3) | 1.73 | (1.40–2.14) | 1.23 | (0.97–1.56) |
| VA priority level* | 0.46 | (0.41–0.52) | 0.88 | (0.75–1.03) |
| Facility-level variables | ||||
| Fiscal year of IMM Index (2003–2009) | 1.15 | (1.13–1.18) | 1.12 | (1.09–1.15) |
| VA encounter frequency (No.) | ||||
| <3 | Ref | Ref | ||
| 3–6 | 4.94 | (3.48–7.00) | 4.96 | (3.48–7.07) |
| 7–12 | 12.60 | (8.99–17.67) | 10.47 | (7.42–14.78) |
| ≥13 | 33.87 | (24.40–47.01) | 22.48 | (6.03–31.52) |
| Facility IBD IMM volume (No. of patients) | ||||
| <11 | Ref | Ref | ||
| 12–21 | 2.31 | (1.48–3.62) | 2.48 | (1.48–4.17) |
| 22–33 | 2.07 | (1.37–3.15) | 1.69 | (1.05–2.73) |
| 34–47 | 3.02 | (2.02–4.51) | 2.51 | (1.59–3.99) |
| ≥48 | 2.84 | (1.93–4.19) | 2.18 | (1.40–3.39) |
| Geographic region of VA facility | ||||
| Midwest | Ref | Ref | ||
| Northeast | 0.82 | (0.70–0.97) | 0.95 | (0.78–1.16) |
| South | 0.93 | (0.82–1.06) | 0.88 | (0.75–1.02) |
| West | 1.02 | (0.87–1.19) | 0.89 | (0.74–1.07) |
| Puerto Rico, Virgin Islands | 1.58 | (0.55–4.57) | 0.66 | (0.20–2.13) |
IMM, immunomodulator; IBD, inflammatory bowel disease; Ref, reference.
Surrogate for socioeconomic status.
Facility-level factors
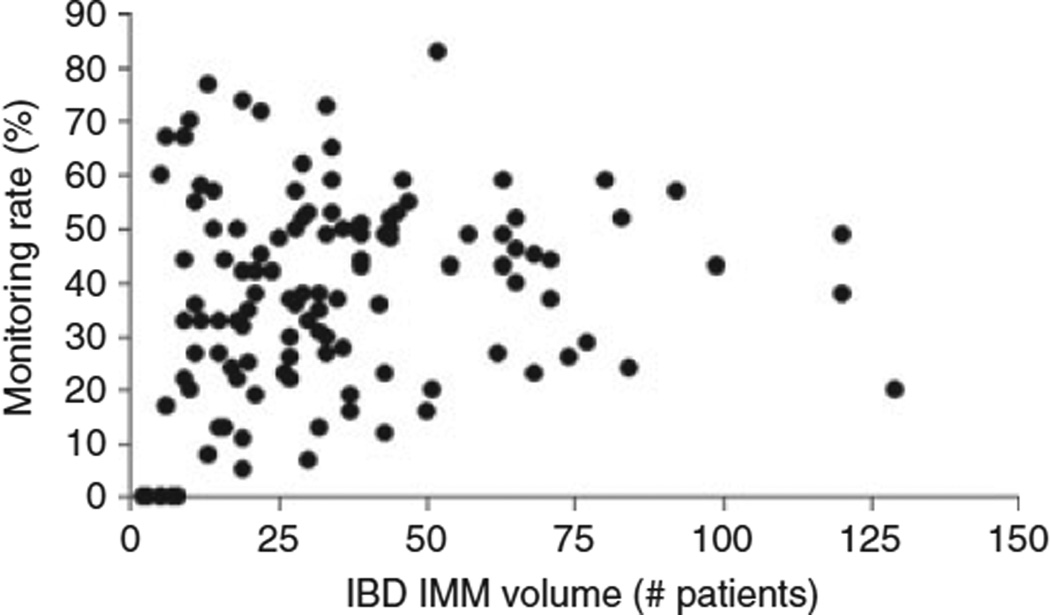
There were no significant differences in monitoring rates based on geographical location of treating VA facility. Frequency of VA encounters was strongly associated with myelosuppression monitoring, with monitoring rates increasing with increasing number of VA encounters. The median number of IBD patients who were IMM users per facility was 34, ranging from 2 to 129 patients. Patients in facilities with very low (<11) IBD IMM patient volume had less frequent WBC monitoring compared with patients in facilities with higher IBD IMM volume. However, myelosuppression monitoring rates did not show a linear or dose–response relationship with IBD IMM volume at facilities with more than 11 patients (Figure 1). There was a high variability in myelosuppression monitoring across facilities from 0 to 83% (interquartile range: 28.25), which did not correlate with IBD IMM patient volume (r = −0.07).
