Abstract
Opiate analgesia in the spinal cord is impaired during neuropathic pain. We hypothesized that this is caused by a decrease in μ-opioid receptor inhibition of neurotransmitter release from primary afferents. To investigate this possibility, we measured substance P release in the spinal dorsal horn as neurokinin 1 receptor (NK1R) internalization in rats with chronic constriction injury (CCI) of the sciatic nerve. Noxious stimulation of the paw with CCI produced inconsistent NK1R internalization, suggesting that transmission of nociceptive signals by the injured nerve was variably impaired after CCI. This idea was supported by the fact that CCI produced only small changes in the ability of exogenous substance P to induce NK1R internalization or in the release of substance P evoked centrally from site of nerve injury.
In subsequent experiments, NK1R internalization was induced in spinal cord slices by stimulating the dorsal root ipsilateral to CCI. We observed a complete loss of the inhibition of substance P release by the μ-opioid receptor agonist [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO) in CCI rats but not in sham-operated rats. In contrast, DAMGO still inhibited substance P release after inflammation of the hind paw with complete Freund’s adjuvant and in naïve rats. This loss of inhibition was not due to μ-opioid receptor downregulation in primary afferents, because their colocalization with substance P was unchanged, both in dorsal root ganglion neurons and primary afferent fibers in the dorsal horn. In conclusion, nerve injury eliminates the inhibition of substance P release by μ-opioid receptors, probably by hindering their signaling mechanisms.
Keywords: inflammation, internalization, opioid receptor, neurokinin 1 receptor, neuropathic pain, substance P
Neuropathic pain affects a large sector of the population and is notoriously difficult to treat (Eisenberg et al., 2005, Campbell and Meyer, 2006, Jackson, 2006, King, 2007). This is due in part to the fact that opiates are less effective in alleviating neuropathic pain than other forms of pain (Arner and Meyerson, 1988, Portenoy et al., 1990, McQuay, 2002, Eisenberg et al., 2005). There is evidence that the analgesic effects of opiates in the spinal cord, but not their supraspinal effects, are impaired during neuropathic pain. Thus, the analgesia produced by intrathecal morphine decreases in nerve injury models (Mao et al., 1995, Wegert et al., 1997). In the spinal cord, μ-opioid receptors (MORs) are present in primary afferent terminals (Li et al., 1998) and in a population of neurons located in laminae I–II of the dorsal horn (Marvizon et al., 1999b, Chen and Marvizon, 2009). MORs in primary afferent terminals inhibit neurotransmitter release, particularly the release of the neuropeptide substance P (Yaksh et al., 1980, Mauborgne et al., 1987, Kondo et al., 2005, Zhang et al., 2010b). Hence, the loss of the analgesic effectiveness of spinal opiates may occur because MORs in primary afferents are downregulated (Zhang et al., 1998, Kohno et al., 2005) or lose their inhibitory coupling to the voltage-gated Ca2+ (CaV) channels (Altier et al., 2007) that mediate neurotransmitter release.
The main objective of this study was to determine whether the ability of MORs to inhibit substance P release is impaired in a rat model of neuropathic pain. Substance P release was measured through the internalization of its receptor, the neurokinin 1 receptors (NK1R). We validated this method by comparing it to conventional immunoassay measures of substance P release and found that it has higher sensitivity (Marvizon et al., 2003a). In addition, NK1R internalization can be measured non-invasively in vivo (Mantyh et al., 1995, Allen et al., 1997, Honore et al., 1999, Kondo et al., 2005, Adelson et al., 2009, Zhang et al., 2010b), allows the spatial location of substance P release (Abbadie et al., 1997, Allen et al., 1999, Hughes et al., 2007, Zhang et al., 2013) and measures substance P release at physiologically relevant concentrations that activate the NK1R (Trafton et al., 1999). NK1R internalization may also detect the release of neurokinin A, but not the release of neurokinin B. These tachykinins were 5–7 times and 64 times less potent than substance P to induce NK1R internalization, respectively (Marvizon et al., 2003b). Since substance P and neurokinin A are co-released from primary afferents (Trafton et al., 2001), their detection is functionally equivalent.
Although multiple studies have investigated substance P release in inflammatory conditions (Abbadie et al., 1997, Allen et al., 1999, Honore et al., 1999, Honore et al., 2002, Zhang et al., 2013), just a few of them have investigated how it is affected by nerve injury. These studies have centered primarily on the type of primary afferents (i.e., Aβ-, Aδ-of C-fibers) that release substance P after nerve injury (Allen et al., 1999, Malcangio et al., 2000, Hughes et al., 2007) and have not investigated changes in the pharmacological modulation of substance P release. Here we show that MOR inhibition of substance P release disappears after chronic constriction injury (CCI) of the sciatic nerve but is not affected by inflammation of the paw with complete Freund’s adjuvant (CFA).
Material and Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Veteran Affairs Greater Los Angeles Healthcare System, and conform to NIH guidelines. Efforts were made to minimize the number of animals used and their suffering. Rats used were male adult (2–4 months old) Sprague-Dawley (Harlan, Indianapolis, IND).
Chemicals and solutions
[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO) and substance P were from Tocris (Ellisville, MO). Other chemicals were from Sigma. Drugs were prepared as stock solutions of 10–100 mM in the appropriate solvent and then diluted in aCSF. Thiorphan was dissolved in DMSO; other compounds were dissolved in water.
Artificial cerebrospinal fluid (aCSF) contained (in mM) 124 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2 and 10 glucose, and was gassed with 95% O2 / 5% CO2. Sucrose-aCSF was the same medium with 5 mM KCl and 215 mM sucrose instead of NaCl. K+-aCSF was aCSF containing 5 mM KCl.
Chronic constriction injury (CCI) of the sciatic nerve
CCI was used as a neuropathic pain model and was performed as described (Bennett and Xie, 1988). Briefly, rats were anaesthetized with isoflurane and their sciatic nerve was exposed at the mid-thigh level proximal to the sciatic trifurcation. Four chromic gut ligatures (4/0) were loosely tied around the nerve, 1–2 mm apart, without compromising the vascular supply. The muscle and the skin were closed with synthetic absorbable surgical suture. Sham surgery consisted in exposing the sciatic nerve without ligation. Rats were given an antibiotic (enrofloxacin) and an analgesic (carprofen) twice daily for 3 days.
Complete Freund’s adjuvant (CFA) injection
Rats were anesthetized (2–3% isoflurane) and injected subcutaneously with 150 μl of undiluted CFA (Sigma) into the plantar surface of the left hindpaw.
Measurement of mechanical allodynia
Allodynia to mechanical stimulation of the hindpaw was used to follow the development of neuropathic pain. Rats moved freely in an acrylic enclosure placed on an elevated metal grid. Rats were habituated to the enclosure for periods of 30 min for 5 days. A set of calibrated plastic von Frey hairs (‘Touch-Test’, North Coast Medical, Inc., San Jose, CA) of increasing size were applied to the hind paw until they bend, and the threshold force (in grams) to elicit paw withdrawal was recorded.
Measurement of thermal hyperalgesia
Paw withdrawal latencies were measured using a “Plantar Analgesia Meter” model 390G (IITC Life Sciences, Woodland Hills, CA), consisting of an acrylic enclosure on an elevated warm glass surface (Cheppudira, 2006). Rats were acclimated to the instrument for 30 min for 3 days. The test consisted in heating the plantar surface of the hind paw from below with a radiant heat source. The intensity of the lamp was set at 30% of maximal power. Cut-off time was 25 s to prevent tissue damage. Paw withdrawal latencies were measured four times at 5 min intervals. Results were calculated as percentage of the maximum possible response (%MPE) (Paronis and Holtzman, 1991):
Noxious mechanical stimulation
Noxious mechanical stimulation was used to induce NK1R internalization in vivo. Rats were anesthetized with isoflurane (2–3%) in an induction box and kept under isoflurane anesthesia until they were euthanized. The hind paw was clamped with a hemostat (closed to the first notch) for 30 sec (Le Bars et al., 1987a). Ten minutes later the rats were euthanized with pentobarbital (100 mg/Kg).
Intrathecal injections
Rats were implanted with chronic intrathecal catheters inserted between the L5 and L6 lumbar vertebrae (Storkson et al., 1996, Chen and Marvizon, 2009). Rats (2–4 months old) were anesthetized with isoflurane (2–4% in oxygen) and kept on a metal platform at 35 °C. The skin and muscle were cut to expose vertebrae L5 and L6. A 20G needle was inserted between the L5 and L6 vertebrae to puncture the dura mater, which was inferred from a flick of the tail or paw and backflow of spinal fluid. The needle was removed and the catheter (20 mm of PE-5 tube heat-fused to 150 mm of PE-10 tube) was inserted into the subdural space and pushed rostrally to terminate over L5-L6. The PE-10 end of the catheter was then tunneled under the skin and externalized over the head. The skin was sutured, and the catheter was flushed with 10 μl saline and sealed. Rats were given an antibiotic (enrofloxacin) and an analgesic (carprofen) twice daily for 3 days after surgery. Rats were housed separately and used for the experiment 5–7 days after surgery. The presence of motor weakness or signs of paresis was established as criteria for immediate euthanasia, but this did not occur in any of the rats in this study.
Intrathecal injection volume was 10 μl of injectate plus 10 μl saline flush (Zorman et al., 1982, Jensen and Yaksh, 1984, Aimone et al., 1987, Kondo et al., 2005). This volume leads to the distribution of the injectate over most of the spinal cord, but not into the brain (Yaksh and Rudy, 1976, Chen et al., 2007). Solutions are preloaded, in reverse order of administration, into a tube (PE-10), and delivered with a 50 μl Hamilton syringe within 1 min. The position of the catheter was examined postmortem and the following exclusion criteria were used: 1) loss of the catheter, 2) termination of the catheter inside the spinal cord, and 3) occlusion of the catheter tip.
Spinal cord slices
Transversal slices (400 μm) were prepared from the L4-L5 segments of the lumbar spinal cord of adult rats using a vibratome (Integraslice 7550PSDS, Lafayette Instruments, Lafayette, IN) as described (Lao et al., 2003, Marvizon et al., 2003a, Song and Marvizon, 2003b, Adelson et al., 2009, Zhang et al., 2010b). Rats were terminally anesthetized with pentobarbital (100 mg/kg). The L4 and L5 dorsal roots were indentified, cut short on the contralateral side to injury and preserved with a minimum length of 1 cm in the ipsilateral side for electrical stimulation. Slices were then cut at the entry zone of these roots. Fiber continuity between the dorsal root and the dorsal horn was assessed by examining the dorsal root and the dorsal surface of the slice with a stereo microscope mounted over the vibratome. Vibratome cutting was done in ice-cold sucrose-aCSF, after which slices were kept at 35°C in K+-aCSF for 1 hr (“recovery incubation”) and in normal aCSF thereafter (maximum 3 hr from the time of preparation).
The dorsal root attached to the slice was electrically stimulated using a custom-made chamber, as previously described (Marvizon et al., 2003b, Adelson et al., 2009). The root was placed on a bipolar stimulation electrode (platinum wire of 0.5 mm diameter, 1 mm pole separation) in a compartment separated from the superfusion chamber by a grease bridge. The root and the electrodes were covered with mineral oil and any excess aCSF was suctioned away. This ensured that electrical current circulated through the root and that the stimulus was consistent between preparations. Electrical stimulation was generated by a Master-8 stimulator and an Iso-Flex stimulus isolating unit in constant voltage mode (A.M.P. Instruments, Jerusalem, Israel), and consisted of 3000 square pulses of 20 V and 0.4 ms delivered at 100 Hz. These stimulation parameters recruited C-fibers (Adelson et al., 2009). Slices were superfused at 3–6 ml/min with aCSF at 35 °C. Drugs were present in the superfusate continuously starting 5 min before root stimulation. Ten minutes after the stimulus, slices were fixed by immersion in ice-cold fixative (4% paraformaldehyde, 0.18% picric acid in 0.1 M sodium phosphate buffer). A round hole was punched in the ventral horn of the slice ipsilateral to the stimulus in order to identify it in the histological sections after immunohistochemistry.
Immunohistochemistry
For NK1R labeling, rats were euthanized with pentobarbital (100 mg/kg) and fixed by aortic perfusion of 100 ml phosphate buffer (0.1 M sodium phosphate, pH 7.4) containing 0.01% heparin, followed by 400 ml of ice-cold fixative (4 % paraformaldehyde, 0.18 % picric acid in phosphate buffer). A L4-L6 segment of the lumbar spinal cord was post-fixed, cryoprotected, frozen and sectioned at 25 μm in the transversal plane using a cryostat (Chen et al., 2007, Lao et al., 2008). Spinal cord slices were fixed and processed similarly (Lao et al., 2003, Marvizon et al., 2003a, Lao and Marvizon, 2005, Adelson et al., 2009). Sections were washed four times with phosphate-buffered saline (PBS) and then incubated overnight at room temperature with the NK1R antiserum diluted 1:3000 in PBS, 0.3 % Triton X-100, 0.001 % thimerosal (PBS-Triton) containing 10 % normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). After three washes, the secondary antibody (1:2000, Alexa Fluor 488 goat anti-rabbit, Molecular Probes-Invitrogen, Eugene, OR) was applied for 2 hours at room temperature in PBS-Triton with 10 % normal goat serum. Sections were washed four more times, mounted on glass slides, and coverslipped with Prolong Gold (Molecular Probes-Invitrogen).
For co-labeling for substance P and the MOR we used a similar procedure. In addition to the transversal spinal cord sections (from the L5 segment), the L4 spinal segment was used to prepare sagittal sections 20 μm thick, and the L4-L5 dorsal root ganglia (DRG) were also collected and sectioned at 20 μm. Sections were washed twice with PBS, incubated for 30 min at room temperature in 50 % ethanol in PBS, washed twice with 5 % normal goat serum in PBS-Triton, and incubated overnight at room temperature with a mixture of the guinea pig substance P antiserum (diluted 1:5000) and the rabbit MOR antiserum (diluted 1:6000) in PBS-Triton with 1 % normal goat serum. Secondary antibodies were goat anti-guinea pig Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 568 (Molecular Probes-Invitrogen) both diluted 1:2000 in PBS-Triton with 1 % normal goat serum.
Antibodies
The NK1R rabbit antisera were # 94168 (CURE: Digestive Diseases Research Center, University of California Los Angeles) or AB-5060 from Millipore (Billerica, MA). Antiserum # 94168 was generated using a peptide corresponding to the C-terminus of the rat NK1R (amino acids 393–407, KTMTESSSFYSNMLA) coupled to KLH (Grady et al., 1996). Antibody #94168 was tested in cell lines transfected with NK1, NK2 and NK3 receptors. The NK1R antibody labeled cells transfected with NK1Rs but not cells transfected with NK2 receptors, NK3 receptors or non-transfected cells (Grady et al., 1996). Staining of the cells transfected with NK1Rs was eliminated by preadsorption with the cogent immunizing peptide. In Western blots from NK1R transfected cells, the antiserum produced a single band corresponding to a molecular weight of 100 kDa (Grady et al., 1996). Both the #98168 and the Millipore AB-5060 antisera labeled the rat dorsal horn with identical patterns. Antibodies against the same epitome of the NK1R are available from various sources and have been used in numerous studies, always producing the same pattern of staining of the dorsal horn (Mantyh et al., 1995, Abbadie et al., 1997, Allen et al., 1997, Honore et al., 1999, Marvizon et al., 2003a, Kondo et al., 2005, Nazarian et al., 2008, Adelson et al., 2009, Chen et al., 2010, Zhang et al., 2010b).
The substance P guinea pig antiserum was purchased from Novus Biologicals (Littleton, CO), catalog no. NB300-187. In a double-label experiment its staining completely colocalized with that of substance P rabbit antiserum CURE-8702 (from CURE: Digestive Diseases Research Center, UCLA, Los Angeles, CA), previously characterized (Marvizon et al., 2009).
The MOR rabbit antiserum was purchased from ImmunoStar (Hudson, WI), catalog no. 24216. It was generated against amino acid residues 384–398 of the rat MOR1. Its specificity was determined in cell lines by its colocalization with epitope-tagged constructs of MOR-1 (Arvidsson et al., 1995). Staining of the rat spinal cord with this antibody was eliminated by preadsorption with its immunizing peptide (Marvizon et al., 1999b). Moreover, its immunoreactivity was present at the surface of dorsal horn neurons in control rats and it internalized in endosomes after application of MOR agonists but not a δ-opioid receptor agonist (Marvizon et al., 1999b, Song and Marvizon, 2003a).
Quantification of NK1R internalization
NK1R neurons in lamina I with and without internalization were counted as reported (Mantyh et al., 1995, Abbadie et al., 1997, Marvizon et al., 1997, Marvizon et al., 1999a, Trafton et al., 2001, Marvizon et al., 2003a, Adelson et al., 2009, Chen et al., 2010) in four sections per spinal segment or slice, using a Zeiss Axio-Imager A1 (Carl Zeiss, Inc., Thornwood, NY) fluorescence microscope with a 63x objective (1.40 numerical aperture). The criterion for internalization was the presence of ten or more NK1R endosomes in the neuronal soma. Counting was done blinded to the treatment. Four sections per spinal segment or slice were used, counting all lamina I NK1R neurons in each section. Roughly, 100–300 neurons were counted per spinal cord slice or per rat. Results were expressed as the percentage of the NK1R neurons in lamina I with NK1R internalization.
Confocal microscopy and image processing
Confocal images were acquired using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Inc., Thornwood, NY), with objectives of 10x, 20x and 63x oil (numerical apertures of 0.3, 0.8 and 1.4, respectively). Laser excitation lines and emission windows were: Alexa Fluor 488 - excitation 488 nm (Ar laser), emission 500–550 nm; Alexa Fluor 568 - excitation 561 nm (diode laser), emission 580–690 nm. The pinhole was 1.0 Airy unit, and its actual width was set to that value for each objective and fluorophore, as determined by the confocal microscope software. Images were acquired as stacks of sections of 1024×1024 pixels, spaced 5.89 μm, 0.85 μm and 0.38 μm for 10x, 20x and 63x, respectively (determined using the Nyquist formula). Photomultiplier gain and offset were adjusted for each confocal stack to avoid pixel saturation.
Imaris 6.1.5 (Bitplane AG, Zurich, Switzerland) was used to crop the images in three dimensions and to generate a two-dimension projection picture. This picture was imported into Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA), which was used to make slight adjustments in the gamma when necessary. Adobe Photoshop was also used to compose the multi-panel figures and to add text and arrows.
Colocalization measures
In dorsal horn images, our goal was to determine whether substance P and MORs were present in the same presynaptic terminals and axons. This was assumed to be the case when the two labels were present in the tissue with enough proximity that they cannot be resolved optically (Hibbs et al., 2006). This type of colocalization was measured using the Colocalization module of Imaris, which analyzes two-channel stacks of confocal sections by measuring the intensity of each label in each voxel. The extent of colocalization was measured as the Pearson’s correlation coefficient of the intensity of the labels in each voxel of the confocal stack. It varies between +1 and −1, with positive values indicating colocalization, values near 0 indicating no colocalization, and negative values indicating mutual exclusion of the labels (Marvizon et al., 2009).
In DRG images, our goal was to determine whether the two labels were present in the same neuronal body regardless of their proximity. It was measured by visually counting neuronal profiles with each of the labels and with the two labels together, and then calculating the percentage of double-labeled profiles relative to each of the single-labeled profiles.
Data analysis
Data were analyzed using Prism 6 (GraphPad Software, San Diego, CA). All data are expressed as mean ± SEM. Statistical significance was set at 0.05. Statistical analyses consisted of one-way ANOVA followed by Sidak’s post-hoc tests, or two-way ANOVA followed by Dunnett’s (comparisons with control) or Tukey’s (multiple comparisons) post-hoc tests. Tests for equivalence were used to determine whether two measures were significantly the same with 95 % confidence (Schuirmann, 1987). Concentration-response data were fitted using non-linear regression by a sigmoidal dose-response function: Y = bottom + (top-bottom) / (1 + 10^(Log EC50-Log X)), where the EC50 is the concentration of drug that produces half of the effect. Baseline measures (zero concentration of drug) were included in the non-linear regression by assigning them a concentration value three log units lower than the estimated EC50. Parameter constraints were: top < 100%, bottom > 0%.