Figure 1.
Myelosuppression monitoring rates by facility. Myelosuppression monitoring rates varied greatly based on facility with no correlation between the volume of inflammatory bowel disease patients on immunomodulators and monitoring rates.
Sensitivity analyses
Secondary endpoint of myelosuppression monitoring within 30 days of IMM index resulted in an overall myelosuppression monitoring rate of 35%. Predictors were similar to the main analysis with the following exceptions: Concomitant biologic and aminosalicylate use was no longer a predictor of monitoring (OR = 1.07; 95% CI: 0.87–1.32 and OR = 1.11; 95% CI: 0.97–1.26 respectively) and higher co-morbidity score was predictive of monitoring (OR = 1.25 95% CI: 1.00–1.56). Analyses of users of only AZA, MP and TG showed a similar myelosuppression monitoring rate of 56.8% within 90 days.
To evaluate the potential bias of using non-VA facilities, we performed a sensitivity analysis in which we limited the evaluation to only subjects with high VA priority level, a surrogate for low socioeconomic status, which has been previously reported to predict low probability of non-VA healthcare utilisation.15 Concomitant IBD medications, VA encounter frequency, facility load greater than 11 IBD IMM users and IMM index date remained significant predictors of myelosuppression monitoring.
DISCUSSION
This is the first study to report screening rates for IMM-associated myelosuppression in a national cohort of patients with IBD. Myelosuppression monitoring after IMM initiation was low, only 57% across the study period even with the most loose interpretation of monitoring recommendations (i.e. WBC within 90 days). Higher VA encounter frequency, younger age at IMM index, concomitant IBD medication use and more recent year of IMM index date were positive predictors of WBC monitoring. Monitoring rates varied greatly between individual VA facilities. Patients at facilities with very low IBD IMM volume were less likely to be monitored for WBC, but there was no linear or dose–response relationship between IBD IMM patient volume and myelosuppression monitoring rates. The absence of modifiable or meaningful patient factors that are associated with low quality of care and the remarkable inter-facility variation collectively point to system-related factors as well as potential solutions.
We a priori chose a 90-day WBC monitoring endpoint to accommodate the variation in guideline recommendations and real-world practice conditions (Table 1). Using a 30-day time frame, which is close to several guideline recommendations, the monitoring rate was only 35%. The increase in monitoring rates during the most recent years of the study period is encouraging, yet reflects low adherence to guideline recommended care.
The strongest predictor of myelosuppression monitoring was higher frequency of VA encounters. Similarly, subjects on concomitant IBD medications of any kind were more likely to be screened. These two variables are indicators of subjects who may already have high resource utilisation and therefore were more likely to have had a WBC performed. The association of higher encounter/visit frequency and concomitant IBD medication use with monitoring patterns was also observed in a study of colonoscopy surveillance patterns among IBD patients in a large managed care practice.16 In univariate analyses, we observed lower WBC monitoring rates among low VA priority (high income) subjects, although this effect did not persist in multivariate analyses. We believe this observation reflects potential non-VA laboratory testing and not a true determinant of monitoring rates. We observed lower WBC monitoring rates among those patients over 65 years of age at IMM index compared with younger patients. Although this effect persisted even when adjusting for priority level, a predictor of non-VA healthcare utilisation, it in part reflects Medicare-eligible patients receiving a portion of care outside the VA system. Although VA users of high priority (low income) may be eligible for Medicaid, there are data (non-IBD-related) showing that Medicaid eligible VA users remain highly likely to use VA services.24
In our study, we observed large variations in WBC screening rates (0–83%) according to VA facility. Part of the inter-facility variation may be explained by facility-level IBD volume. Facilities with very low counts of IBD patients who are IMM users (less than 11) were less likely to screen patients. However, among VA facilities with higher patient volumes, there was no significant association between WBC screening rates and facility total patient volume, IBD volume, or IBD IMM volume. The wide practice variation by facility combined with the few patient-related factors implies the presence of general provider or facility-level barriers to adherence to guidelines.