Results
Nerve injury affected the ability of noxious stimulation of the hind paw to induce NK1R internalization
Noxious mechanical or thermal stimulation of the hind paw has been used in numerous studies to evoke substance P release in the spinal dorsal horn, which was then measured in situ as NK1R internalization (Mantyh et al., 1995, Allen et al., 1997, Kondo et al., 2005, Zhang et al., 2010a). This has the advantage of mimicking the acute pain events that trigger substance P release. However, after CCI of the sciatic nerve the action potentials elicited by the noxious stimulus have to cross the injured part of the nerve, which could affect the efficacy of the stimulus to induce substance P release. Hence, we first investigated whether delivering a noxious stimulus to the paw affected by nerve injury is reliable way to evoke substance P release and NK1R internalization.
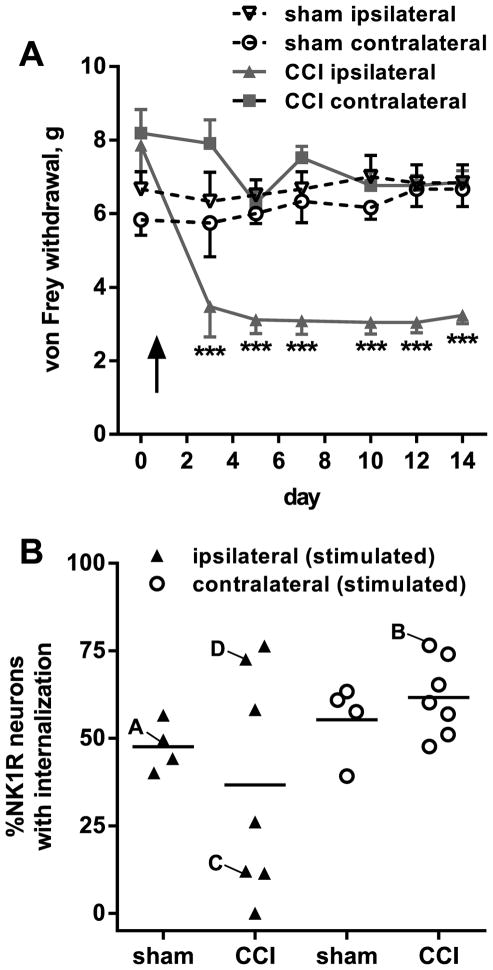
As the stimulus to evoked NK1R internalization we chose clamping with a hemostat, a noxious mechanical stimulus previously used in several studies (Kondo et al., 2005, Chen and Marvizon, 2009, Zhang et al., 2010b). We clamped both paws in order to be able to compare the evoked NK1R internalization between the dorsal horns ipsilateral and contralateral to CCI, in addition to comparing the CCI rats to the sham-operated rats. The CCI rats, but not the sham-operated rats, developed mechanical allodynia in the ipsilateral hindpaw from day 3 to day 14 (Fig. 1A). On day 14 the rats were anesthetized and both of their hind paws were sequentially clamped with a hemostat for 30 s. Ten min later, the rats were sacrificed, fixed by aortic perfusion and their spinal cord was extracted to quantify NK1R internalization in lamina I of spinal segments L4-L5. As shown in Fig. 1B, this stimulus evoked NK1R internalization in about 50% of the NK1R neurons in the sham-operated rats (ipsilateral and contralateral dorsal horns) and in the contralateral dorsal horn of the CCI rats. However, in the ipsilateral dorsal horn of the CCI rats the evoked NK1R internalization was highly variable: in 3 out of 7 rats it was comparable to that found in the sham-operated rats, but in the remaining 4 rats it was substantially lower. Indeed, the scatter of the data differed substantially between the ipsilateral and the contralateral sides of the CCI rats, as revealed by an F test to compare the variances of these two sets of data in an unpaired t-test with Welsh’s correction. Although the mean NK1R internalization was not significantly different between the ipsilateral and the contralateral sides (p = 0.0869), the variances were significantly different in the F-test (p = 0.0208, F6,6 = 8.339). Because of this, we analyzed the data in Fig. 7B using a non-parametric Kruskal-Wallis analysis, which indicated that there were no significant differences between the groups (p=0.25).
Figure 1. NK1R internalization induced by hind paw clamp after CCI.
A. Responses of the rats to von Frey hair stimulation of the hind paws were followed every 2 days for 14 days after CCI (n = 7 rats) or sham surgery (n = 4). Two-way ANOVA revealed a significant effect of CCI (p < 0.0001), time (p = 0.0001), interaction (p < 0.0001) and subject matching (p = 0.0018). Dunnett’s multiple comparison test: *** p<0.001 compared with CCI contralateral. B. On day 14, rats were anesthetized and both hind paws were clamped sequentially for 30 s. Ten min later, the spinal cord was extracted and NK1R internalization was quantified in lamina 1 of spinal segments L4-L5. Letters next to the symbols indicate data from rats corresponding to the panels in Fig. 2.
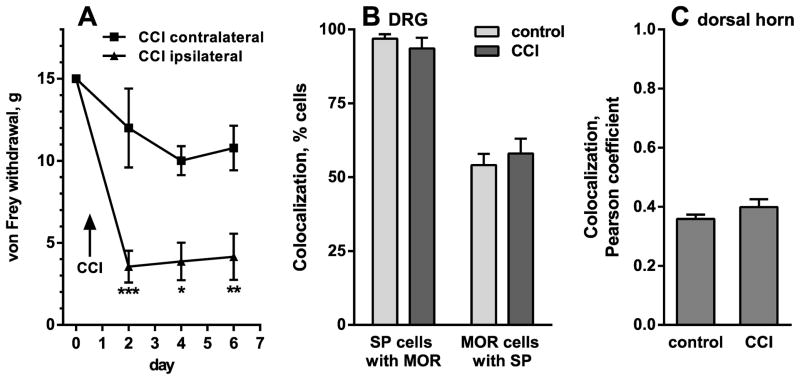
Figure 7. Quantification of substance P and MOR colocalization in DRG and spinal cord.
A. Responses of 3 rats to von Frey hair stimulation in the hind paws were followed every 2 days for 6 days after unilateral CCI. Two-way ANOVA revealed a significant effect of CCI (p = 0.0182), time (p < 0.0001), interaction (p = 0.0036) and subject matching (p = 0.0198). Sidak’s post-hoc tests compared CCI ipsilateral to CCI contralateral: * p < 0.05, ** p < 0.01, *** p < 0.001. B. In the L4-L5 DRG from those 3 rats or from 4 naïve rats (“control”), colocalization of substance P (SP) with MORs was measured by counting cell bodies with each label and with both labels; n = 12 confocal stacks from control rats, n = 9 confocal stacks from CCI rats (3 per rat). C. In the dorsal horn of the same rats, colocalization of SP with MORs was measured as the Pearson’s correlation coefficient of the intensity of these two labels in 12 (control) or 9 (CCI) confocal stacks (3 per rat).
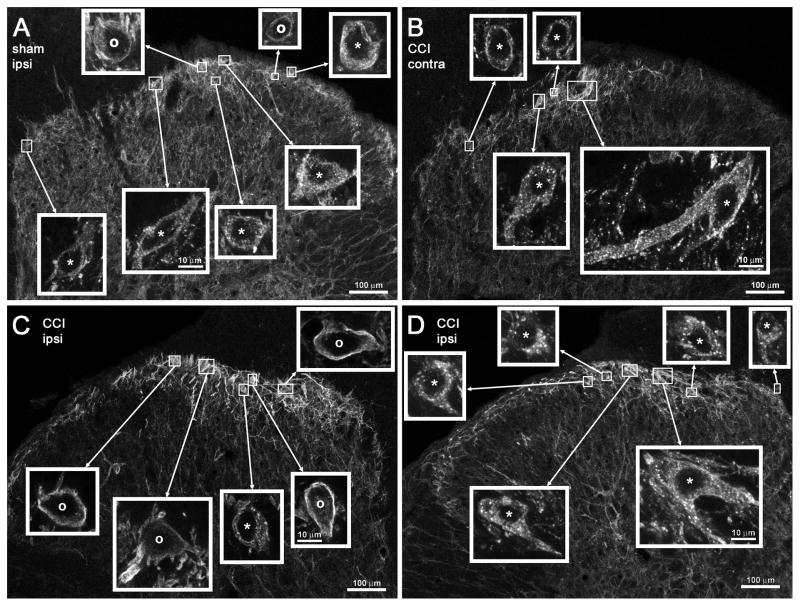
Fig. 2 shows confocal microscope images of NK1R neurons in lamina I of the L4 dorsal horn of these rats. Letters next to the data points in Fig. 1B indicate the rats chosen for the images in the corresponding panels in Fig. 2. Images of the entire dorsal horn obtained with the 10x objective were used to show the location of each of the NK1R neurons imaged with the 63x objective. Clamping the hind paw produced NK1R internalization in many of these neurons in the dorsal horn ipsilateral to sham surgery (Fig. 2A) or contralateral to CCI (Fig. 2B). Panels C and D of Fig. 2 correspond to the dorsal horn ipsilateral to CCI of two different rats. In the rat of panel C clamping the hind paw largely failed to evoke NK1R internalization, whereas in the rat of panel D it evoked extensive NK1R internalization.
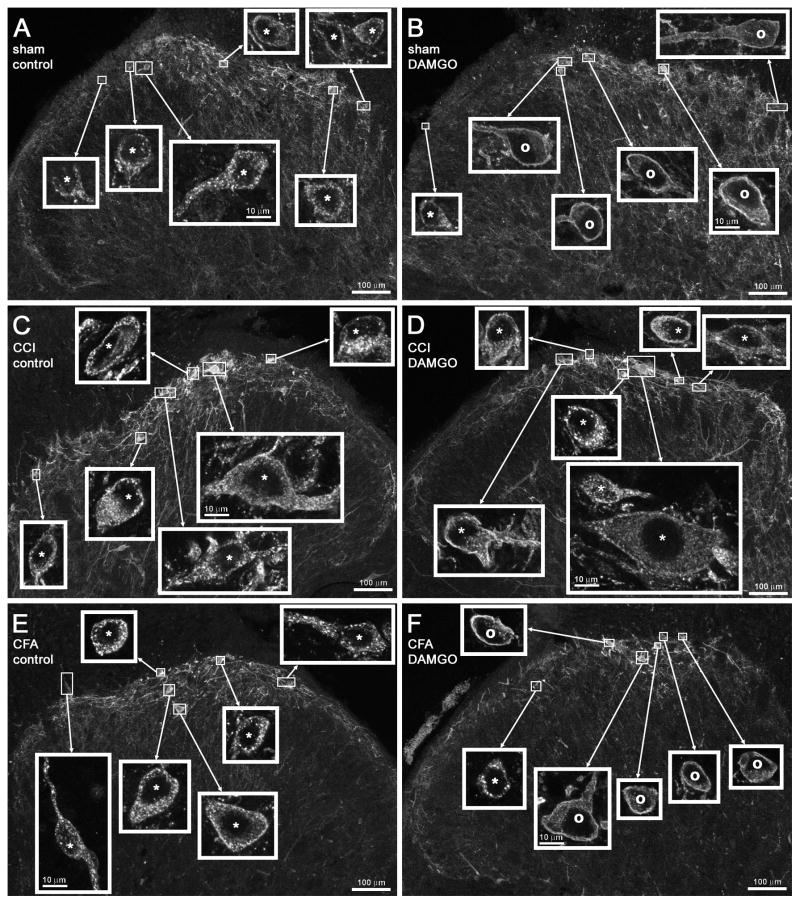
Figure 2. Confocal images of NK1R neurons in lamina I after CCI and paw clamp.
Rats received unilateral sham surgery (A) or CCI (B–D), and 7 days later both paws were clamped to evoke substance P release. Images were taken from sections of the L4 spinal segment ipsilateral (A, C, D) or contralateral (B) to the operated paw. Paw clamp produced NK1R internalization in numerous cells ipsilaterally to sham surgery (A) and contralaterally to CCI (B); however, ipsilaterally to CCI some rats presented abundant NK1R internalization (D) whereas others showed little internalization (C). The percentage of NK1R lamina 1 neurons with internalization in these rats in given in Fig. 2B, where the letters next to the symbols correspond to the same rats as the panels of this figure. Main panels: images taken with a 10x objective; voxel size of 830 × 830 × 5983 nm, 1 confocal plane. Insets: images taken with a 63x objective of the lamina I neurons indicated by the frames in the main panels; voxel size of 132 × 132 × 383 nm, 3–6 confocal planes. Neurons with NK1R internalization are indicated with “*” and neurons without internalization by “o”, with the symbol placed over the nucleus. Scale bars indicate 100 μm in the main panels and 10 μm in the insets.
This experiment shows that a noxious stimulus applied to the hind paw affected by CCI is not a reliable way to evoke substance P release. Possible explanations for this are that CCI caused: 1) changes in the ability of NK1Rs to undergo internalization; 2) a decrease in the amount of substance P released by each action potential, or 3) a decrease in the ability of substance P-containing fibers in the sciatic nerve to conduct action potentials. These explanations were considered in the next experiments.
Nerve injury did not affect the ability of exogenous of substance P to induce NK1R internalization, but decreases its potency
To investigate whether nerve injury hinders the ability of NK1Rs to undergo internalization, we determined whether exogenous substance P is able to induce NK1R internalization in spinal cord slices from CCI and sham-operated rats. In addition, we obtained concentration-response curves for substance P to detect possible changes in its potency at the NK1R after nerve injury.
Rats underwent CCI of the sciatic nerve (3 rats) or sham surgery (3 rats). The development of mechanical allodynia was followed by measuring paw withdrawal responses to von Frey hair application every 2 days for 14 days. After day 2, paw withdrawal threshold was markedly reduced in the CCI rats ipsilaterally to CCI, but not contralaterally or in sham-operated rats (Fig. 3A). On day 14, the rats were sacrificed and six spinal cord slices per rat were prepared from the lumbar spinal cord. Each of these 6 slices was incubated with a different concentration of substance P. By averaging the data of the 3 rats in each group, concentration-response curves for substance P were obtained separately for the ipsilateral and contralateral dorsal horns. After CCI there was a decrease in the potency of substance P to induce NK1R internalization in the ipsilateral dorsal horn (Fig. 3B): from an EC50 of 5.6 nM (95% CI = 2.9–10.5 nM) in the sham rats to and EC50 of 22.0 nM (95% CI = 11.8–40.9 nM) in the CCI rats. In the contralateral dorsal horn there was a less pronounced decrease in the potency of substance P (Fig. 3C): from an EC50 of 6.0 nM (95% CI = 3.7–9.7 nM) in the sham rats to an EC50 of 14.6 nM (95% CI = 9.1–23.4 nM) in the CCI rats. The statistical significance of these differences was studied using Akaike’s Information Criterion applied to the non-linear regression fitting, which yielded a probability that the EC50 was different between the sham and the CCI rats of 99.67% (ipsilateral dorsal horn) and 99.05% (contralateral dorsal horn). The probability that the EC50s for substance P was different between the ipsilateral and the contralateral dorsal horns was 51% in the CCI rats and 21.6 % in the sham rats.
Figure 3. Concentration-responses for substance P to induce NK1R internalization after CCI.
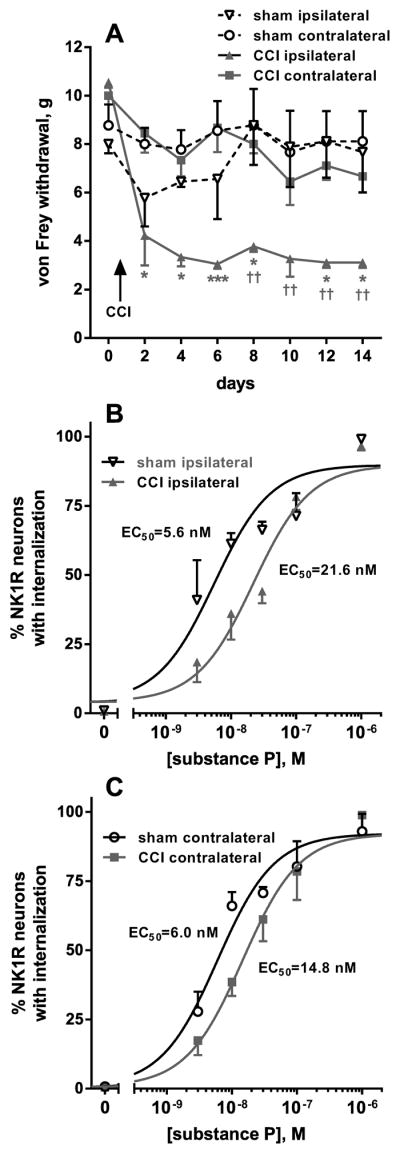
A, B. After CCI or sham surgery (n = 3 rats per group), responses of the rats to von Frey hair stimulation of the hind paws were followed every 2 days for 14 days. Two-way ANOVA of all data in panels A and B revealed a significant effect of CCI (p = 0.0253), time (p < 0.0001), interaction (p = 0.0006) and subject matching (p < 0.0001). Dunnett’s post-hoc tests compared CCI ipsilateral to CCI contralateral (* p < 0.05, *** p < 0.001) or CCI ipsilateral to sham ipsilateral (†† p < 0.01). C, D. On day 14, rats were sacrificed and used to prepare spinal cord slices (6 slices/rat). Each slice was incubated for 10 min with the concentrations of substance P indicated, so that a full concentration-response was obtained for each rat. Points represent the mean ± SEM of 3 rats / group. Curves correspond to non-linear regression fittings to a dose-response function. Fitting was done simultaneously for the two sets of data in each panel (the ‘bottom’ and ‘top’ parameters were constrained to be the same), and Akaike’s Information Criterion was used to test whether the two curves had the same or different EC50. The probability that the EC50s are different is 99.67% for the curves in panel B (ipsilateral) and 99.05% for the curves in panel C (contralateral). EC50 values were: sham ipsilateral, 5.6 nM (95% CI = 2.9–10.5 nM); CCI ipsilateral, 22.0 nM (95% CI = 11.8–40.9 nM); sham contralateral, 6.0 nM (95% CI = 3.7–9.7 nM); CCI contralateral, 14.6 nM (95% CI = 9.1–23.4 nM).
In conclusion, nerve injury decreases the potency of substance P to induce NK1R internalization, although this decrease is relatively small. The concentration of substance P in the dorsal horn can be obtained from NK1R internalization measures by interpolating them in the concentration-response curves of Fig. 3A–B, allowing a comparison of substance P release between CCI and sham-operated rats.
Nerve injury slightly decreased NK1R internalization induced by dorsal root stimulation
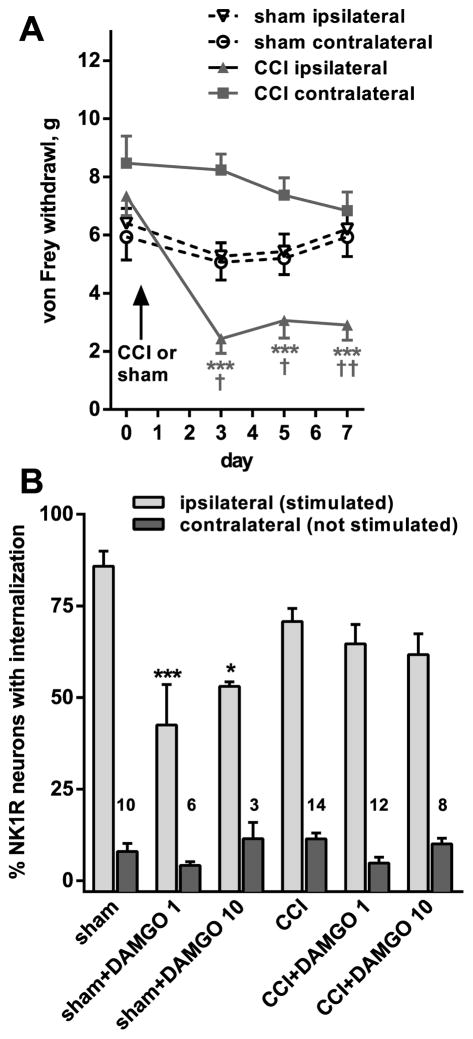
To investigate whether nerve injury has a direct effect on substance P release from the central terminals of primary afferents, we evoked substance P release with a stimulus delivered centrally from the point of CCI injury, namely to the dorsal root. The development of neuropathic pain in these rats after CCI was confirmed by measuring mechanical allodynia to von Frey hair stimulation, which was detected in the ipsilateral hind paw of the CCI rats (Fig. 4A). Two-way ANOVA comparing 10 sham-operated rats with 10 CCI rats revealed a significant effect of CCI-side (p = 0.0002, F3,36 = 8.518), time (p < 0.0001, F3,108 = 19.11), interaction (p < 0.0001, F9,108 = 7.269) and subject matching (p < 0.0001, F36,108 = 7.360).