Variations in IBD practice patterns were previously reported in screening for osteoporosis, tuberculosis and colorectal cancer. Wagnon et al. found that only 49% of 304 surveyed gastroenterologists followed AGA guidelines on osteoporosis screening in patients with IBD.17 Similarly, in a study from France, only 25% of 46 gastroenterologists surveyed in practice reported appropriate assessment prior to initiation of anti-tumor necrosis factor medications.18 In a large managed healthcare organisation, surveillance colonoscopy was performed on only 24.6% of eligible patients with IBD.16 Conversely, in a referral hospital in Canada, 90% of patients with UC had undergone surveillance colonoscopy.19 These examples highlight the disconnect between practice guidelines and clinical practice and implies sub-optimal quality of care across various aspects of IBD care. Variations in practice patterns and adherence to guideline recommendations have been similarly used to assess quality of care in patients with cirrhosis and hepatitis C in the VA.20, 21 Our study is the first to report on the practice patterns related to monitoring for myelosuppression after IMM initiation and the first to evaluate practice according to IBD guideline at a national level.
Uniform guidelines among different societies and professional organisations may serve as one approach to improve adherence to recommended care in IBD. The Barriers to Physician Adherence to Practice Guidelines model proposed by Cabana provides a framework to approach adherence to guidelines in IBD. Barriers to adherence may be classified in relationship to physician (i) knowledge; (ii) attitudes; and (iii) external barriers.22 Lack of consistency in practice guidelines contributes to all three barrier domains.
This study has limitations inherent to an administrative dataset. We used a diagnostic algorithm of ICD-9 codes used previously and validated for IBD within the VA with PPV 86% for UC and 88% for CD, similar to that derived in other administrative databases.13, 14, 23 However, IMM index dates have not been validated to reflect initiation of IMM as opposed to subjects on maintenance IMM, which may affect practice patterns of myelosuppression monitoring. We included a washout period of one fiscal year of pharmacy data availability to reduce the likelihood of including subjects on maintenance IMM. The VA is an integrated healthcare system where laboratory and prescription data at any VA facility is included in our dataset. However, healthcare services provided outside the VA are not captured. As specialty IBD care may be referred out of the VA system, our findings may reflect underreporting of true monitoring practices. We performed a sensitivity analysis excluding low VA priority patients who are most likely to afford and receive care outside the VA, which did not change our conclusions. Our endpoint was defined as completed WBC testing as indicated by test results, but we are unable to determine if non-adherence was due to lack of physician ordering or of lack of patient follow-up. Data regarding characteristics of individual providers were unavailable. Therefore, data were analysed at the facility level. Lastly, our results reflect the VA IBD population and may not be representative of the non-VA IBD population in age, gender or ethnic distribution.
In conclusion, myelosuppression monitoring among veterans with IBD after IMM initiation is low in the VA with wide variability based on VA facility. IBD volume per facility may explain only a small part of the variation. Patient factors including frequency of healthcare encounters and concomitant IBD medications were predictors of monitoring; however, there were no linear or dose–response relationships between facility IBD IMM patient volume and monitoring. Unifying the recommended published guidelines with provider- and system-wide interventions is needed to improve adherence and reduce variability of IMM monitoring across facilities.
ACKNOWLEDGEMENTS
Jennifer R. Kramer contributed to study design, data analysis and editorial input in the manuscript. She has approved the final draft submitted. Peter Richardson and Shubhada Sansgiry contributed to study design, programming, data abstraction, data analysis and editorial input in the manuscript. They have approved the final draft submitted. Hashem El-Serag contributed to study design, data interpretation and editorial input in the manuscript. He has approved the final draft submitted. Declaration of funding interests: The research reported here was supported in part by the American College of Gastroenterology Junior Faculty Development Award to J. K. Hou and by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service, grant MRP05-305 to J. R. Kramer. No writing assistance was used in the preparation of this manuscript.
Footnotes
Jason Hou contributed to study design, data analysis and primary authorship of manuscript. He has approved the final draft submitted.
Declaration of personal interests: Jason Hou has served as a speaker for UCB pharmaceuticals and has received research funding from Aptalis pharmaceuticals.