Figure 4. Loss of DAMGO inhibition of substance P release after CCI.
A. Responses of the rats to von Frey hair stimulation in the hind paws were followed every 2 days for 7 days after unilateral CCI (n = 10 rats) or sham surgery (n = 10 rats). Two-way ANOVA revealed a significant effect of CCI-side (p = 0.0002), time (p < 0.0001), interaction (p < 0.0001) and subject matching (p < 0.0001). Tukey’s post-hoc tests compared CCI ipsilateral to CCI contralateral (*** p < 0.001) or to sham ipsilateral († p < 0.05, †† p < 0.01). B. On day 7, 2–3 spinal cord slices (L4-L5 segments) were prepared from each rat (10 with sham surgery and 14 with CCI): one was used served as control and the others were superfused with DAMGO (1 μM or 10 μM). The dorsal root ipsilateral to CCI or sham surgery was stimulated electrically (3000 pulses at 100 Hz) to elicit substance P release. Peptidase inhibitors (captopril 10 μM and thiorphan 10 μM) were present in the superfusate to decrease substance P degradation. NK1R internalization was quantified in lamina I. Numbers represent the number of slices (n). Two-way ANOVA restricted to the ipsilateral dorsal horn revealed a significant effect of DAMGO (p < 0.0001), no significant effect of CCI (p = 0.32) and significant interaction (p = 0.0041). Tukey’s post-hoc test revealed significant inhibition by DAMGO only in sham-operated rats (* p = 0.012, *** p < 0.0001).
Substance P release was measured in slices from spinal cord segments L4-L5 with attached dorsal roots, obtained seven days after CCI or sham surgery. NK1R internalization was evoked by electrically stimulating the dorsal root ipsilateral to the surgery with 3000 pulses of 20 V and 0.4 ms delivered at 100 Hz (Adelson et al., 2009). The slices were superfused with the peptidase inhibitors captopril and thiorphan (10 μM) to reduce substance P degradation (Marvizon et al., 2003b). Root stimulation produced slightly less NK1R internalization in slices from CCI rats (70.7 ± 3.59 %, n = 14 slices) than in slices from the sham rats (85.8 ± 4.13 %, n = 10 slices, Fig. 4B). An unpaired t-test with Welsh’s correction comparing NK1R internalization between the sham rats and the CCI rats revealed significant differences between the means (p = 0.0121, t = 2.76, df =19.9). However, an F-test showed no significant differences between the variances (p = 0.9554, F13,9 = 1.061), in contrast to what we observed when using noxious stimulation of the paw (Fig. 1B).
Since CCI also decreased the potency of substance P to induce NK1R internalization (Fig. 1B), it is possible that this, and not a decrease in substance P release, caused the decrease in evoked NK1R internalization after CCI. To investigate this possibility, we interpolated the NK1R internalization values obtained in the sham rats and in the CCI rats in the respective concentration-response curves for substance P shown in Fig. 1B. In the sham rats, 85.8 % NK1R internalization corresponded to a concentration of substance P of 114 nM, log [substance P] = −6.94, 95% CI = −7.394 to infinity (because the value is close to the saturation part of the curve). In the CCI rats, 70.7 % NK1R internalization corresponded to a concentration of substance P of 76.8 nM, log [substance P] = −7.11, 95% CI = −7.346 to −6.884. Since the lower limits of the 95% CIs extensively overlap, we concluded that the decrease in NK1R internalization was likely due to a decrease in the potency of substance P to induce NK1R internalization produced by CCI and not to an actual decrease in substance P release.
CCI eliminated MOR inhibition of substance P release
The objective of this experiment was to determine whether nerve injury changes the ability of MORs to inhibit substance P release from primary afferent terminals (Yaksh et al., 1980, Kondo et al., 2005, Zhang et al., 2010b). The same rats as in the experiment described above were used. Additional spinal cord slices from those rats were superfused with the selective MOR agonist DAMGO (1 μM or 10 μM) while electrically stimulating the ipsilateral dorsal root (3000 pulses at 100 Hz) to evoke substance P release in the presence of peptidase inhibitors.
We found that while in the sham-operated rats DAMGO inhibited the evoked NK1R internalization, in the CCI rats this inhibition was lost (Fig. 4B). DAMGO failed to decrease the evoked NK1R internalization even at 10 μM, a supersaturating concentration (Marvizon et al., 1999b), indicating that there was a loss of its efficacy and not a mere decrease in its potency. Two-way ANOVA of the data from the ipsilateral dorsal horn revealed a significant effect of DAMGO (p < 0.0001, F2,47 = 11.99), no significant effect of CCI (p = 0.319, F1,47 = 1.015) and significant interaction (p = 0.0041, F2,47 = 6.197).
Tests for equivalence were used to determine the whether these measures were significantly the same. A zone of indifference was defined as 20 % change in NK1R internalization. The 90% CI of the difference between the means of control and 1 μM DAMGO was −16.7 % to 4.60 %, which fell within the zone of indifference, indicting that the means were the same with 95 % confidence (Schuirmann, 1987). The 90% CI of the difference between the means of control and 10 μM DAMGO was −20.0 % to 1.98 %, which was equal to the zone of indifference.
Examples of lamina I NK1R neurons from this experiment are shown in the confocal images in Fig. 6A–D. The left panels (A, C) show the abundant NK1R internalization evoked in the slices by dorsal root stimulation. The right panels (B, D) show that DAMGO decreases the number of NK1R neurons showing internalization in the sham-operated rats (B), but not in the CCI rats (D).
Figure 6. Confocal images of NK1R neurons in lamina I - DAMGO after CCI or CFA.
Rats received sham surgery (A, B), CCI (C, D) or CFA in the hind paw (E, F). Seven days after sham or CCI, or 3 days after CFA, spinal cord slices prepared from these rats were electrically stimulated at the ipsilateral dorsal root to induce substance P release. In the absence of DAMGO, dorsal root stimulation produced NK1R internalization in most lamina I cells in the sham (A), CCI (C) and CFA-injected rats (E). However, 1 μM DAMGO inhibited the NK1R internalization evoked by dorsal root stimulation in the sham (B) and the CFA-injected rats (F), but not in the CCI rats (D). The percentage of NK1R lamina 1 neurons with internalization in these rats is given in Figs. 3B and 4B. Main panels: images taken with a 10x objective; voxel size of 830 × 830 × 5983 nm, 1 confocal plane. Insets: images taken with a 63x objective of the lamina I neurons indicated by the frames in the main panels; voxel size of 132 × 132 × 383 nm, 3–7 confocal planes. Neurons with NK1R internalization are indicated with “*” and neurons without internalization by “o”, with the symbol placed over the nucleus. Scale bars indicate 100 μm in the main panels and 10 μm in the insets.
CFA inflammation did not affect MOR inhibition of substance P release
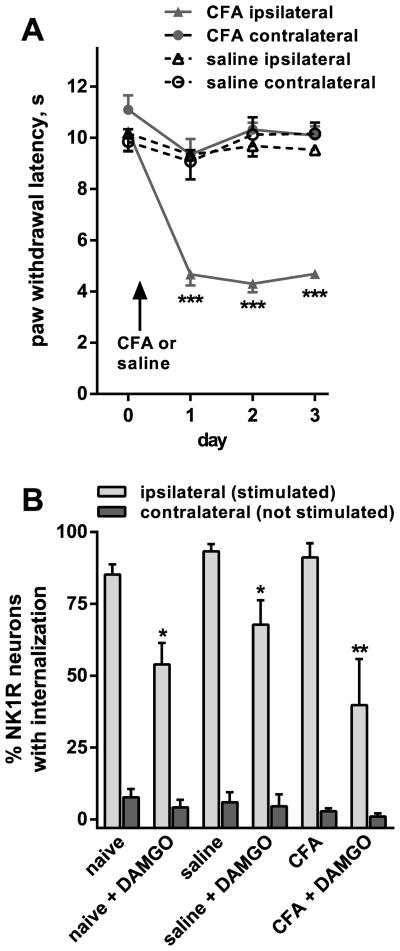
The objective of this experiment was to determine whether the loss of MOR inhibition of substance P release was specific to neuropathic pain or also occurs in inflammatory pain. In one group of rats (“CFA”, n = 3), we induced unilateral inflammation of the hind paw by injecting it with CFA (150 μl, undiluted). Another group of rats (“saline”, n = 5) received similar injection of saline (150 μl). The CFA-injected rats developed thermal hyperalgesia in the injected hind paw, determined as reduced latencies in their paw responses to radiant heat (Fig. 5A). The hyperalgesia was present one day after the injection and persisted for at least 3 days. No thermal hyperalgesia was found contralaterally to CFA or in the saline-injected rats. Two-way ANOVA revealed a significant effect of CFA-side (p < 0.0001, F3,8 = 33.93), time (p < 0.0001, F3,24 = 17.14), interaction (p < 0.0001, F9,24 = 7.821) but not subject matching (p = 0.0815, F8,24 = 2.062).
Figure 5. DAMGO inhibition of substance P release after CFA.
A. Responses of the rats to thermal stimulation of the hind paws were followed daily for 3 days after injecting one hind paw with CFA (n = 3 rats) or saline (n = 5 rats). Two-way ANOVA revealed a significant effect of CFA (p < 0.0001), time (p < 0.0001), interaction (p < 0.0001) but not subject matching (p = 0.0815). Tukey’s post-hoc tests compared CFA ipsilateral to CFA contralateral or to saline ipsilateral (*** p < 0.001). B. Three days after the injection in the hindpaw, two spinal cord slices with the dorsal root ipsilateral to the injection were prepared from each rat (and also from 4 naïve rats). That root was electrically stimulated (3000 pulses at 100 Hz) while the slice was superfused with aCSF containing peptidase inhibitors (captopril 10 μM and thiorphan 10 μM). One of the slices from each rat was also superfused with 1 μM DAMGO, while the other slice from that rat served as a control. NK1R internalization was quantified in lamina 1, and found to be negligible in the contralateral side. One-way ANOVA restricted to data from the ipsilateral dorsal horn revealed a significant effect of DAMGO (p < 0.0001), no significant effect of CFA (p = 0.139), and no significant interaction (p = 0.266). Sidak’s multiple comparison test revealed significant inhibition by DAMGO: * p < 0.05, ** p < 0.01.
Three days after the injection, the rats were euthanized and their spinal cords (L4-L5 spinal segment) were used to prepare two slices per rat with one dorsal root attached. We also prepared slices from rats that did not receive any injection (“naïve”, n = 4). In the “CFA” and “saline” rats, the root was ipsilateral to the injected paw. The slices were electrically stimulated at the dorsal root with (3000 pulses at 100 Hz) to evoke substance P release. One of the slices from each rat was superfused with 1 μM DAMGO, while the other served as a control. The superfusate contained peptidase inhibitors (captopril 10 μM and thiorphan 10 μM) for all the slices. Fig. 5B shows that DAMGO (1 μM) inhibited the evoked NK1R internalization in the naïve, saline-injected and CFA-injected rats. Two-way ANOVA revealed a significant effect of DAMGO (p < 0.0001, F1,18 = 32.82), no significant effect of CFA (p = 0.139, F2,18 = 2.20), and no significant interaction (p = 0.266, F2,18 = 1.427).
Examples of lamina I NK1R neurons from a CFA-injected rat are shown in the confocal microscope images of Fig. 6E–F: there was abundant NK1R internalization evoked by dorsal root stimulation in a control slice (Fig. 6E) but little NK1R internalization in a slice superfused with DAMGO (Fig. 6F).
Therefore, unlike what we observed after CCI, MORs were still able to inhibit substance P release during CFA inflammation.
CCI did not affect MOR staining in DRG or the dorsal horn
The loss of MOR inhibition of substance P release after CCI could be caused by the downregulation of MORs in primary afferent neurons. To determine whether this is the case, we performed MOR immunohistochemistry in lumbar (L4, L5) DRG and spinal cord sections from rats that underwent CCI or naïve rats. CCI resulted in the development of mechanical hyperalgesia (Fig. 7A). In the spinal dorsal horn, MORs are present both in primary afferent terminals and in intrinsic dorsal horn neurons. To detect changes in MORs occurring specifically in the primary afferents that contain substance P, spinal cord and DRG sections were double-labeled using antibodies directed against the MOR and substance P. In naïve rats (Fig. 8A), MOR immunoreactivity was found in most of the DRG cell bodies labeled for substance P and in some cells that do not contain it. In CCI rats (Fig. 8B), the DRG ipsilateral to the injured nerve presented a staining pattern identical to the naïve rats. Substance P and MOR immunoreactivities were also found together in axon fibers inside the DRG (Fig. 8C), although they were present in different puncta within these fibers. In the dorsal horn, substance P and MOR labels were most abundant in laminae I and II (Fig. 8D). High magnification images (63x objective) of these laminae were obtained from sagittal spinal cord sections in order to observe the substance P-containing fibers, which course rostro-caudally. MOR and substance P immunoreactivities colocalized in many of these fibers, which were found interspersed with the bodies and dendrites of MOR-expressing dorsal horn neurons. This colocalization did no differ between the dorsal horn of naïve rats (Fig. 8E) and the dorsal horn ipsilateral to nerve injury of the CCI rats (Fig. 8F).
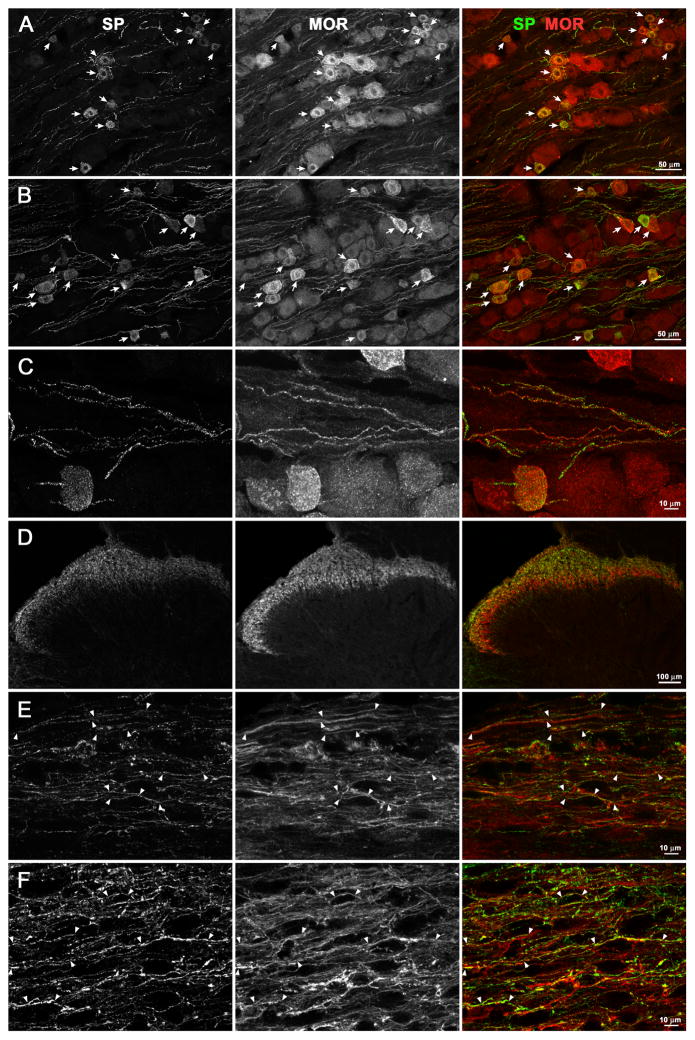
Figure 8. Colocalization of substance P and MORs in DRG and spinal cord in naïve and CCI rats.
Naïve rats or rats that received CCI 7 days earlier were euthanized and fixed. Sections from the L4 or L5 DRG and L4-L5 spinal segments were labeled with antibodies recognizing substance P (SP, green) and MOR (red). A, B. DRG from a naïve rat (A, 20x objective, 4 optical sections) or a CCI rat (B, ipsilateral to CCI, 20x objective, 6 optical sections); arrows indicate neuron bodies with SP/MOR colocalization. C. DRG ipsilateral to CCI (63x objective, 19 optical sections); SP and MOR colocalize in numerous primary afferent fibers. D. Dorsal horn ipsilateral to CCI (coronal section, segment L5, 10x objective, 1 optical section); SP and MOR labels are present mostly in laminae I and II. E, F. Laminae I–II (sagittal sections, segment L4, 63x objective, 6 optical sections) from a naïve rat (E) or a CCI rat (F, ipsilateral to CCI); arrowheads bracket fibers with SP/MOR colocalization.
To confirm that MOR expression in substance P-containing primary afferents did not decrease after CCI, we performed quantitative analyses of images similar to the ones shown in Fig. 8. In the DRG, we counted cell bodies stained for substance P, for MORs and for both labels together. We used 4 naïve rats and 3 CCI rats, obtaining confocal stacks from 3 DRG sections from each rat. Therefore, we studied a total of 12 confocal stacks from the naïve rats and 9 confocal stacks from the CCI rats. The percentage of substance P profiles that contained MORs was high (~95%, Fig. 7B) and not significantly different (p = 0.36, t-test) between the naïve and the CCI rats. Conversely, the percentage of MOR profiles that contained substance P was lower (~55%, Fig. 7B), but also not significantly different (p = 0.53, t-test) between the naïve and the CCI rats. Tests for equivalence were used to determine the whether these measures were significantly the same. A zone of indifference was defined as a difference of 15% of the DRG cells. The 90% CI of the difference between the means was −9.54 % to 2.83 % for substance P cells that contained MOR and −6.78 % to 14.61 % for MOR cells that contained substance P. Since both 90% CIs fell within the zone of indifference, colocalization of MORs and substance P in the DRG was deemed the same of between naïve and CCI rats.
In the dorsal horn we adopted a different approach. The colocalization module of Imaris (see Methods) was used to quantify the colocalization of the two labels voxel by voxel in whole confocal stacks as the Pearson’s correlation coefficient (Fig. 7C). Again, we analyzed 12 confocal stacks from 4 naïve rats and 9 confocal stacks from 3 CCI rats (3 confocal stacks per rat). Each confocal stack was taken from a different histological section and consisted of 7–12 optical sections of 1024 × 1024 pixels each. In the CCI rats, all measures were taken from the dorsal horn ipsilateral to the injured nerve. The Pearson’s coefficients obtained were positive and large enough (~0.4, Fig. 7C) to indicate colocalization. There were no significant differences in the Pearson’s coefficients between naïve and CCI rats, p = 0.175 (t-test). A tests for equivalence was used to determine the whether these measures were significantly the same. A zone of indifference was defined as a difference of 0.1 in the Pearson correlation coefficient. The 90% CI of the difference between the means was −0.0091 to 0.089, which fell within the zone of indifference. Therefore, CCI did not affect the colocalization of MORs and substance P in the dorsal horn. Taken together, results in the DRG and the dorsal horn show that nerve injury does not change the amount of MORs present in substance P-containing primary afferents.
Discussion
The main finding of this study is that in the CCI model of neuropathic pain there is a complete loss of the ability of MORs to inhibit substance P release in the dorsal horn. This loss of inhibition is not due to a downregulation of MORs in the primary afferents, because there was no decrease of MOR immunoreactivity in substance P-containing DRG neurons or primary afferent fibers in the dorsal horn.