REFERENCES
- 1.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1051–1063. doi: 10.1038/ajg.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010;16:112–124. doi: 10.1002/ibd.21048. [DOI] [PubMed] [Google Scholar]
- 3.van Asseldonk DP, Sanderson J, de Boer NK, Sparrow MP, et al. Difficulties and possibilities with thiopurine therapy in inflammatory bowel disease–proceedings of the first Thiopurine Task Force meeting. Dig Liver Dis. 2011;43:270–276. doi: 10.1016/j.dld.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081–1085. doi: 10.1136/gut.34.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783–1800. doi: 10.1111/j.1572-0241.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JD, Abramson O, Pascua M, et al. Timing of myelosuppression during thiopurine therapy for inflammatory bowel disease: implications for monitoring recommendations. Clin Gastroenterol Hepatol. 2009;7:1195–1201. doi: 10.1016/j.cgh.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombel JF, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000;118:1025–1030. doi: 10.1016/s0016-5085(00)70354-4. [DOI] [PubMed] [Google Scholar]
- 8.González-Lama Y, Bermejo F, López-Sanromán A, et al. Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) Thiopurine methyl-transferase activity and azathioprine metabolite concentrations do not predict clinical outcome in thiopurine-treated inflammatory bowel disease patients. Aliment Pharmacol Ther. 2011;34:544–554. doi: 10.1111/j.1365-2036.2011.04756.x. [DOI] [PubMed] [Google Scholar]
- 9.Mowat C, Cole A, Windsor A, et al. IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Hanauer SB. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein GR, Abreu MT, Cohen R American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Berwick DM. Controlling variation in health care: a consultation from Walter Shewhart. Med Care. 1991;29:1212–1225. doi: 10.1097/00005650-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hou JK, Kramer JR, Richardson P, et al. Risk of colorectal cancer among Caucasian and African American veterans with ulcerative colitis. Inflamm Bowel Dis. 2012;18:1011–1017. doi: 10.1002/ibd.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thirumurthi S, Chowdhury R, Richardson P, et al. Validation of ICD-9-CM diagnostic codes for inflammatory bowel disease among veterans. Dig Dis Sci. 2010;55:2592–2598. doi: 10.1007/s10620-009-1074-z. [DOI] [PubMed] [Google Scholar]
- 15.Morgan RO, Petersen LA, Hasche JC, et al. VHA pharmacy use in veterans with Medicare drug coverage. Am J Manag Care. 2009;15:e1–e8. [PubMed] [Google Scholar]
- 16.Velayos FS, Liu L, Lewis JD, Allison JE, et al. Prevalence of colorectal cancer surveillance for ulcerative colitis in an integrated health care delivery system. Gastroenterology. 2010;139:1511–1518. doi: 10.1053/j.gastro.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Wagnon JH, Leiman DA, Ayers GD, et al. Survey of gastroenterologists’ awareness and implementation of AGA guidelines on osteoporosis in inflammatory bowel disease patients: are the guidelines being used and what are the barriers to their use? Inflamm Bowel Dis. 2009;15:1082–1089. doi: 10.1002/ibd.20857. [DOI] [PubMed] [Google Scholar]
- 18.Peyrin-Biroulet L, Oussalah A, Boucekkine T, et al. TNF antagonists in the treatment of inflammatory bowel disease: results of a survey of gastroenterologists in the French region of Lorraine. Gastroenterol Clin Biol. 2009;33:23–30. doi: 10.1016/j.gcb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Kottachchi D, Yung D, Marshall JK. Adherence to guidelines for surveillance colonoscopy in patients with ulcerative colitis at a Canadian quaternary care hospital. Can J Gastroenterol. 2009;23:613–617. doi: 10.1155/2009/691850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanwal F, Hoang T, Kramer J, et al. The performance of process measures in hepatitis C. Am J Gastroenterol. 2012 doi: 10.1038/ajg.2012.201. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the department of veterans affairs. Gastroenterology. 2012;143:70–77. doi: 10.1053/j.gastro.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 23.Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis. 2007;13:451–461. doi: 10.1002/ibd.20021. [DOI] [PubMed] [Google Scholar]
- 24.French DD, Margo CE. Factors associated with the utilization of cataract surgery for veterans dually enrolled in Medicare. Mil Med. 2012;177:752–756. doi: 10.7205/milmed-d-12-00046. [DOI] [PubMed] [Google Scholar]