Nerve injury hinders the ability of noxious stimuli to induce substance P release
An initial finding in our study was that after CCI a noxious stimulus applied to the affected paw produced inconsistent effects in evoking substance P release in the dorsal horn. The use of noxious stimuli to evoke substance P release in vivo has the advantage of mimicking naturally-occurring pain events (Mantyh et al., 1995, Allen et al., 1997, Kondo et al., 2005, Zhang et al., 2010b). However, in four out of seven rats with CCI, clamping the paw with nerve injury produced much less NK1R internalization than clamping the contralateral paw or the paws of sham-operated rats. In the remaining three rats, paw clamp produced the expected amount of NK1R internalization. This decrease in NK1R internalization evoked by the noxious stimulus was not due to the inability of substance P to induce NK1R internalization, because exogenous substance P applied to spinal cord slices prepared from CCI rats reliably induced NK1R internalization, albeit with reduced potency. To determine whether the decrease in NK1R internalization was due to alterations in substance P release itself we stimulated the dorsal root, so that action potentials do not have to cross the injured portion of the nerve. Previous studies on substance P release in nerve injury models used stimuli applied centrally from the nerve injury (Allen et al., 1999, Malcangio et al., 2000, Hughes et al., 2007). In spinal cord slices prepared from CCI rats, stimulating the dorsal root reliably induced NK1R internalization without the variability observed when using noxious paw stimulation. Although the evoked NK1R internalization was somewhat lower in the CCI rats than in the control rats, this was probably not due to a decrease in substance P release but to the decrease in the potency of substance P to induce NK1R internalization. Therefore, CCI seems to compromise the propagation of action potentials across the injured section of the sciatic nerve, decreasing the drive for substance P release.
Nevertheless, the same rats that had decreased paw clamp-induced NK1R internalization (Fig. 1B) had allodynia to von Frey hair application (Fig. 1A). This suggests that the nociceptive signals responsible for allodynia were transmitted by Aβ-fibers that do not release substance P. Although there are some reports of substance P expression in Aβ-fibers after nerve injury (Marchand et al., 1994, Noguchi et al., 1994), they were contradicted by later studies (Fukuoka et al., 1998, Hughes et al., 2007). In normal rats, we previously found that NK1R internalization evoked by sciatic nerve stimulation is due to substance P release from C-fibers (Adelson et al., 2009). In nerve injured rats, Malcangio et al. (2000) found that Aβ-fiber stimulation could evoke substance P release in spinal cord slices from rats with spinal nerve transection but not rats with sciatic nerve transection. More detailed studies reported that Aβ-fiber stimulation in vivo did not evoke substance P release after sciatic nerve transection (Allen et al., 1999) and after CCI or sciatic nerve ligation (Hughes et al., 2007).
Our observation that nerve injury hinders the ability of noxious stimuli to elicit substance P release may be related to paradoxical deficits in the perception of noxious stimuli that are often associated with allodynia and spontaneous pain in neuropathic pain syndromes (Campbell and Meyer, 2006).
Nerve injury eliminated the ability of MORs to inhibit substance P release
Our main finding was that in rats with CCI there was a complete loss of the inhibition of substance P release by the selective MOR agonist DAMGO. In contrast, in naïve and sham-operated rats DAMGO readily inhibited substance P release. After CCI, DAMGO failed to decrease substance P release even at 10 μM, a concentration three orders of magnitude higher than its potency at MORs: it induces MOR internalization with an EC50 of 30 nM (Marvizon et al., 1999b), it stimulates [γ35S]GTP binding with an EC50 of 28 nM (Yabaluri and Medzihradsky, 1997) and it inhibits adenylyl cyclase with an IC50 of 5–13 nM (Keith et al., 1998). Therefore, the loss of inhibition by DAMGO cannot be attributed to a right-shift of its concentration-response curve, but rather to a complete loss of its efficacy. This is consistent with the finding that spinal nerve ligation drastically decreased DAMGO inhibition of dorsal root-stimulated EPSCs in dorsal horn neurons (Kohno et al., 2005).
By using dorsal root stimulation, we studied substance P release from primary afferent terminals, which is where MOR inhibition of its release takes place. Some descending bulbospinal axons (Magoul et al., 1988, Ceccatelli et al., 1989, Nicholas et al., 1992) and possibly dorsal horn neurons (Noguchi and Ruda, 1992) also contain substance P. However, they could not have been recruited by our electrical stimulus, which was completely isolated from the spinal cord slice. The fact that a glutamate receptor antagonist did not inhibit substance P release evoked by dorsal root stimulation (Marvizon et al., 1997) rules out the possibility that substance P was released from dorsal horn neurons receiving synapses from primary afferents. Moreover, substance P immunoreactivity in laminae I and II, next to the neurons used to measure NK1R internalization, colocalizes extensively with CGRP and therefore is mainly in primary afferents (Tuchscherer and Seybold, 1989, Marvizon et al., 2009).
A likely explanation for the loss of MOR inhibition of substance P release is the decoupling of MORs from their signaling machinery to inhibit CaV channels in primary afferent terminals. MORs decrease substance P release by inhibiting CaV2.2 channels by two mechanisms: voltage-dependent inactivation by Gβγ (Dolphin, 2003, Evans and Zamponi, 2006, Dai et al., 2009) and voltage-independent inactivation by Tyr-phosphorylation by a Src family kinase (Diverse-Pierluissi et al., 1997, Strock and Diverse-Pierluissi, 2004, Evans and Zamponi, 2006, Dai et al., 2009). MOR inhibition of substance P release in our conditions is likely mediated by Src family kinase inactivation of CaV2.2, because high frequency stimulation of the dorsal root depolarizes primary afferent terminals and therefore removes the voltage-dependent Gβγ inactivation. Indeed, we recently reported that MOR inhibition of substance P release, elicited either by high frequency dorsal root stimulation in slices or by a noxious stimulus in vivo, is mediated by a Src family kinase (Zhang et al., 2010b).
MORs are still present in substance P-containing primary afferents after CCI
An alternative explanation for the loss of MOR inhibition of substance P release would be the downregulation of MORs in substance P-containing primary afferents. Indeed, there are multiple reports of reduced expression of MORs in primary afferents in different models of nerve injury, including peripheral axotomy (Zhang et al., 1998), partial sciatic nerve ligation (Rashid et al., 2004), spinal nerve ligation and spared nerve injury (Kohno et al., 2005), spinal nerve ligation (Lee et al., 2011), CCI (Hervera et al., 2011) and streptozotocin-induced diabetes (Shaqura et al., 2013). The extent of the reduction in MORs seems to depend on the severity of the nerve injury, with the greater loss in MORs occurring in the axotomy models. Although we didn’t measure total MOR content in the DRG, we found that the number of substance P-containing DRG neurons that also express MORs did not change after CCI. Most important, there were no changes in the colocalization of MORs with substance P in primary afferent fibers in the dorsal horn. Therefore, the complete loss of the inhibition of substance P release by MORs cannot be attributed to the disappearance of MORs from the substance P-containing primary afferents.
The fact that we found little decrease of MOR immunoreactivity in the DRG is probably due to the mild nature of the lesion produced by CCI. Indeed, one study that also used CCI (Hervera et al., 2011) found only a small decrease in MOR protein in Western blots, which may be limited to DRG neurons that do not contain substance P or may have gone undetected in our immunohistochemistry assays.
Paw inflammation did not affect MOR inhibition of substance P release
In contrast with the effect of CCI, inflammation of the hindpaw with CFA did not affect the ability of the MOR agonist DAMGO to inhibit substance P release. This indicates that the alteration in MOR signaling responsible for the loss of inhibition of substance P release is specific to neuropathic pain.
In neuropathic pain there is a decrease in the analgesia produced by spinally administered opiates. Thus, the analgesia produced by intrathecal morphine was substantially decreased after root ligation (Bian et al., 1995, Ossipov et al., 1995, Wegert et al., 1997), sciatic nerve ligation (Mao et al., 1995) or partial sciatic nerve injury (Rashid et al., 2004). This is also specific to neuropathic pain and could be due to the loss of MOR inhibition of substance P release. These phenomena are likely related to the low efficacy of opiates to treat neuropathic pain. In future studies, it will be interesting to determine whether the inhibition of substance P release by GABAB and α2A adrenergic receptors (Marvizon et al., 1999a, Zhang et al., 2010b) is also affected by nerve injury, which would be important to inform the development of therapies to treat neuropathic pain.
Highlights.
μ-Opioid receptor inhibition of substance P release is lost in neuropathic pain.
This inhibition is still present in normal rats and rats with inflammation.
The loss of inhibition was not due to μ-opioid receptor downregulation.
Acknowledgments
Supported by grants 1I01RX000378 from the Rehabilitation Research & Development Service, Department of Veterans Affairs to J.C.M. and R01-DA033059 from the National Institute of Drug Abuse to J.C.M and J.A.M. This study was done under the umbrella of the following UCLA institutes: Brain Research Institute, CURE: Digestive Diseases Research Center and the Oppenheimer Family Center for Neurobiology of Stress. We thank Wendy Walwyn for her valuable advice.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- CaV channels
voltage-gated calcium channels
- CCI
chronic constriction injury
- CFA
complete Freund’s adjuvant
- DAMGO
[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin
- DRG
dorsal root ganglion
- K+-aCSF
artificial cerebrospinal fluid with 5 mM KCl
- MOR
μ-opioid receptor
- NK1R
neurokinin 1 receptor
- PBS
phosphate-buffered saline
- PBS-Triton
phosphate-buffered saline with 0.3 % Triton X-100 and 0.001 % thimerosal
- sucrose-aCSF
artificial cerebrospinal fluid with 5 mM KCl and 215 mM sucrose instead of NaCl
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson DW, Lao L, Zhang G, Kim W, Marvizón JC. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increases with the firing frequency of C-fibers. Neuroscience. 2009;161:538–553. doi: 10.1016/j.neuroscience.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, Zamponi GW. Differential role of N-type calcium channel splice isoforms in pain. J Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bian D, Nichols ML, Ossipov MH, Lai J, Porreca F. Characterization of the antiallodynic efficacy of morphine in a model of neuropathic pain in rats. Neuroreport. 1995;6:1981–1984. doi: 10.1097/00001756-199510010-00007. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccatelli S, Millhorn DE, Hokfelt T, Goldstein M. Evidence for the occurrence of an enkephalin-like peptide in adrenaline and noradrenaline neurons of the rat medulla oblongata. Exp Brain Res. 1989;74:631–640. doi: 10.1007/BF00247366. [DOI] [PubMed] [Google Scholar]
- Chen W, Marvizon JC. Acute inflammation induces segmental, bilateral, supraspinally mediated opioid release in the rat spinal cord, as measured by μ-opioid receptor internalization. Neuroscience. 2009;161:157–172. doi: 10.1016/j.neuroscience.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Song B, Lao L, Perez OA, Kim W, Marvizon JCG. Comparing analgesia and μ-opioid receptor internalization produced by intrathecal enkephalin: Requirement for peptidase inhibition. Neuropharmacology. 2007;53:664–667. doi: 10.1016/j.neuropharm.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marvizon JC. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience. 2010;166:924–934. doi: 10.1016/j.neuroscience.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheppudira BP. Characterization of hind paw licking and lifting to noxious radiant heat in the rat with and without chronic inflammation. J Neurosci Methods. 2006;155:122–125. doi: 10.1016/j.jneumeth.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diverse-Pierluissi M, Remmers AE, Neubig RR, Dunlap K. Novel form of crosstalk between G protein and tyrosine kinase pathways. Proc Natl Acad Sci U S A. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA. 2005;293:3043–3052. doi: 10.1001/jama.293.24.3043. [DOI] [PubMed] [Google Scholar]
- Evans RM, Zamponi GW. Presynaptic Ca2+ channels--integration centers for neuronal signaling pathways. Trends Neurosci. 2006;29:617–624. doi: 10.1016/j.tins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Grady EF, Baluk P, Bohm S, Gamp PD, Wong H, Payan DG, Ansel J, Portbury AL, Furness JB, McDonald DM, Bunnett NW. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervera A, Negrete R, Leanez S, Martin-Campos JM, Pol O. Peripheral effects of morphine and expression of mu-opioid receptors in the dorsal root ganglia during neuropathic pain: nitric oxide signaling. Mol Pain. 2011;7:25. doi: 10.1186/1744-8069-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs AR, MacDonald G, Garsha K. Practical Confocal Microscopy. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. New York, NY: Springer; 2006. pp. 650–671. [Google Scholar]
- Honore P, Kamp EH, Rogers SD, Gebhart GF, Mantyh PW. Activation of lamina I spinal cord neurons that express the substance P receptor in visceral nociception and hyperalgesia. J Pain. 2002;3:3–11. doi: 10.1054/jpai.2002.27001. [DOI] [PubMed] [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal cord substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Scott DT, Riddell JS, Todd AJ. Upregulation of substance P in low-threshold myelinated afferents is not required for tactile allodynia in the chronic constriction injury and spinal nerve ligation models. J Neurosci. 2007;27:2035–2044. doi: 10.1523/JNEUROSCI.5401-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KC., 2nd Pharmacotherapy for neuropathic pain. Pain Pract. 2006;6:27–33. doi: 10.1111/j.1533-2500.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984;321:287–297. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- King JC. Therapeutic options for neuropathic pain. J Trauma. 2007;62:S7. doi: 10.1097/TA.0b013e3180653a71. [DOI] [PubMed] [Google Scholar]
- Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao L, Marvizon JCG. GABAA receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord. Neuroscience. 2005;130:1013–1027. doi: 10.1016/j.neuroscience.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Lao L, Song B, Chen W, Marvizon JC. Noxious mechanical stimulation evokes the segmental release of opioid peptides that induce μ-opioid receptor internalization in the presence of peptidase inhibitors. Brain Res. 2008;1197:85–93. doi: 10.1016/j.brainres.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao L, Song B, Marvizon JCG. Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: relationship with NMDA and GABAB receptors. Neuroscience. 2003;121:667–680. doi: 10.1016/s0306-4522(03)00501-3. [DOI] [PubMed] [Google Scholar]
- Lee CY, Perez FM, Wang W, Guan X, Zhao X, Fisher JL, Guan Y, Sweitzer SM, Raja SN, Tao YX. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain. 2011;15:669–675. doi: 10.1016/j.ejpain.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JL, Ding YQ, Li YQ, Li JS, Nomura S, Kaneko T, Mizuno N. Immunocytochemical localization of μ-opioid receptor in primary afferent neurons containing substance P or CGRP. A light and electron microscope study in the rat. Brain Res. 1998;794:347–352. doi: 10.1016/s0006-8993(98)00332-1. [DOI] [PubMed] [Google Scholar]
- Magoul R, Oblin A, Calas A, Araneda S. Serotonergic projections to the spinal cord but not those to the olfactory bulb also contain substance P. A combined immunocytochemical and autoradiographic study following retrograde axonal transport of [3H]serotonin labeled products. Neuroscience. 1988;26:959–969. doi: 10.1016/0306-4522(88)90112-1. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Jones MG, McMahon SB. Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur J Neurosci. 2000;12:397–399. doi: 10.1046/j.1460-9568.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- Marchand JE, Wurm WH, Kato T, Kream RM. Altered tachykinin expression by dorsal root ganglion neurons in a rat model of neuropathic pain. Pain. 1994;58:219–231. doi: 10.1016/0304-3959(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Chen W, Murphy N. Enkephalins, dynorphins and β-endorphin in the rat dorsal horn: an immunofluorescence colocalization study. J Comp Neurol. 2009;517:51–68. doi: 10.1002/cne.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABA(B) receptors. Eur J Neurosci. 1999a;11:417–426. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Waszak-McGee J, Mayer EA. Internalization of μ-opioid receptors in rat spinal cord slices. Neuroreport. 1999b;10:2329–2334. doi: 10.1097/00001756-199908020-00020. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003a;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Marvizon JCG, Wang X, Lao L, Song B. Effect of peptidases on the ability of exogenous and endogenous neurokinins to produce neurokinin 1 receptor internalization in the rat spinal cord. Br J Pharmacol. 2003b;140:1389–1398. doi: 10.1038/sj.bjp.0705578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauborgne A, Lutz O, Legrand JC, Hamon M, Cesselin F. Opposite effects of delta and mu opioid receptor agonists on the in vitro release of substance P-like material from the rat spinal cord. J Neurochem. 1987;48:529–537. doi: 10.1111/j.1471-4159.1987.tb04125.x. [DOI] [PubMed] [Google Scholar]
- McQuay HJ. Neuropathic pain: evidence matters. Eur J Pain. 2002;6(Suppl A):11–18. doi: 10.1053/eujp.2001.0316. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Gu G, Gracias NG, Wilkinson K, Hua XY, Vasko MR, Yaksh TL. Spinal NMDA receptors and nociception-evoked release of primary afferent substance P. Neuroscience. 2008;152:119–127. doi: 10.1016/j.neuroscience.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Arvidsson U, Hokfelt T. Serotonin-, substance P- and glutamate/aspartate-like immunoreactivities in medullo-spinal pathways of rat and primate. Neuroscience. 1992;48:545–559. doi: 10.1016/0306-4522(92)90401-m. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA. Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J Neurosci Res. 1994;37:596–603. doi: 10.1002/jnr.490370506. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ruda MA. Gene regulation in an ascending nociceptive pathway: inflammation-induced increase in preprotachykinin mRNA in rat lamina I spinal projection neurons. J Neurosci. 1992;12:2563–2572. doi: 10.1523/JNEUROSCI.12-07-02563.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci Lett. 1995;199:87–90. doi: 10.1016/0304-3940(95)12022-v. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. Increased analgesic potency of mu agonists after continuous naloxone infusion in rats. J Pharmacol Exp Ther. 1991;259:582–589. [PubMed] [Google Scholar]
- Portenoy RK, Foley KM, Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain. 1990;43:273–286. doi: 10.1016/0304-3959(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- Schuirmann D. A comparison of the Two One-Sided Tests Procedure and the Power Approach for assessing the equivalence of average bioavailability. Journal of Pharmacokinetics and Biopharmaceutics. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- Shaqura M, Khalefa BI, Shakibaei M, Winkler J, Al-Khrasani M, Furst S, Mousa SA, Schafer M. Reduced number, G protein coupling, and antinociceptive efficacy of spinal mu-opioid receptors in diabetic rats are reversed by nerve growth factor. J Pain. 2013;14:720–730. doi: 10.1016/j.jpain.2013.01.776. [DOI] [PubMed] [Google Scholar]
- Song B, Marvizon JC. Peptidases prevent μ-opioid receptor internalization in dorsal horn neurons by endogenously released opioids. J Neurosci. 2003a;23:1847–1858. doi: 10.1523/JNEUROSCI.23-05-01847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG. Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce μ-opioid receptor internalization in the rat spinal cord. J Neurosci. 2003b;23:9171–9184. doi: 10.1523/JNEUROSCI.23-27-09171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Strock J, Diverse-Pierluissi MA. Ca2+ channels as integrators of G protein-mediated signaling in neurons. Mol Pharmacol. 2004;66:1071–1076. doi: 10.1124/mol.104.002261. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Basbaum AI. Differential contribution of substance P and neurokinin A to spinal cord neurokinin-1 receptor signaling in the rat. J Neurosci. 2001;21:3656–3664. doi: 10.1523/JNEUROSCI.21-10-03656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? J Neurosci. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherer MM, Seybold VS. A quantitative study of the coexistence of peptides in varicosities within the superficial laminae of the dorsal horn of the rat spinal cord. J Neurosci. 1989;9:195–205. doi: 10.1523/JNEUROSCI.09-01-00195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegert S, Ossipov MH, Nichols ML, Bian D, Vanderah TW, Malan TP, Jr, Porreca F. Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats. Pain. 1997;71:57–64. doi: 10.1016/s0304-3959(97)03337-x. [DOI] [PubMed] [Google Scholar]
- Yabaluri N, Medzihradsky F. Down-regulation of mu-opioid receptor by full but not partial agonists is independent of G protein coupling. Mol Pharmacol. 1997;52:896–902. doi: 10.1124/mol.52.5.896. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen W, Lao L, Marvizon JC. Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as NK1R internalization. Eur J Neurosci. 2010a;31:225–237. doi: 10.1111/j.1460-9568.2009.07075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chen W, Marvizon JC. Src family kinases mediate the inhibition of substance P release in the rat spinal cord by μ-opioid receptors and GABAB receptors, but not α2 adrenergic receptors. Eur J Neurosci. 2010b;32:963–973. doi: 10.1111/j.1460-9568.2010.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Ji W, Pei LY, Wang W, Chen T, Li H, Zhang T, Wu SX, Li YQ. Acute colitis induces neurokinin 1 receptor internalization in the rat lumbosacral spinal cord. PloS one. 2013;8:e59234. doi: 10.1371/journal.pone.0059234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hokfelt T. Downregulation of μ-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82:223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]
- Zorman G, Belcher G, Adams JE, Fields HL. Lumbar intrathecal naloxone blocks analgesia produced by microstimulation of the ventromedial medulla in the rat. Brain Res. 1982;236:77–84. doi: 10.1016/0006-8993(82)90035-x. [DOI] [PubMed] [Google Scholar]